Abstract
Background
The rising increase in the incidence of breast cancer among women is worrisome and a great concern to all. More disturbing is that the incidence of breast cancer and death has been attributed to exposure to imaging modalities that utilize ionizing radiation such as computed tomography. The aim of this study was to estimate the lifetime attributable risk (LAR) incidence and mortality for breast cancer for female patients who had head computed tomography in two imaging facilities (centres) in the Niger Delta region of Nigeria.
Result
The overall estimated mean effective dose in centres G1 and G2 is 5.76 mSv and 1.54 mSv, respectively. There was a statistical significant difference in the mean effective dose between centres G1 and G2 (P < 0.001). The LAR breast cancer incidence obtained in this study ranged between 0.5 and 26.45 per 100,000 population in centre G1, while in centre G2, it ranged between 0.14 and 6.56 per 100,000 population. The LAR breast cancer mortality obtained in this study ranged between 0.07 and 6.25 per 100,000 population in centre G1, while in centre G2, it ranged between 0.03 and 1.55 per 100,000 population.
Conclusion
The estimated mean LAR of breast cancer incidence and mortality for the women who had head CT examination in the two study centres was found to be minimal to negligible among the female patients of the different age groups in the study population. The overall mean risk of breast cancer incidence was very low in centre G1 and minimal in centre G2. The obtained risk values can be used to optimize the dose delivered to patients and also ensure that CT examinations are justified.
Similar content being viewed by others
Background
The radiation dose received by the patient undergoing computed tomography (CT) is high compared to the dose delivered from the other imaging modalities that utilize ionizing radiation such as plain radiography [1]. Since the introduction of the first CT scanner at the University College Hospital (UCH) Ibadan, Nigeria, in 1987 [2], there has been a continuous rise in the acquisition of CT scanners in the country as well as an increase in the provision and delivery of CT service as more patients are being referred for CT examinations based on different clinical indications on a daily basis. Concerns have been raised on the probability of an increase in the risk of developing cancers from exposure to ionizing radiation during computed tomography (CT) examinations [3, 4]. Sadly, there is no corresponding increase in inculcating the knowledge of radiation hazards and effects among health care providers who refer patients for examinations which utilize ionizing radiation [5]. This study therefore set out to examine this probability by estimating the mean LAR of breast cancer incidence and mortality for the women who had head CT examination.
There are many types of cancer that can occur in the human body but the commonest amongst females is the cancer of the breast [6]. The International Agency for Research on Cancer reported in 2012 that breast cancer death is higher than deaths from other cancer types [6]. This fact has also been established in Nigeria by different researchers [7, 8]. Interestingly, it has been reported that the incidence of cancer in Africa is low compared to other parts of the world, while the mortality rate from cancer is higher than other parts of the world [9]. A study by Azubuike et al. [10] postulated that breast cancer incidence and mortality in Africa can be greatly reduced if there is a great reduction of breast cancer incidence and mortality in Nigeria since Nigeria has the highest population in Africa.
The effective dose (measured in Sievert) is one of the quantities used to assess the risk of radiation that patients are exposed to when they undergo examinations that utilize ionizing radiation such as CT scanning [11, 12]. There are available data to estimate cancer risk from exposure to low dose of ionizing radiation. These data were sourced mainly from the initial research involving a group of people that survived the nuclear bomb explosion in Hiroshima and Nagasaki Japan in 1945 [13]. Other studies have used the data obtained from this research to develop models that were used to estimate cancer incidence and mortality risks. The most recent of these studies is the latest report from the Biological Effects of Ionizing Committee (BEIR) of the National Academy of Science in the USA in 2006. This report is also known as the BEIR VII [11]. The BEIR VII report provided lifetime attributable risk (LAR) estimates for cancer incidence and mortality for different anatomical sites such as the thyroid, breast, lungs, prostate, uterus, ovary, colon, liver, stomach and urinary bladder as well as leukaemia. The LAR of cancer incidence and mortality from this report is mainly dependent on the age of the patient at exposure, patient’s gender and the radiation dose to the patient [11].
The aim of this study was to estimate the LAR for breast cancer incidence and mortality attributed to CT of the head in two centres in the Niger Delta region of Nigeria.
Methods
Study design
This study was a prospective, cross-sectional study that involved 125 female adult patients that underwent CT examination of the head over a one year period in two CT centres in the Niger Delta region of Nigeria labelled as G1 and G2, respectively.
Study technique
The protocol of the CT examination of the head was unenhanced scans (done without administration of contrast agent) and enhanced scans (done following intravenous administration of contrast agent). The relevant information for the study was obtained from the records of the patients (age, weight, height), while parameters collated from the console of the CT scanners during the scan of each of the patients were age at exposure, volume computed tomography dose index (CTDIvol) and dose-length product (DLP) values.
Eligibility criteria
The eligibility criteria included all adult female patients who were referred for head CT examination in the two centres during the study period and whose records had the relevant information required for the study. Also eligible for inclusion into the study were only the female patients whose weight were within 70 ± 10 kg [14] and those who had information on the technical parameters displayed on the CT console.
Study size
The sample size for this study was based on the European commission recommendation of a minimum of sample size of 10 standard-sized patients [14]. However, data from more than 10 patients in each CT examination was used to broaden the base of the study and increase the statistical relevance of the data.
The technical characteristics of the CT scanners are described in Table 1. Scan protocols for the head examinations were set at a potential tube voltage of 120kvp for all the patients in both centres. The tube current, slice thickness, pitch, rotation time and scan length varied among the two centres as displayed in Table 2.
Estimation of effective dose
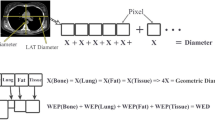
The effective dose of all the patients was calculated using the expression in Eq. 1:
where k is the conversion coefficient based on the head (k = 0.0021 mSvm Gy−1 cm−1 for head) [15].
Estimation of lifetime attributable risk (LAR) for breast cancer incidence and mortality
Estimation of LAR for breast cancer incidence and mortality was based on the effective dose, patient’s sex and age at exposure using the BIER VII report. This was estimated from tables 12D-1 and 12D-2, respectively, documented in the BEIR VII report (displayed as Tables 3 and 4 in this study) for each patient based on the age of the patient at exposure and sex using an equivalent dose of 0.1 Gy [11]. Data for specific ages that were not available in the tables were linearly interpolated using the nearest two ages in the tables.
The categorization of the risk level for LAR for breast cancer incidence and mortality displayed in Table 5 was determined as documented in the BIER VII report.
Statistical analysis
The mean, standard error of mean and standard deviation values were analysed for the effective dose, while, the minimum, maximum and mean values of the LAR incidence and mortality of breast cancer in the patients were analysed using statistical package for the social sciences (SPSS) version 22.0 (SPSS Inc., Chicago, IL, USA). Descriptive and independent sample t test was used at a 95% level of significance for the effective dose. P < 0.05 was considered statistically significant.
Results
Data from a total of 125 female patients who underwent CT head examination were collated. The number of female patients in centres G1 and G2 was 47 (37.6%) and 78 (62.4%), respectively. The highest number of patients was recorded in the age group of 60–69 years in centre G1 and 70–79 years in centre G2 (Table 6).
The calculated effective dose for the study participants is displayed in Table 7.
The highest effective dose in centre G1 was observed in age group 70–79 years, while the lowest effective dose was recorded in the patients in age group 60–69 years. The highest effective dose in centre G2 was observed in the 20–29 years and 70–79 years age groups, while the lowest effective dose was recorded in the patients in the 80–89 years age group (Table 7).
There was a statistical significant difference in the mean effective dose between centres G1 and G2 (P < 0.001) (Table 8).
The estimates of LAR of breast cancer incidence are displayed in Table 9.
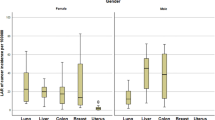
The minimum, maximum and mean values of the estimated LAR of breast cancer incidence for the different age groups as well as the approximate risk in centres G1 and G2 are displayed in Table 9. Patients in centre G1 had higher LAR of breast cancer incidence compared to the patients in centre G2. Higher LAR of breast cancer incidence was observed in patients within the age group of 20–29 years in both centres G1 and G2.
The estimates of LAR of breast cancer mortality are displayed in Table 10.
The minimum, maximum and mean values of the estimated LAR of breast cancer mortality for the different age groups as well as the approximate risk in centres G1 and G2 are displayed in Table 10. Patients in centre G1 have higher LAR of breast cancer mortality compared to the patients in centre G2. Higher LAR of breast cancer mortality was observed in patients within the age group of 20–29 years in both centres G1 and G2.
Discussion
The overall estimated mean effective dose in centres G1 and G2 is 5.76 mSv and 1.54 mSv, respectively. The overall mean LAR of breast cancer incidence in centre G1 was 7.17 per 100, 000 population (about 1 in 1426). The overall mean LAR of breast cancer incidence in centre G2 was 1.35 per 100, 000 population or about 1 in 74,074 (Table 9). The overall mean LAR of breast cancer mortality in centre G1 was 1.73 per 100, 000 population (about 1 in 57,143).The overall mean LAR of breast cancer mortality in centre G2 was 0.35 per 100, 000 population (about 1 in 285,714) (Table 10). There was a wide variation in the effective dose and cancer risks estimates amongst the different age groups in the two centres.
The study observed that the mean risk of breast cancer incidence in centre G1 ranged between moderate and minimal in all the age groups, while the mean of breast cancer incidence in centre G2 ranged from low to very low. The overall mean risk of breast cancer incidence was low in centre G1 and very low in centre G2 (Table 3).
The study also observed that the mean risk of breast cancer mortality in centre G1 and G2 ranged between minimal and negligible in all the age groups. The overall mean risk of breast cancer incidence was very low in centre G1 and minimal in centre G2 (Table 3).
The LAR value describes the risk of cancer incidence and mortality from exposures to ionizing radiation. The risk of cancer incidence and mortality can be greatly reduced when CT examinations are done using doses that are as low as reasonably achievable. The LAR incidence and mortality of breast cancer for adult female patients aged 20–89 years that underwent CT head examinations were estimated in this study. This study observed that the risk of breast cancer reduced as the age of the patients increased for both LAR incidence and mortality. The study also observed that the LAR of breast cancer incidence and mortality in both centres G1 and G2 increased in younger patients and decreased in older patients. That is, younger patients have a greater risk of developing and dying from cancer than the older patients since breast cancer risk depends on the age at exposure [11, 16]. The result obtained from this study also showed that the LAR of breast cancer incidence is higher than the LAR of breast cancer mortality which means that a greater number of the patients who underwent CT scan examination are of a greater risk of developing breast cancer than dying from breast cancer.
The study observed a higher risk of breast cancer incidence and mortality in centre G1 compared to centre G2. This is mostly due to the higher effective dose values recorded in centre G1 since the radiation dose delivered to the patient increases with increased tube current as observed in Table 2 [17]. Although some studies have reported that Toshiba CT scanners deliver high radiation dose [18, 19], other recent studies have reported higher radiation dose from higher slice CT scanners [20, 21].
Some of the limitations of this study include (a) the effective dose was estimated using DLP and k conversion factor. A study by Kobayashi et al. reported that there is a 20% difference in the value obtained from the estimated effective dose using DLP and k conversion factor and measured effective dose [22]. (b) LAR estimations were done using data from the BEIR VII report since the study did not have access to real data from epidemiological studies of female breast cancer in the study centres covered; therefore, the LAR values obtained in this study are just approximates values and not the precise risk values. (c) The CT scanners in the two study centres are from different manufacturers and also have different slices, thereby affecting the comparison of the obtained LAR values.
A study by Ikubor et al. [5] stated that the knowledge of radiation dose and risk from most doctors were obtained during their undergraduate studies in medical school and so there is a need for constant training and review on radiation risk and safety for all doctors. The data obtained in this study can serve as part of the resource data for the training and continuing medical education for referring doctors on the justification of the CT examination.
Conclusions
This study has estimated breast cancer incidence and mortality in two CT centres in the Niger Delta region of Nigeria. Although the two centres recorded low to minimal LAR of breast cancer incidence and mortality, the risk data presented here can be used to optimize the dose delivered to patients and also ensure that CT examinations are justified. This is the first time breast cancer risk has been estimated from CT imaging in these centres.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Brenner DJ, Elliston CD (2004) Estimated radiation risks potentially associated with full-body CT screening. Radiology 232:735–738
Adejoh T, Onwujekwe EC, Abba M, Ali AM (2018) Computed tomography scanner census and adult head dose in Nigeria. Egypt J Radiol Nuclear Med 49(1):66–70
Tesoriero HW. Worries mount over excessive CAT scans. Wall Street J. November 2, 2006:D1. CT scans may cause extra cancer: study. American Coll Radiol. 2004.
McCollough C, Bruesewitz MR, McNitt-Gray MF (2004) The phantom portion of the American College of Radiology (ACR) Computed Tomography (CT) accreditation program: practical tips, artifact examples, and pitfalls to avoid. Med Phys 31(9):2423–2442
Ikubor JE, Awunor NS, Atare EE (2021) Radiation safety knowledge and practice amongst doctors in a teaching hospital in the Niger Delta Region of Nigeria. West J Med and Biomed Sci 2(1):13–22
International Agency for Research on Cancer. Globacon 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. 2012.
Abdulrahman GO, Rahman GA (2012) Epidemiology of breast cancer in Europe and Africa. J Cancer Epidemiol 2012:915–6105
Jedy-Agba E, Curado MP, Ogunbiyi O, Oga E, Fabowale T, Igbinoba F et al (2012) Cancer incidence in Nigeria: a report from population-based cancer registries. Cancer Cancer Epidemiol 36:e271–e8
International Agency for Research on Cancer. Global cancer burden rises to 14.1 million new cases in 2012: marked increase in breast cancers must be addressed. (Press release). 2013.
Azubuike SO, Muirhead C, Hayes L, McNally R (2018) Rising global burden of breast cancer: the case of sub-Saharan Africa (with emphasis on Nigeria) and implications for regional development: a review. World J Surg Oncol 16(63):526
National Research Council of the National Academies (2006) Health risks from exposure to low levels of ionizing radiation: BEIR VII, phase 2—Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. National Academies Press, Washington
Shrimpton PC, Wall BF (2009) Effective dose and dose-length product in CT. Radiology 250(2):604–605
Brenner DJ, Hall EJ (2007) Computed tomography-An increasing source of radiation exposure. N Engl J Med 357(22):2277–2284
European Commission (EC). Guidance on diagnostic reference levels (DRLs) for medical exposures. European commission, Brussels. Radiation protection, 1999; 109.
AAPM. American Association of Physicists in Medicine AAPM , The measurement, reporting, and management of radiation dose in CT. 2008,AAPM report No.93. Report of AAPM Task Group 23 of the Diagnostic Imaging Council CT Committee. College Park.
Smith-Bindman R, Lipson J, Marcus R et al (2009) Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 169(22):2078–2086
Aweda MA, Arogundade RA (2007) Patient dose reduction methods in computerized tomography procedures: a review. Int J Phys Sci 2(1):001–009
Elmahdi A, Abuzaid MM, Babikir E, Sulieman A (2017) Radiation dose associated with multi-detector 64-slice computed tomography brain examinations in Khartoum State. Sudan Pol J Radiol 82:603–606
Smith-Bindman R, Wang Y, Chu P, Hung R, Einstein AJ, Balcombe J et al (2019) International variation in radiation dose for computed tomography examinations: prospective cohort study. Br Med J 364:k493
Al Ewaidat H, Zheng X, Khader Y, Abdelrahman M, Mustafa AMK, Rawashdeh MA, Al Mousa DS, Alawneh KZA (2018) Assessment of radiation dose and image quality of multidetector computed tomography. Iran J Radiol 15(3):e59554
Pera CM, Girjoaba OI, Cucu A, Iosif M (2016) Comparison of radiation dose in abdomen-pelvis and trunk imaging between 64 slice and 16 slice CT. Phys Med 3(3):295
Kobayashi M, Ootsuka T, Suzuki S (2013) Evaluation and examination of accuracy for the conversion factors of effective dose per dose-length product. Nippon Hoshasen Gijutsun Gakkaizasshi 69(1):19–27
Acknowledgements
The authors acknowledge with thanks the management and radiological staff of Delta State University Teaching Hospital, Oghara, Delta State and Raytouch Diagnostic Centre, Benin-city, Edo State.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
JEI and ACT contributed to conception, acquisition of data, interpretation of data and writing the manuscript. ACT was involved in analysis of data. JEI contributed to review of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate.
Ethical clearance for this study was obtained from the Health Research and Ethics Committee of each study centre before commencement of the study. Permission to access the required data from the CT scan machines was also sought from the management of the respected hospitals.
Consent for publication
Not applicable. Verbal or written consent was not obtained from the patient as the information required for the study was obtained from the records. However, confidentiality was strictly adhered to. Names of the patients were not written on the data spread sheet; instead study numbers were assigned as identifiers.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ikubor, J.E., Tobi, A.C. Estimation of lifetime attributable risk incidence and mortality for breast cancer attributed to computed tomography of the head in the Niger Delta Region of Nigeria. Egypt J Radiol Nucl Med 53, 105 (2022). https://doi.org/10.1186/s43055-022-00786-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00786-0