Abstract
Background
Recently, vascular shear wave elastography (SWE) applications have gained the interest and shown potential clinical value. However, there is limited evidence about the variability in Young’s modulus (YM) estimates. The purpose of this study was to quantify sources of variability associated with SWE measurements in common carotid artery (CCA).
Results
Mean YM of both anterior proximal and distal region of interest (ROIs) of the right CCA was significantly different (61 kPa vs 55.6 kPa; p < 0.001). Mean YM of anterior and posterior proximal and distal ROIs of the left side was significantly different (58.9 kPa vs 52.4 kPa; p < 0.001). Mean YM of right anterior and posterior proximal and distal ROIs was significantly different (58.3 kPa vs 51 kPa; p < 0.001).
Conclusions
US SWE was able to assess YM in CCAs although YM measurements were highly variable. The difference between YM between anterior and posterior walls is attributed to an artifact of the SWE technique that has not previously been reported and requires further investigation.
Similar content being viewed by others
Background
Shear wave elastography (SWE) is a quantitative imaging tool that provides a color SWE map over a greyscale B-mode image. SWE has been used in a wide range of non-vascular applications and found to provide reliable and clinically valuable estimates of tissue stiffness. There are three potential sources of variability: fundamental technical limitations associated with measurement accuracy and repeatability, imaging-related variability, and patient-related confounders [1]. Several previous studies have demonstrated that region of interest size, unit, probe load, anatomical area, breathing, pulsation, and depth can all contribute to variability in SWE measurements [2,3,4]. Acoustic nonlinearity within a tissue can also be a confounder [5]. Although these sources of variability have been reported, the relative contributions of confounders have not been fully investigated. Understanding and identifying sources of variability in SWE measurements will be essential in determining whether the SWE technique is sufficiently sensitive to small differences in YM associated with pathology. Additionally, acquisition standardization may help reduce manufacturer variability which may improve our ability to evaluate scanner performance.
Studies have shown that SWE can be used to distinguish between disease and non-disease tissue based on their elasticities [6,7,8]. However, there are currently no guidelines for standardization of ROIs and different ROI sizes are used without considering the impact of such variation. The influence of ROI size has been investigated extensively in clinical applications [9,10,11]). In some breast studies, small ROIs (up to 3 mm) [12, 13] were used to determine lesion YM values, while others have used relatively large ROIs to cover the whole lesion [9, 14]. A previous study by Yoon et al. [15] investigated factors related to inter-observer differences using SWE, and an inverse relationship was observed between the sizes of ROI and the inter-observer measurement. A smaller ROI is therefore thought to increase the chance of a sampling error, whereas a larger ROI is more vulnerable to heterogeneity. Studies have not so far investigated factors such as tissue depth, and other potential sources of variability such as observer experience. Variations of elastic modulus over different ROI sizes have also been reported in muscle elastography [16]. A larger ROI size is recommended for accurate SWE measurement, and variability in YM estimates is associated with smaller ROI [17]. Most vascular SWE applications have focused on the carotid artery (which has an intima-media thickness of about 0.6 mm), so the ROI size adopted needs to be larger than the artery. This means that YM estimates will be inevitably affected by adjacent structures with differing elasticity. This is likely to reduce the reliability of SWE measurement of vessel wall elasticities. The minimum ROI size of 2 mm is usually applied for vascular applications in order to minimize errors in YM estimates due to tissue heterogeneity [7, 18]
This study aimed to quantify sources of variability associated with SWE measurements.
Methods
Participants and study protocol
This study was approved by Research Ethics Committee (REC reference 14/EM/0056). SWE images obtained from our spontaneous coronary artery dissection (SCAD) patients and healthy subjects (n = 127) were analyzed retrospectively. All participants provided written, fully informed consent.
Measurement setup
SuperSonic Imagine Aixplorer ultrasound imaging system was used to assess the variability of SWE measurements at different CCA locations in Young’s modulus (kilopascal, kPa) using a curvilinear 4–15 MHz transducer. Measurement was obtained by an experienced certified clinical sonographer with efficient training on ultrasound SWE.
SWE image analysis
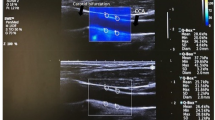
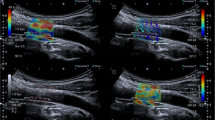
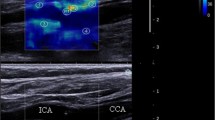
The SWE scans were analyzed retrospectively, and arterial wall YM was quantified using the Aixplorer’s in-built analysis software for 2 mm ROIs. ROIs were placed in different CCA locations to assess the variability of YM measurements with ROI position as shown in Fig. 5. The locations include anterior, posterior, left, right, distal, and proximal. Criteria for inclusion of measurements in further statistical analysis were as follows: (1) good image acquisition; (2) complete filling of the shear wave elastogram; and (3) adequate cine-loops. Exclusion criteria included are as follows: (1) poor image acquisition; (2) insufficient shear wave elastogram filling; and (3) cine-loops with less than five SWE frames.
Statistical analysis
Data were analyzed using statistics software (GraphPad Prism® version 7 (Prism, California, USA) and SPSS software version 24 (IBM Corporation, New York, USA). The Shapiro–Wilk test was performed to test for normality. Mean coefficient of variation (CV) and Bland–Altman analysis were used to assess the variability. A p value of p < 0.05 was considered statistically significant.
Results
Dependence of mean YM on ROI location
Mean YM measurements within the different ROIs were analyzed to assess variability and agreement of YM measurements within different ROIs, as well as between consecutive imaging frames to assess inter-frame variability. Table 1 provides a summary of mean YM measurements from different ROIs, comparing differences between ROIs adjacent (proximal and distal to the carotid bifurcation), anterior and posterior walls, and left and right sides.
Paired differences between the measurements from each of the 127 participants were quantified, and a paired t test was used to identify significant differences (p < 0.05). The mean difference in mean YM between adjacent ROIs, for each participant, is shown in the Bland–Altman plots in Fig. 1.
Bland–Altman analysis showed that the 95% CI for differences in left and right sides was between − 48 and 50 kPa with 1 kPa estimated bias, which was not a significant bias (Fig. 2).
Anterior and posterior wall measurements from each participant showed a statistically significant difference in mean anterior YM compared to the posterior estimate using a paired t test on the 127 participants; the average anterior YM was 7 kPa [95% CI:4–10 p = < 0.001] higher than the posterior wall.
Bland–Altman analysis reveals very wide 95% limits of agreement, ranging from 41 to − 28 kPa and a bias of 7 kPa (Fig. 3). The absolute mean difference in estimated YM between the participants’ anterior and posterior walls was 7 kPa. The Box and Whisker plots in Fig. 4 show that the anterior and posterior wall measurements were also significantly different between the population groups (p < 0.01).
Inter-frame YM reproducibility
Table 2 shows that left CCAs are more variable than right CCAs with a difference of 14%. Variability is more obvious in the posterior wall compared to the anterior wall of CCAs.
Discussion
SWE technology has been used in many applications, but its suitability for vascular applications remains hotly debated. It has been proposed that SWE might have potential clinical value in the diagnosis of vascular disease, and in particular, detection of early changes in arterial vessel elasticity [19]. However, concerns have arisen over the reliability of SWE YM estimates and the potential impact of factors such as ROI size and position on YM estimates. Ultimately, if measurement variability is greater than typical clinically significant differences, this will limit the usefulness of SWE in clinical practice. This study focused on the identification and quantification of sources of variability in arterial wall SWE measurements (Fig. 5).
Our analysis of individual ROI data may help to optimize protocols for the estimation of arterial wall stiffness using SWE. This provides a more detailed evaluation of the potential value of SWE which may help implementation of SWE in the vascular clinic. This study found a wide range (1–259 kPa with a mean of 55 kPa) in the estimated mean YM within each of the 1016 ROIs ((2 anterior + 2 posterior) * 2 (left and right CCAs) *127 (participants) = 1016 ROIs). Similarly, mean YM measurements within a single 2 mm ROI across 5 frames varied widely, with a coefficient of variation from 1 to 135% and a mean of 26%.
The challenges associated with the measurement of YM of arteries are widely recognized, but this is the first study to systematically estimate variability in SWE parameters as a function of ROI position (between adjacent ROIs, different positions in the vessel walls, and left vs right carotid arteries, within and between subjects, with varying machine settings and physiology, and considering inter-observer variability) [18, 20,21,22,23,24]. Previous researchers have cited small heterogeneous vessel walls, wall motion due to the blood flow pulsatility, and nonlinear elastic properties of tissue. Assumptions made by commercial scanners that calculate the YM from the measured shear wave velocity may be valid for bulk homogeneous tissues but not for vessels, which support Lamb wave propagation and require a different theoretical model [24]. CCA stiffness also varies with the cardiac cycle which our study did not consider, and which may have added to the variability in our measurements. A previous study of the YM in the CCA of one healthy volunteer throughout the cardiac cycle showed a higher YM in systole (130 kPa) compared to diastole (80 kPa) [25]. Unfortunately, our measurements were not synchronized with ECG data; however, a comparison of successive frames shows variations between frames when measuring the same ROI.
A previous study by Ramnarine et al. [18] investigated YM measurements in the presence of steady and pulsatile flow conditions. They found that YM estimates were not significantly affected by the pulsatile flow. However, YM measurements over the cardiac cycle were estimated using a single SWE frame instead of cardiac gating. There is considerable scope for improvement in SWE technology for vascular applications and our study highlights wide variability in YM measurements, which would limit the clinical sensitivity and potential clinical implementation of YM measurements.
Mean YM measurements between adjacent ROIs were expected to have the closest agreement in view of their close proximity. The mean difference between adjacent regions of interest was 14 kPa. The right posterior wall showed significant differences between adjacent ROIs with the ROI proximal to the carotid bifurcation slightly higher than the distal ROI (bias of 5 kPa). Rather than a true difference in YM between adjacent ROIs, differences could be attributed to SWE imaging artifacts such as a vertical banding sometimes observed during scans and placement of adjacent ROIs in these regions.
There was no significant difference between YM measurements between the left and right CCA, with a bias of 0.6 in the anterior wall (mean 59 ± 25 kPa) and a bias of 1.4 between the left and right posterior walls (mean 52 ± 26 kPa). The high variability between these YM measurements (95% limits of agreement from -28 to 41 kPa) shows that measurement variability is far higher than the bias. Differences in results between ROIs may be attributed to an artifact of the SWE technique related to the shear wave propagation or implementation in the scanner, depth of the ROI, or the position of the ROI within the anterior wall where the surrounding tissues including muscles may be of high YM. A real difference due to different tethering between anterior and posterior walls would also explain the findings and requires further investigation. Preliminary, unpublished data from our vessel phantom models surrounded by water or a tissue mimic highlighted the importance of the surrounding medium, supporting this observation. It showed that in a submerged phantom YM anterior wall measurements were significantly higher than posterior wall. Moreover, results from our phantom test object specifically clarify this aspect in which YM measurements decrease with increasing depth, and the maximum penetration depth was dependent on the pipe diameter [26]
Previous studies have reported reproducibility of YM measurements with a coefficient of variation (COV) of 22% compared to a mean of 26% in this study [18]. Our finding that inter-frame variability of the anterior wall was lower than for the posterior wall (CoV 22% vs 30%) suggests the anterior wall YM estimate may be a more robust and sensitive biomarker. Considering the higher YM estimate in the anterior wall, future study protocols may benefit from assessing anterior and posterior walls separately.
Conclusions
This study has investigated the influence of ROI positioning on YM measurements across different CCA positions. US SWE was able to assess YM in CCAs though YM measurements were highly variable. The difference between YM between anterior and posterior walls can be attributed to an artifact of the SWE technique that has not previously been reported. Further investigation is required to study the source of the variability associated with SWE vascular application.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- CCA:
-
Common carotid artery
- SWE:
-
Shear wave elastography
- ROI:
-
Region of interest
- YM:
-
Young’s modulus
References
Tang X, Zhu B, Tian M, Guo R, Huang S, Tang Y, Qiu L (2021) Preliminary study on the influencing factors of shear wave elastography for peripheral nerves in healthy population. Sci Rep 11(1):1–9
Alfuraih AM, O’Connor P, Tan AL, Hensor E, Emery P, Wakefield RJ (2017) An investigation into the variability between different shear wave elastography systems in muscle. Med Ultrason 19(4):392–400
Almutairi FF, Abdeen R, Alyami J, Sultan SR (2022) Effect of depth on ultrasound point shear wave elastography in an elasticity phantom. Appl Sci 12(13):6295
Mulabecirovic A, Mjelle AB, Gilja OH, Vesterhus M, Havre RF (2018) Liver elasticity in healthy individuals by two novel shear-wave elastography systems—Comparison by age, gender, BMI and number of measurements. PLoS ONE 13(9):e0203486
Rominger MB, Kälin P, Mastalerz M, Martini K, Klingmüller V, Sanabria S, Frauenfelder T (2018) Influencing factors of 2D shear wave elastography of the muscle–an ex vivo animal study. Ultrasound Int Open 4(02):E54–E60
Alis D, Durmaz ESM, Civcik C, Tutuncu M, Saip S, Kocer N, Kizilkilic O (2018) Assessment of the common carotid artery wall stiffness by shear wave elastography in Behcet’s disease. Med Ultrason 20(4):446–452
Lou Z, Yang J, Tang L, Jin Y, Zhang J, Liu C, Li Q (2017) Shear wave elastography imaging for the features of symptomatic carotid plaques: a feasibility study. J Ultrasound Med 36(6):1213–1223
Marais L, Pernot M, Khettab H, Tanter M, Messas E, Zidi M, Boutouyrie P (2019) Arterial stiffness assessment by shear wave elastography and ultrafast pulse wave imaging: comparison with reference techniques in normotensives and hypertensives. Ultrasound Med Biol 45(3):758–772
Berg WA, Cosgrove DO, Doré CJ, Schäfer FK, Svensson WE, Hooley RJ, Tourasse C (2012) Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology 262(2):435–449
Bilgili Y, Unal B (2004) Effect of region of interest on interobserver variance in apparent diffusion coefficient measures. Am J Neuroradiol 25(1):108–111
Evans A, Whelehan P, Thomson K, Brauer K, Jordan L, Purdie C, Thompson A (2012) Differentiating benign from malignant solid breast masses: value of shear wave elastography according to lesion stiffness combined with greyscale ultrasound according to BI-RADS classification. Br J Cancer 107(2):224–229
Skerl K, Vinnicombe S, Giannotti E, Thomson K, Evans A (2015) Influence of region of interest size and ultrasound lesion size on the performance of 2D shear wave elastography (SWE) in solid breast masses. Clin Radiol 70(12):1421–1427
Youk JH, Son EJ, Han K, Gweon HM, Kim JA (2018) Performance of shear-wave elastography for breast masses using different region-of-interest (ROI) settings. Acta Radiol 59(7):789–797
Chang JM, Moon WK, Cho N, Yi A, Koo HR, Han W, Kim SJ (2011) Clinical application of shear wave elastography (SWE) in the diagnosis of benign and malignant breast diseases. Breast Cancer Res Treat 129(1):89–97
Yoon K, Jeong WK, Kim Y, Kim MY, Kim TY, Sohn JH (2017) 2-dimensional shear wave elastography: interobserver agreement and factors related to interobserver discrepancy. PLoS ONE 12(4):e0175747
Kot BCW, Zhang ZJ, Lee AWC, Leung VYF, Fu SN (2012) Elastic modulus of muscle and tendon with shear wave ultrasound elastography: variations with different technical settings. PLoS ONE 7(8):e44348
Ateş F, Hug F, Bouillard K, Jubeau M, Frappart T, Couade M, Nordez A (2015) Muscle shear elastic modulus is linearly related to muscle torque over the entire range of isometric contraction intensity. J Electromyogr Kinesiol 25(4):703–708
Ramnarine KV, Garrard JW, Kanber B, Nduwayo S, Hartshorne TC, Robinson TG (2014) Shear wave elastography imaging of carotid plaques: feasible, reproducible and of clinical potential. Cardiovasc Ultrasound 12(1):1–9
Pruijssen JT, de Korte CL, Voss I, Hansen HH (2020) Vascular shear wave elastography in atherosclerotic arteries: a systematic review. Ultrasound Med Biol 43:2145
Al-Mutairi FF, Al-Hussaini A, Marsh AM, Samani N, McCann G, Adlam D, Ramnarine KV (2022) Ultrasound shear wave elastography imaging of common carotid arteries in patients with spontaneous coronary artery dissection (SCAD). J Ultrasound. https://doi.org/10.1007/s40477-021-00627-2
Bruce M, Kolokythas O, Ferraioli G, Filice C, O’Donnell M (2017) Limitations and artifacts in shear-wave elastography of the liver. Biomed Eng Lett 7(2):81–89
Maksuti E, Widman E, Larsson D, Urban MW, Larsson M, Bjällmark A (2016) Arterial stiffness estimation by shear wave elastography: validation in phantoms with mechanical testing. Ultrasound Med Biol 42(1):308–321
Urban MW, Nenadic IZ, Pislaru C, Greenleaf JF (2013) Measurement of longitudinal and circumferential waves in tubes and artery excited with ultrasound radiation force. In: 2013 IEEE International Ultrasonics Symposium (IUS), pp 1765–1768.
Widman E, Maksuti E, Larsson D, Urban MW, Bjällmark A, Larsson M (2015) Shear wave elastography plaque characterization with mechanical testing validation: a phantom study. Phys Med Biol 60(8):3151
Couade M, Pernot M, Prada C, Messas E, Emmerich J, Bruneval P, Tanter M (2010) Quantitative assessment of arterial wall biomechanical properties using shear wave imaging. Ultrasound Med Biol 36(10):1662–1676
Al-Mutairi FF, Chung EM, Moran CM, Ramnarine KV (2021) A novel elastography phantom prototype for assessment of ultrasound elastography imaging performance. Ultrasound Med Biol 47(9):2749–2758
Acknowledgements
Not applicable.
Funding
This research work was funded by the Institutional Fund Projects under grant no. (IFPDP-114-22). Therefore, the author gratefully acknowledges technical and financial support from the Ministry of Education and the Deanship of Scientific Research (DSR), King Abdulaziz University (KAU), Jeddah, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
The author confirms sole responsibility for the following: study conception and design, data collection, analysis and interpretation of results, and manuscript preparation. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the University Hospitals of Leicester NHS Trust under NHS Research Ethics Committee Health Research Authority approval (REC reference 14/EM/0056; 2017). All participants provided written, fully informed consent.
Consent for publication
All participants provided written, fully informed consent.
Competing interests
The author declares that he has no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Almutairi, F.F. Measurement variability in two-dimensional shear wave elastography (SWE) of common carotid artery (CCA). Egypt J Radiol Nucl Med 53, 216 (2022). https://doi.org/10.1186/s43055-022-00902-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00902-0