Abstract
Background
Breast cancer is known to be the most common cancer in women; in the last decade, contrast-enhanced magnetic resonance imaging has become an important tool in the diagnosis of cancer breast. Numerous studies have analyzed associations between imaging and histopathological features as well as the proliferation potential of breast cancer. The purpose of this study was to evaluate the relationship between the apparent diffusion coefficient (ADC) and expression of Ki-67 as well as tumor molecular subtype in breast cancer.
Results
No significant difference between the mean ADC value of tumors of grade I, II, and III was found. However, there was a significant difference between the mean ADC value of tumors of molecular type A and molecular type B (P = 0.000), HER2 overexpression (P = 0.018), and TN (P = 0.000), respectively. However, there was no significant difference between molecular type B, HER2 overexpression and TN. Also, no significant difference was found between the Ki-67 value of tumors of grade I, II, and III. Yet there was a significant difference between the mean ADC value of tumors of molecular type A and molecular type B (P = 0.000), HER2 overexpression (P = 0.014), and TN (P = 0.000), respectively. However, there was no significant difference between molecular type B, HER2 overexpression, and TN.
Conclusions
There is a significant inverse correlation between ADC values and Ki-67 expression. DWI and Ki-67 could be a good discriminator between tumors of molecular subtype A from other subtypes, yet it did not show a correlation with the tumor grade.
Similar content being viewed by others
Background
A common malignancy among women is breast cancer (BC). Using DNA microarray techniques, the gene pattern of expression in breast cancer has been classified into different molecular types with clinical, biologic, and treatment effects based on estrogen (ER), progesterone (PR) and HER-2 receptor analysis. Breast cancer has four primary molecular subtypes, defined mainly by hormone receptors (HR) and other types of proteins involved (or not) in each cancer: Luminal A or HR + /HER2- (HR-positive/HER2-negative); Luminal B or HR + /HER2 + (HR-positive/HER2-positive); triple negative or HR-/HER2- (HR/HER2-negative); and HER2-positive [1].
Ki-67 is a nuclear protein related to cellular proliferation and was first identified by Gerdes et al. in the early 1980s [2]. Ki-67 is found in all proliferating cells and is used as a proliferation marker; it is also considered a prognostic factor for breast cancer [3, 4]. Compared with other markers, Ki- 67 immuno-staining is a suitable method for testing proliferating index [5, 6].
Various imaging modalities including mammography, ultrasound, and magnetic resonance (MRI) play a vital role in diagnosing and staging breast cancer. Imaging cannot only detect breast tumors but can also predict their histopathological characteristics, yet none of these imaging modalities can provide accurate information about tissue cellularity, which is considered the main indicator of tumor grade. Therefore, many investigators try to assess by using diffusion-weighted breast imaging and without intravenous contrast material injection, diffusion-weighted breast imaging can facilitate the diagnosis of breast lesions, yet may require some increase in examination time [7, 8, and 9].
Diffusion-weighted imaging of the breast is an MRI sequence that gives several advantages compared to dynamic contrast-enhanced sequence as it does not require intravenous contrast, short duration, and easy to perform. Moreover, integrating quantitative imaging biomarkers gives us the ability to measure the underlying pathological mechanisms in vivo noninvasively, identifying properties that are important for detection, diagnosis, prognosis, or response to therapy [10].
In this study, we aimed to assess the status of apparent diffusion coefficient (ADC) in breast cancer patients and to evaluate its association with other factors including Ki- 67, molecular subtype, and grade of breast tumor to validate its significance as a prognostic factor of breast cancer.
Methods
This was a retrospective study. It was approved by the institutional review board. Between April 2016 and February 2020, the total number of included patients was 55.
Inclusion criteria: (1) patients with breast malignancy proved by pathology either by surgery or by biopsy; (2) patients who had a standard MRI breast study, including axial T1WI, fat-suppressed T2 WI, axial fat-saturated T1WI pre- and post-enhancement, and DWI sequences; and (3) patients with complete clinical data; immunohistochemistry and Ki-67 values in their histopathology reports. Exclusion criteria: (1) operated/ treated breast before MRI; (2) poor image quality; and (3) incomplete immunohistochemistry or Ki-67 values in their reports.
MRI technique
DCE-MRI breasts were performed on a 1.5-T system (MAGNETOM Aera; Siemens, Erlangen, Germany). Women lying prone with their breasts fixed in a dedicated four-channel phased-array breast coil. All written consents were obtained before the study was performed. Patients were advised not to move to avoid motion artifacts.
The following MRI sequences were taken: slice thickness 4 mm, FOV 350 mm, and matrix 512 × 512: Axial Turbo Inversion Recovery Magnitude (TIRM) with TR 7700 ms and TE 74 ms; Axial T2 fast spin-echo (FSE) with TR 6160 ms and TE 76 ms; and Axial T1 fast spin-echo (FSE) with TR 415 ms and TE 4.6 ms. Diffusion-weighted imaging (DWI) was done at TR 8200 ms and TR 85 ms in the axial plane bilaterally with b values of 0, 500, and 1000 s/mm2. ADC map was systematically performed. ADC value was measured in mm2/s using a rounded ROI with a diameter of 5–10 mm2 placed on the darkest point of the mass which shows diffusion restriction on the DW sequences. Areas of T2 shine-through, such as cystic or necrotic portions of the tumor shown as high signal intensity on T2-weighted images and ADC maps, were avoided. When comparing with DCE MR images, the enhancing solid portion of the mass was used to locate ADC measurements. The ADC value was automatically calculated when the ROI was drawn. Two radiologists with 20 and 25 years of experience in MRI breast analyzed the images. The diagnosis was reached by consensus.
Histological analysis
According to the numerical scoring pattern to define tubule formation, pleomorphism, and mitotic count, Nottingham combined grading was used to assess histological grades of invasive ductal carcinoma, NOS (non-otherwise specified). The score ranges from 3 to 9, with a total score of 3–5 representing low grade (grade 1), a score of 6 or 7 representing grade 2, and a score of 8 or 9 representing grade 3 (Table 1): Nottingham combined histological grade [11] (Figs. 1, 2, 3, 4).
Statistical analysis
Data were coded and entered data using SPSS (Statistical Package for the Social Sciences) version 23. Data were summarized using the mean and standard deviation. For quantitative data, we used median, minimum, and maximum; for qualitative data, frequency (number) and relative frequency (%) were used for categories. A comparison of quantitative variables was made using nonparametric Kruskal–Wallis and Mann–Whitney tests [12]. ROC curve was plotted with an area under the curve analyzed to detect the specific cutoff value of ADC for the detection of high-grade tumors. P values below 0.05 (< 5%) were considered statistically significant.
Results
Fifty-five female patients were included in this study, with an age range from 21 to 83 years (mean age 50.8 ± 14). The included carcinomas included 48 (87.3%) invasive ductal carcinomas; 26 (54%) of them were NST (no specific type), 3 (5.5%) mucinous carcinoma, 2 (3.6%) invasive lobular carcinoma, 1 (1.8%) NEC (neuroendocrine carcinoid), and 1 (1.8%) carcinosarcoma/metaplastic carcinoma.
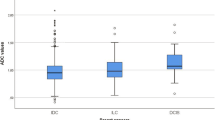
Their grading was as follows: 11 lesions (20%) grade I, 38 lesions (69.1%) grade II, and 6 (10.9%) lesions grade III. Ten (18.2) lesions were molecular type A, 35 (63.6) lesions were molecular type B, 5 (9.1) were HER2 overexpression, and 5 (9.1) were triple negative (Table 2).
Analysis of diffusion-weighted images and comparison of the mean ADC value as well as Ki-67 with the pathological grade and molecular subtype of the tumor were performed. In all the studied lesions, 55 (100%) showed a bright signal in DWI and low/intermediate signal in ADC map denoting diffusion restriction. ADC values ranged from 0.5 to 1.6 × 10–3 mm2/s (mean 1 ± 0.2 × 10-3mm2/s). The mean ADC value of grade I was 1.0 ± 0.2 × 10–3 mm2/s, grade II was 0.9 ± 0.1 × 10–3 mm2/s, and grade III was 0.9 ± 0.2 × 10–3 mm2/s (Table 3). There was no significant difference regarding the mean ADC values of tumors grade I, II, and III. The mean ADC value of molecular type A, molecular type B, HER2 overexpression, and triple-negative subtypes was 1.2 ± 0.2 × 10–3 mm2/s, 0.9 ± 0.1 × 10–3 mm2/s, 0.9 ± 0.1 × 10–3 mm2/s, and 0.8 ± 0.1 × 10–3 mm2/s, respectively. Yet a significant difference between the mean ADC value of tumors of different molecular subtypes (P = 0.000) was found. A significant difference between the mean ADC value of tumors of molecular type A as opposed to molecular type B (P = 0.000), HER2 overexpression (P = 0.018), and TN (P = 0.000), respectively, was found. However, there was no significant difference between molecular type B, HER2 overexpression, and TN (Tables 4, 5, 6).
Ki-67 values ranged from 6 to 92.5 with a mean of 42.9 ± 17.3; the mean Ki-67 of grade I, grade II, and grade III was 41.3 ± 18.2, 40.9 ± 16.2, and 58.8 ± 17.2, respectively. There was no significant difference between the Ki-67 value of tumors of grade I, II, and III (Table7).
The Ki-67 value of tumors of different molecular subtypes showed a significant difference (P = 0.000) (Table 8). A significant difference was found between the mean ADC value of tumors of molecular type A as opposed to molecular type B (P = 0.000), HER2 overexpression (P = 0.014), and TN (P = 0.000), respectively. However, there was no significant difference between the last three types together: molecular type B, HER2 overexpression, and TN (Tables 5 and 6).
Correlation between ADC and Ki-67 value showed an R value of -0.779 and R2 value of 0.599 (P = 0.000) (significant/good negative correlation) (Tables 9 and 10).
Discussion
Different molecular types of breast carcinoma show different biological characteristics, clinical outcomes, and prognoses [13]. An accurate assessment of the disease course and prognosis preoperatively is essential for accurate diagnosis planning for treating breast cancer [14].
The value of breast MRI in the preoperative assessment of patients is important owing to its value as a means of describing pattern, size, and number of breast lesions. The early proliferation of tumor accompanies changes in both anabolism and catabolism affecting its size and altering the intra- and extracellular environment. Functional MR as diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) assist the radiologist in finding certain changes related to tumor proliferation [15].
In the current study, the calculated mean ADC value of breast malignant masses measured 1 ± 0.2 × 10–3 mm2/s. This was similar to mean ADC values reported by previous studies, such as Belli et al. [13] and Costantini et al. [16], where mean ADC values measured 1.02 × 10–3 mm2/s and 1.03 × 10–3 mm2/s, respectively. However; other studies reported lower ADC including Kato et al. [17] who reported the mean ADC value of 0.894 ± 0.20 × 10–3 mm2/s; it was 0.85 ± 0.12 × 10–3 mm2/s in Gouhar et al. [18] study, 0.91 ± 0.20 × 10–3 mm2/s in Park et al. [19] study, 0.93 ± 0.27 × 10–3 mm2/s in Ulghaffara et al. [20] study, and 0.91 ± 0.151 × 10–3 mm2/s in Matsubayashi et al. [21] study.
ADC values ranged from 0.5 to 1.6 × 10–3 mm2/s in our study, thus lying above the cutoff value differentiating benign from malignant lesions suggested by previous studies, such as Sharma et al. [15] with a cutoff ADC value of 1.23 × 10−3 mm2/s, and Tan et al. [22] where the cutoff ADC value for benign lesions was 1.21 × 10−3 mm2/s for b = 500 s/mm2 and for malignant lesions was 1.22 × 10−3 mm2/s for b value:1000 s/mm2. Studying the correlation between the mean ADC value and histological grades of breast carcinoma, we found that there was no significant relation between them.
This coincided with the previous research done by Park et al. [19] and Tan et al. [22] who found no significant correlation between the measured ADC values and tumor grades.
On the contrary, Belli et al. [13], Abdel Razek et al. [23], Costantini et al. [16], Gouhar et al. [18], and Guo Yan et Al. [24] found that there was a significant yet inverse relation between them.
In the current study, the mean ADC value for grade I masses was 1.0 ± 0.2 × 10–3 mm2/s, that of grade II was 0.9 ± 0.1 × 10–3 mm2/s, and that of grade III was 0.9 ± 0.2 × 10–3 mm2/s. Kindly view Figs. 1, 2, 3 and 4 (case presentations).
A 42-year-old female with multifocal left breast ca A MLO low-energy CESM (B), (C) recombined CESM MLO and CC showing multiple enhancing masses lower inner quadrant D DCE-MRI shows marginal enhancement with central necrosis E and F DWI b value = 800 and ADC, respectively, showing peripheral diffusion restriction. ADC value = 0.989 × 10−3mm2/s. HER-2 positive BC. IDC GRADE III
A 52-year-old female with recently diagnosed left breast LOQ IDC Grade II (Luminal type A) A MRI Wash-in map B, C DWI b value = 800 and ADC, respectively, showing diffusion restriction ADC value = 0.123 × 10−3mm2/s D 3D Tomo slice CC view a single spiculate mass (arrow) E DCE-MRI sagittal shows intense marginal enhancement
A 49-year-old female with bifocal right breast IDC Grade II (luminal subtype B) A DWI b value:800 B ADC showing diffusion restriction ADC value = 0.994 × 10−3mm2/s. C DCE-MRI showing intense enhancement. D 3D MIP E delayed sagittal MRI showing central wash-out with marginal enhancement. Ki-67 high 25%
A 72-year-old female with an incidentally discovered single circumscribed left breast mass with homogenous enhancement as seen on mammogram CC view (A) and recombined CESM (B). C and D DWI b value = 800 and ADC, respectively, showing diffusion restriction. ADC value = 0.789 × 10−3mm2/s E Axial MRI Left breast shows hypointense mass with smooth margin F DCE-MRI 3D MIP shows the left breast mass with feeding vessels (arrow) G US shows the mass with circumscribed margin, posterior enhancement and advancing anterior edge. Note peripheral vascularity by color Doppler. Core biopsy revealed metastatic NEC, triple negative. H Axial CT scan shows the primary mesenteric carcinoid tumor with calcifications
This is equal to the results of Costantini et al. [16] reporting mean ADC values for grade I, II, and III tumors to be: 1.25 × 10−3 mm2/s, 1.02 × 10−3 mm2/s, and 0.92 × 10−3 mm2/s, respectively. However, it differs from the results obtained by Gouhar et al. [18] who reported that the mean ADC values of grades I, II, and III were 0.96 ± 0.12 × 10–3 mm2/s, 0.87 ± 0.07 × 10–3 mm2/s, and 0.75 ± 0.12 × 10–3 mm2/s, respectively. This difference may be attributed to the discrepancy in patient number and diffusion technique using different b values.
High ADC values can be useful in diagnosing TNBC [25]. In our study, we had 5 cases of TNBC with a mean ADC value measuring 0.8 × 10−3mm2/s. It is postulated that TNBC shows higher ADC values due to necrotic tissue.
In previous studies, breast carcinomas showing high expression of Ki 67 had lower ADC values as opposed to tumors with low Ki 67 expression (26). In breast cancer, an insignificant correlation between both was found. Thus, ADC cannot be used as a proliferation marker in breast cancer [27]. This differs from our results which showed a good negative correlation between both ADC and Ki-67. Low ADC values were associated with tumors with high Ki-67 expression which is a predictor of proliferation and prognosis. This difference may be attributed to a limited number of patients, uneven distribution of the variable histological grades, and molecular subtypes.
Limitations of this study
The limited number of patients might have affected our results, especially that all our patients were pathologically proven malignant breast lesions. No cutoff value for luminal subtypes has been elicited during our study result analysis; this might be due to overlap between readings among molecular subtypes. Further studies on a larger scale of patients should be encouraged.
Conclusions
There was a significant inverse correlation between ADC values and Ki-67 expression. ADC values could be a good discriminator between tumors of molecular subtype A from other subtypes. Ki 67 could also be a good discriminator between tumors of molecular subtype A from other subtypes. Both ADC values and Ki- 67 did not show a correlation with the pathological grade of the tumor.
Availability of data and materials
All data and materials used in this research are available.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- BC:
-
Breast cancer
- DWI:
-
Diffusion-weighted imaging
- ER:
-
Estrogen receptors
- HR:
-
Hormone receptor
- MRI:
-
Magnetic resonance imaging
- NOS:
-
Non-otherwise specified
- NST:
-
No specific type
- NEC:
-
Neuroendocrine carcinoid
- PR:
-
Progesterone receptors
- TNBC:
-
Triple-negative breast cancer
References
Yadav BS, Sharma SC, Chanana P, Jhamb S (2014) Systemic treatment strategies for triple-negative breast cancer. World J Clin Oncol 5(2):125–133
Gerdes J, Schwab U, Lemke H, Stein H (1983) Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31(1):13–20
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA (2010) Ki67 in breast cancer Prognostic and predictive potential. Lancet Oncol 11(2):174–183
de Azambuja E, Cardoso F, de Castro G Jr, Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M (2007) Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 96(10):1504–1513
Ellis MJ, Suman VJ, Hoog J, Goncalves R, Sanati S, Creighton CJ, DeSchryver K, Crouch E, Brink A, Watson M, Luo J, Tao Y, Barnes M, Dowsett M, Budd GT, Winer E, Silverman P, Esserman L, Carey L, Hunt K (2017) Ki67 proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results from the American College of Surgeons Oncology Group Z1031 Trial (Alliance). J Clin Oncol 35(10):1061–1069
Carbognin L, Sperduti I, Fabi A, Dieci MV, Kadrija D, Griguolo G, Pilotto S, Guarneri V, Zampiva I, Brunelli M, Orvieto E, Nortilli R, Fiorio E, Parolin V, Manfrin E, Caliò A, Nisticò C, Pellini F, Scarpa AB, E. (2017) October). Prognostic impact of proliferation for resected early stage ‘pure’ invasive lobular breast cancer: cut-off analysis of Ki67 according to histology and clinical validation. The Breast 35:21–26
Surov A, Clauser P, Chang YW, Li L, Martincich L, Partridge SC, Kim JY, Meyer HJ, Wienke A (2018) Can diffusion-weighted imaging predict tumor grade and expression of Ki-67 in breast cancer? A multicenter analysis. Breast Cancer Res 20(1):58
Suo S, Cheng F, Cao M, Kang J, Wang M, Hua J, Hua X, Li L, Lu Q, Liu J, Xu J (2017) Multiparametric diffusion-weighted imaging in breast lesions: Association with pathologic diagnosis and prognostic factors. J Magn Reson Imag 46(3):740–750
Woodhams R, Ramadan S, Stanwell P, Sakamoto S, Hata H, Ozaki M, Kan S, Inoue Y (2011) Diffusion-weighted imaging of the breast: Principles and clinical applications. Radiographics 31(4):1059–1084
European Society of Radiology (ESR) (2013) ESR statement on the stepwise development of imaging biomarkers. Insights Into Imag 4(2):147–152
Zbytek B, Cohen C, Wang J, Page A, Williams DJ, Adams AL (2013) Nottingham-defined mitotic score: comparison with visual and image cytometric phosphohistone H3 labeling indices and correlation with Oncotype DX recurrence score. Appl Immunohistochem Mol Morphol 21:48–53
Chan YH (2003) Biostatistics102: quantitative data—parametric and non-parametric tests. Singap Med J 44(8):391–396
Belli P, Costantini M, Bufi E, Giardina GG, Rinaldi P, Franceschini G, Bonomo L (2015) Diffusion magnetic resonance imaging in breast cancer characterisation: correlations between the apparent diffusion coefficient and major prognostic factors. Radiol Med (Torino) 120(3):268–276
Baba S, Isoda T, Maruoka Y, Kitamura Y, Sasaki M, Yoshida T, Honda H (2014) Diagnostic and prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. J Nucl Med 55(5):736–742
Sharma U, Sah RG, Agarwal K, Parshad R, Seenu V, Mathur SR, Hari S, Jagannathan NR (2016) Potential of diffusion-weighted imaging in the characterization of malignant, benign, and healthy breast tissues and molecular subtypes of breast cancer. Front Oncol 6:126
Costantini M, Belli P, Rinaldi P, Bufi E, Giardina G, Franceschini G, Petrone G, Bonomo L (2010) Diffusion-weighted imaging in breast cancer: relationship between apparent diffusion coefficient and tumour aggressiveness. Clin Radiol 65(12):1005–1012
Kato F, Kudo K, Yamashita H, Wang J, Hosoda M, Hatanaka KC, Mimura R, Oyama-Manabe N, Shirato H (2016) Differences in morphological features and minimum apparent diffusion coefficient values among breast cancer subtypes using 3-tesla MRI. Eur J Radiol 85(1):96–102
Gouhar GK, El-Hariri MA, Lotfy WE (2011) Malignant breast tumours: Correlation of apparent diffusion coefficient values using diffusion-weighted images and dynamic contrast enhancement ratio with histologic grading. Egypt J Radiol Nucl Med 42:451–460
Park EK, Cho KR, Seo BK, Woo OH, Cho SB, Bae JW (2016) Additional value of diffusion-weighted imaging to evaluate prognostic factors of breast cancer: correlation with the apparent diffusion coefficient. Iran J Radiol 13(1):e33133
Ulghaffara WA, Magdy M (2013) Role of diffusion-weighted imaging in assessment of breast lesions. Med J Cairo Univ 81(2):87–93
Matsubayashi RN, Fujii T, Yasumori K, Muranaka T, Momosaki S (2010) Apparent diffusion coefficient in invasive ductal breast carcinoma: correlation with detailed histologic features and the enhancement ratio on dynamic contrast-enhanced MR images. J Oncol. https://doi.org/10.1155/2010/821048
Tan SL, Rahmat K, Rozalli FI, Mohd-Shah MN, Aziz YF, Yip CH, Vijayananthan A, Ng KH (2014) Differentiation between benign and malignant breast lesions using quantitative diffusion-weighted sequence on 3 T MRI. Clin Radiol 69:63–71
Razek AA, Gaballa G, Denewer A, Nada N (2010) Invasive ductal carcinoma: correlation of apparent diffusion coefficient value with pathological prognostic factors. NMR Biomed 23:619–623
Guo Y, Kong Q, Li L, Tang W, Zhang W, Ning G, Xue J, Zhou Q, Liang Y, Wu M, Jiang X (2021) Whole volume apparent diffusion coefficient (ADC) histogram as a quantitative imaging biomarker to differentiate breast lesions: correlation with the Ki-67 proliferation index. BioMed Res Int. https://doi.org/10.1155/2021/4970265
Youk JH, Son EJ, Chung J, Kim JA, Kim EK (2012) Triple-negative invasive breast cancer on dynamic contrast-enhanced and diffusion-weighted MR imaging: comparison with other breast cancer subtypes. Eur Radiol 22(8):1724–1734
Choi SY, Chang YW, Park HJ, Kim HJ, Hong SS, Seo DY (2012) Correlation of the apparent diffusion coefficiency values on diffusion-weighted imaging with prognostic factors for breast cancer. Br J Radiol 85:e474–e479
Mori N, Ota H, Mugikura S, Takasawa C, Ishida T, Watanabe G, Tada H, Watanabe M, Takase K, Takahashi S (2015) Luminal-type breast cancer: correlation of apparent diffusion coefficients with the Ki-67 labeling index. Radiology 274:66–73
Acknowledgements
Thanks are due to patients, radiographers, and histopathologists who contributed to this work.
Funding
Self-funding.
Author information
Authors and Affiliations
Contributions
RH and HA made equal sharing in data collection and analysis; RH made the initial manuscript writing; and HA was involved in editing and adding. Both RH and HA shared data analysis. Both RH and HA shared in writing, editing, and revising data and manuscript. HA performed statistical analysis and impact on results and discussion. Both authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Local Ethics Committee. Vancouver ethical standards were followed in the conduct of the study. Ethics committee reference number is not available. All patients signed a written consent to perform the procedure.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained and/or analyzed within this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hegazy, R., Azzam, H. Value of apparent diffusion coefficient factor in correlation with the molecular subtypes, tumor grade, and expression of Ki-67 in breast cancer. Egypt J Radiol Nucl Med 53, 198 (2022). https://doi.org/10.1186/s43055-022-00881-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00881-2