Abstract
Background
Spinal dural arteriovenous fistulas (dAVF) remain a rare, diagnostically challenging and possibly correctable condition with important prognostic outcomes dependent primarily on early detection and treatment.
Case presentation
We present a case of a 73-year-old male with progressively worsening neurological symptoms after steroid administration for a presumed diagnosis of transverse myelitis. This case is extremely unique, as the administration of steroids helped unmask an underlying spinal dural arteriovenous fistula by revealing characteristic imaging findings of a dAVF, not seen on the original MRI study.
Conclusion
In the setting of an unclear cause of transverse myelitis and worsening symptoms following steroid administration, the possibility of a ‘masked’ dural AVF should be considered and repeat imaging performed, which might help in the eventual diagnosis and definitive treatment of this elusive entity.
Similar content being viewed by others
Background
Spinal dural AVFs are rare disease entities, with many different established causes. Although they comprise approximately 60–80% of total spinal malformations, their overall yearly incidence is about 5–10 cases per million [1]. Patients typically experience a slowly progressive course of symptoms, and less commonly rapid progression. The average patient will present 1–3 years prior to a correct diagnosis [2]. At the time of diagnosis, the triad of motor deficits, sensory disturbances and cauda syndrome are present in 70% of patients [3].
There are 5 types of spinal AV shunts, with the most common type being a Type 1 dural AVF. The typical location for these lesions is in the lower thoracic and the upper lumbar spine [1, 4]. The exact etiology remains unknown, with a small percentage of possible multifactorial causes attributed to infection, syringomyelia, trauma, and surgery [2]. Irrespective of the cause, the disproportionate arterial inflow and venous outflow creates increased subarachnoid venous plexus pressure and congestion of the radial veins draining the spinal cord with resultant cord edema and eventual ischemia [4].
Although imaging characteristics may be non-specific, the multisegmental centromedullary T2 hyperintense signal with associated subarachnoid flow voids, particularly dorsally, are pathognomonic for this condition. As there is increased venous pressure, cord volume expansion becomes evident. These findings classically span approximately 5–7 vertebral levels [5]. Enhancement is typically present, however, the length of enhancement does not correlate with clinical symptoms. The majority of lesions involve the thoracolumbar junction, and 80% involve the conus [6]. The relatively lower venous outflow channels in the thoracic spine relative to the cervical level may explain why venous congestion is transmitted craniocaudally, and presenting symptoms may be conus medullaris related, even from a shunt higher up [6]. Primary differential diagnoses include transverse myelitis, neuromyelitis optica, vasculitis, demyelinating lesions, or tumors. CSF analysis is helpful in narrowing this differential, with a bland CSF profile excluding several etiologies and favoring a vascular etiology [2]. If the shunt volume is small, the perimedullary vessels may not be seen, or only noted on post contrast timed MRA sequences. First pass contrast enhanced MRA (CE MRA) can demonstrate early venous filling, confirming the shunt. CE-MRA is also useful in localizing the spinal level of the shunt, which can be very helpful in guiding the interventionalist while performing the angiogram to confirm the diagnosis. However, MRAs are often technically limited, limiting interpretation [7]. A pinal angiogram remains the gold standard for localizing and treatment planning of the lesion. Open ligation and endovascular embolization are the mainstays of treatment, currently [8]. The treatment usually stops the progression of symptoms or improves neurological deficits.
We present a case of a patient with suspected transverse myelitis, with worsening symptoms after steroid administration which unmasked an underlying dural arteriovenous fistula as seen on follow-up imaging.
Case presentation
A 73-year-old male presented to an outside hospital for acute bilateral lower extremity weakness with progressively worsening urinary and bowel incontinence. The patient had a prior history of cervical and lumbar myelopathy with lower lumbar canal decompressive laminectomy and fusion, remote history of radiation status post right nephrectomy 20 years prior, and leukemia status post chemo-radiation, currently in remission. MRI at the outside institution reported edema in the lower thoracic cord concerning for possible transverse myelitis.
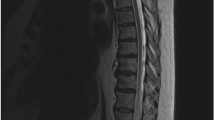
MRI (Siemens Avanto 1.5 T) performed following transfer to our institution demonstrated cord enlargement with a long segment of hyperintense T2 signal and cord enhancement spanning from the T9-T10 level to the conus on the sagittal and axial T2 sequences (Fig. 1). No definite flow voids were appreciated along the surface of the cord or within the spinal canal. Primary differential considerations included transverse myelitis, neuromyelitis optica, MOG or subacute cord infarct. CSF obtained via a lumbar puncture was bland with a mild increase in protein. No occult blood cells or WBCs were noted. Antibody testing for MOG, NMO (Aquaporin 4 Ab), Paraneoplastic syndrome (Hu Ab) was performed and came back negative after 10–14 days. Cytology and cytometry testing came back negative as well. Based on the CSF picture, a probable diagnosis of transverse myelitis was made. Given the lack of obvious flow voids on the MRI, an MRA was not performed at this time. The patient was empirically treated with IV steroids while in the hospital. The patient started developing worsening neurological symptoms and a follow-up MRI/MRA of the spine was performed, approximately 1 week after the original MRI. Interestingly, besides the findings of cord edema and enhancement from the T9-T10 level to the conus as seen on the prior MRI, the new MRI study demonstrated numerous clearly visualized tortuous vessels along the dorsal aspect of the lower thoracic cord, findings which were highly suggestive of a dural AVF. In the subsequently performed MRA study a tortuous medullary vein was noted within the right L1-L2 foramen, suggesting the site of the dAVF (Fig. 2).
Sagital Stir (a) and post contrast T1W fat saturated (b) images through the thoracic spine, sagital Stir image through the lumbar spine (c) and axial T2 weighted image at the T12 level (d), demonstrate a long segment of thoracolumbar expansile intramedullary cord signal abnormality and enhancement (arrows). Post surgical changes of prior decompressive laminectomy in the lumbar spine are noted
Based on the MRI/MRA study, a diagnostic angiogram was performed which demonstrated a type 1 dAVF with the fistulous point at the inferior surface of the right L2 pedicle, supplied by the radicular artery off the right L2 segmental artery and with an engorged draining spinal vein that traversed cranially along the dorsal surface of the spinal cord (Fig. 3). The patient underwent a L1-L2 laminectomy with intra-dural exploration and obliteration of the dAVF. Postoperatively, the patient reported improved but persistent neurological symptoms, with persistent bladder and bowel incontinence. At the last documented follow-up the patient had an uneventful rehabilitation stay and was discharged home with home therapies.
Conclusion
In the patient described above, several possible etiologies were considered based on the initial MRI appearance of the diffuse intramedullary cord signal abnormality and enhancement and the absence of flow voids. These were excluded based on the CSF picture and other tests leading to a probable diagnosis of transverse myelitis. Administration of IV steroids for the presumed diagnosis of transverse myelitis resulted in acute worsening of neurological symptoms. A repeat MR showed prominent subarachnoid flow voids, not seen on the original MR, leading to the eventual diagnosis and treatment of a dural AVF with subsequent resolution of symptoms. Absence of flow voids as seen on a spinal MR, in the setting of a dural AVF, although rare has been reported before in the literature [9], particularly in the case of drainage solely relying on the anterior spinal veins which are subpial in location and therefore may not be visualized [10]. Steroid administration resulting in worsening clinical symptoms and appearance of flow voids on MRI is even rarer with reports of anecdotal cases, in the literature [11,12,13]. The cause of this phenomenon is not entirely clear. The prevailing hypothesis is that the mineral-corticoid effects of the corticosteroids and saline solution in which they are administered results in fluid retention thereby causing engorgement of the veins of the dural AVF, worsening cord edema and neurological symptoms [12].
The non-specific clinical and sometimes unclear radiological findings of spinal dAVFs continue to hinder early diagnosis and treatment of this entity. In the setting of an unclear cause of transverse myelitis and worsening symptoms following steroid administration, the possibility of a ‘masked’ dural AVF should be considered and repeat imaging performed, which might help in the eventual diagnosis and definitive treatment of this elusive entity.
Availability of data and materials
All data (figures) generated for this study and included in this published article are available on the University of Miami PACS system.
Abbreviations
- dAVF:
-
Dural arteriovenous fistula
- CE MRA:
-
Contrast enhanced MRA
References
Thron A (2001) Spinal dural arteriovenous fistulas [in German]. Radiologe 41(11):955–960
Gemmete JJ, Chaudhary N, Elias AE, Toma AK, Pandey AS, Parker RA, Davagnanam I, Maher CO, Brew S, Robertson F (2013) Spinal dural arteriovenous fistulas: clinical experience with endovascular treatment as a primary therapy at 2 academic referral centers. AJNR Am J Neuroradiol 34:1974–1979
Rain S, Udding J, Broere D (2016) Acute clinical worsening after steroid administration in cervical myelitis may reveal a subdural arteriovenous fistula. Case Rep Neurol 8(3):234–242
Koch C (2006) Spinal dural arteriovenous fistula. Curr Opin Neurol 19(1):69–75
Jellema K, Tijssen CC, Fijnheer R, De Groot PG, Koudstaal PJ, Van Gijn J. Spinal dural arteriovenous fistulas are not associated with prothrombotic factors. Stroke 2004; 35(9):2069–2071. This careful research paper reveals an important difference in predisposing factor between cranial and spinal dural arteriovenous fistula.
Koch C, Kucinski T, Eckert B, Röther J, Zeumer H (2003) Spinal dural arteriovenous fistula: clinical and radiological findings in 54 patients [in German]. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 175(8):1071–1078
Chibbaro S, Gory B, Marsella M, Tigan L, Herbrecht A, Orabi M, Bresson D, Baumann F, Saint-Maurice JP, George B, Kehrli P, Houdart E, Manisor M, Pop R (2015) Surgical management of spinal dural arteriovenous fistulas. J Clin Neurosci 22:180–183
Morris JM (2012) Imaging of dural arteriovenous fistula. Radiol Clin N Am 50:823–839
Thiex R, Mayfrank L, Krings T, Mull M (2006) Delayed diagnosis of spinal dural arteriovenous fistula in the absence of pathological vessels on MRI. Zentralbl Neurochir 67:94–98
Strowd RE, Geer C, Powers A, Abou-Zeid N (2012) A unique presentation of a spinal dural arteriovenous fistula exacerbated by steroids. J Clin Neurosci 19:466–468
Tadié M, Hemet J, Freger P, Clavier E, Creissard P (1985) Morphological and functional anatomy of spinal cord veins. J Neuroradiol 12:3–20
Bowen BC, Saraf-Lavi E, Pattany PM (2003) MR angiography of the spine: update. Magn Reson Imaging Clin N Am 11:559–584
Chibbaro S, Gory B, Marsella M, Tigan L, Herbrecht A, Orabi M et al (2015) Surgical management of spinal dural arteriovenous fistulas. J Clin Neurosci 22:180–183
Acknowledgments
Not applicable.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
GS contributed to conception, draft. JR, NN, JM contributed to acquisition, analysis, draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
University of Miami IRB waives the need for ethics approval and consent for single patient case reports.
Consent for publication
NA (there are no personal details of participant which might compromise anonymity.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rodriguez, J., Nagornaya, N., Margolesky, J. et al. Unmasking of a spinal dural AV fistula on MRI following steroid administration. Egypt J Radiol Nucl Med 53, 184 (2022). https://doi.org/10.1186/s43055-022-00863-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00863-4