Abstract
Background
Breast microcalcifications are one of the most difficult mammographic findings to assess. The purpose of this study is to assess the ability of contrast-enhanced spectral mammography in the assessment of suspicious microcalcification and in predicting the grade of DCIS.
Methods
Three hundred and forty cases with suspicious microcalcification were reviewed in this study. We excluded 160 cases associated with masses. We enrolled 180 cases for analysis of suspicious microcalcification on mammograms with no underlying masses. We reviewed the microcalcification for their morphology, distribution, and associated pathological enhancement according to BI-RADS lexicon with pathology results reviewed and classified into benign and malignant which subdivided into low, intermediate, or high-grade DCIS or invasive carcinoma.
Results
Three hundred and forty cases with suspicious microcalcification were reviewed in this study. We excluded 160 cases associated with masses. Forty-five of 180 cases were benign, and 135/180 cases were malignant. Twenty-five of 135 cases were diagnosed as invasive breast carcinomas while 110/135 were ductal carcinoma in situ. From the latter, 110 patients with DCIS, 22/110 cases were low grade, 11/110 cases were intermediate grade, and 77/110 cases were high grade (44 with micro-invasion). A total of 25 invasive carcinomas showed pathological non-mass enhancement, 76/77 cases of high-grade DCIS, and 6/11 cases of intermediate-grade DCIS. No abnormal enhancement appeared with benign entities, low-grade DCIS, and 5/11 cases of intermediate DCIS. The diagnostic performance of CESM in anticipation of high grade in DCIS patients was sensitivity of 98%, specificity of 81.8%, and accuracy of 93.1%. CESM sensitivity, specificity, and accuracy in prediction of invasiveness or high-grade DCIS were 98.5%, 81.8%, and 87.5%, respectively.
Conclusion
CESM can provide a fundamental contribution in the evaluation of suspicious microcalcification as high-grade DCIS or invasive component can present by non-mass enhancement, but enhancement paucity is favorable to diagnose benign lesion or non-invasive/low-grade DCIS.
Similar content being viewed by others
Background
Breast calcifications are one of the common mammographic findings in screening and symptomatic populations. An approach to discriminate between benign and malignant breast calcifications to image analysis includes morphology, distribution, size, stability, and the number of calcifications [1].
Most of them have a benign origin presenting with characteristic benign morphology and need no further workup. Nevertheless, suspicious grouped calcifications can occur in ductal carcinoma in situ [1].
The American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS) classifies calcifications on mammograms into three categories: typical benign, intermediate concern, and higher probability of malignancy [1,2,3].
Certain suspicious calcifications being micro <0.5 mm, with amorphous, coarse heterogeneous, fine pleomorphic, and fine linear or fine linear branching morphologies and grouped, segmental, or regional distribution were found to be of significant concern [4].
Most high-grade DCIS and low- or intermediate-grade invasive cancers can be observed by screening mammography. The diagnosis of high-grade DCIS before developing into high-grade invasive carcinoma was one of the added beneficial values of screening mammography programs [2].
CESM highlights the enhancement related to the breast cancer neovascularity similarly to dynamic contrast-enhanced breast MRI. This neovascularity is rapidly formed and causing contrast agent leakage [5].
The iodine uptake within the calcification calls attention to the plausible underlying pathologies. Nevertheless, the added value is still vague [6].
CESM is offering an accessible substitute for suspicious findings that may need CE-MRI with the benefit to conceive microcalcifications in low-energy images and accompanied enhancement [7,8,9].
CESM can be specifically indicated as annual screening for women who underwent chest radiation therapy during young age, who have a higher incidence of DCIS with possible low neoangiogenesis that may be overlooked at CE-MRI [10, 11].
This study aims to assess the ability of CESM in the assessment of suspicious microcalcification and in predicting the grade of DCIS.
Methods
This study is a prospective analytical study. Three hundred and thirty females were incorporated with 340 suspicious microcalcifications. The multidisciplinary “Breast Cancer Hospital” ethical committee approved the study. Enlightened written consent was taken from all participants.
Patients
Patients included were females with suspicious microcalcifications. We excluded 160 cases associated with masses. We enrolled 180 cases for analysis of suspicious non-mass-associated microcalcification on mammograms. CESM was requested aiming for both clarifications of its significance and prediction of related malignancy.
Image evaluation
A thorough review of the low-energy mammography images and the post-processed contrast images was performed. Two radiologists analyzed and interpreted CESM in accordance with the latest MRI BI-RADS lexicon update (because of no contrast-enhanced mammographic ACR lexicon) [12]. All patients underwent biopsy+/- surgery.
Calculation of diagnostic indices, i.e., sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy, was done.
Histopathologic examination
Histopathologic diagnosis of all cases and grading of DCIS were carried out based on morphological examination of either tissue biopsies or surgical specimens according to the WHO Classification of Breast Tumours, fifth Edition, 2019. The final histopathologic result of malignant cases was based on examination of the surgical specimen, while that of benign/atypical cases was based on either tissue biopsies or surgical specimen examination [13].
Results
Analysis of the CESM findings of 340 suspicious microcalcifications in 330 patients was done. Ten patients had bilateral suspicious microcalcifications (Fig. 1). Their ages ranged from 27 to 77 years (mean age 47 years).
A Bilateral breast MLO view mammogram showing bilateral upper outer quadrant segmental amorphous microcalcifications, more delineated in zoomed images (B). C CESM shows right breast UOQ intense segmental non-mass enhancement with no pathological enhancement on the left side. D Hx&E, Mag. 200× pathology images revealing right breast invasive duct carcinoma grade III. E Hx&E, Mag. 100× pathology images showing left breast fibrocystic disease with calcifications
According to histopathology results, 45/180 (25%) were non-malignant lesions, and 135/180 (75%) were malignant lesions (Table 1).
According to microcalcification distribution, 73 (40.5%) were classified as grouped, 65 (31.1%) as segmental, 12 (6.6%) regional, 21 (11.6%) linear, and 9 (5%) diffuse. Morphology analysis classified 49 (27.2%) as amorphous, 25 (13.8%) fine linear+/-branching, 38 (21.1%) as round, 33 (18.3%) as coarse heterogeneous, and 34 (18.8%) as fine pleomorphic (Table 2 and Figs. 2, 3, 4, 5, and 6).
A Left breast MLO view showing left upper outer quadrant segmental pleomorphic microcalcifications, more delineated in zoomed images (C). B CESM shows left breast UOQ segmental non-mass enhancement reaching to retro-areolar region. D H&E, Mag. 100× pathology images revealing high-grade DCIS, showing severe atypical cells, with comedo necrosis and microcalcifications. Associated microinvasive carcinoma (≤1 mm) is also seen (the arrow)
A Left breast MLO view showing left upper outer quadrant segmental coarse heterogeneous microcalcifications, more delineated in zoomed images (C). B CESM shows left breast UOQ segmental non-mass enhancement. D H&E, Mag. 200× pathology images revealing high-grade DCIS, solid with comedo necrosis and calcifications
A Right breast CC view showing lower outer quadrant regional coarse heterogeneous microcalcifications, more delineated in zoomed images (C). B CESM show related mild lower outer non-mass enhancement with a focal area of more intense enhancement at the retro-areolar location. The patient underwent right simple mastectomy and pathology revealed invasive duct carcinoma grade II (0.8×0.3 cm). The intervening stroma is markedly desmoplastic entangling mild lymphocytic cell infiltrate with scattered epithelial microcalcific foci. There are associated scattered foci of intermediate grade DCIS component (solid pattern with comedo necrosis and calcifications), constituting 10% of the tumor area. D Intermediate grade DCIS, showing mild to moderate atypical cells with luminal calcifications. H&E, ×200
Most of the microcalcifications (n=163, 90.5%) were classified as BI-RADS 4 and 5, and only 17 (9.4%) were classified as BI-RADS 3.
Pathological non-mass enhancement was associated with all 25 invasive carcinomas, 76/77cases of high-grade DCIS, and 6/11 cases of intermediate grade DCIS. No pathological enhancement was elicited with benign entities, all low-grade DCIS, and 5/11 cases of intermediate DCIS (Table 3).
We identified contrast enhancement in 109/180 microcalcifications. The presence of enhancing lesions underlying microcalcifications was significantly higher in malignant than in benign lesions (p-value 0.05).
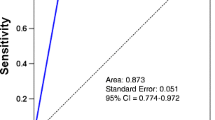
Diagnostic performance of CESM in the prediction of high grade in DCIS patients was sensitivity of 98%, specificity of 81.8%, and accuracy of 93.1%. CESM sensitivity, specificity, and accuracy in the prediction of invasiveness or high-grade DCIS were 98.5%, 81.8%, and 87.5% respectively (Tables 4 and 5).
Discussion
Mammography remains the gold standard imaging modality in detecting breast microcalcifications. Discrimination between benign and malignant microcalcifications according to the morphology and distribution has been strengthened and gives a confident diagnosis if associated with pathological contrast enhancement in a rapid CESM modality compared to breast MRI.
Studies have shown that CESM is superior to FFDM in overall performance in cancer detection; the estimated sensitivity of CESM was 98% (95% CI = 96–100), with a reported estimated specificity of 58% (95% CI = 38–77) [14]. However, most studies included all breast lesions, not only specific entities as suspicious calcifications.
Houben et al. included 147 women in their study; diagnostic performances of CESM in non-mass microcalcifications were sensitivity of 93.8%, specificity of 36.6%, PPV of 54%, and NPV of 88.2% [15].
Cheung et al. on the diagnostic performance of CESM held two studies in calcifications; sensitivity was 89%, specificity 87%, PPV 77%, and NPV 95% [6].
In the current study, pathological non-mass enhancement was associated with all invasive carcinoma, almost all cases of high-grade DCIS, and some cases of intermediate grade DCIS. No pathological enhancement was elicited with benign entities, all low-grade DCIS, and some cases of intermediate DCIS. As compared to Cheung et al., all IDC (100%) and some DCIS (84.21%) showed enhancement, but the other 15.79% DCIS did not show enhancement, while Houben et al. did not observe any differences between the amounts of enhancement between invasive and in situ breast cancers, and approximately 11% of the high-grade DCIS did not show any enhancement [6, 15].
In the current study, the grouped amorphous and fine pleomorphic microcalcifications associated with enhancement were associated with high-grade DCIS and invasive carcinoma (46%) compared to the other morphological entities, as compared to Cheung et al., who found that the pleomorphous microcalcifications with enhancement showed higher positive predictive value (90.00% vs 46.15%, p = 0.013) and higher cancer probability than the amorphous microcalcifications (46.3% vs 15.1%) [6].
Conclusion
CESM can provide a fundamental contribution in the evaluation of suspicious microcalcification as high-grade DCIS or invasive component can present by non-mass enhancement, but enhancement paucity is favorable to diagnose benign lesion or non-invasive/low-grade DCIS.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DCIS:
-
Ductal carcinoma in situ
- BI-RADS:
-
Breast Imaging-Reporting and Data System
- CESM:
-
Contrast-enhanced spectral mammography
- ACR:
-
American College of Radiology
- CE-MRI:
-
Contrast-enhanced-magnetic resonance imaging
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- UDH:
-
Usual ductal hyperplasia
- FFDM:
-
Full-field digital mammography
References
Demetr LA, Slanetz PJ, Eisenberg RL (2012) Breast calcifications: the focal group. Am J Roentgenol 198(4):325–343. https://doi.org/10.2214/AJR.10.5732
Luiten JD, Voogd AC, Luiten EJT, Duijm LEM (2017) Trends in incidence and tumour grade in screen-detected ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res Treat 166(1):307–314. https://doi.org/10.1007/s10549-017-4412-4
Breast Imaging Reporting & Data System | American College of Radiology. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads. Accessed 4 May 2020
Cheung YC, Tsai HP, Lo YF, Ueng SH, Huang PC, Chen SC (2016) Clinical utility of dual-energy contrast-enhanced spectral mammography for breast microcalcifications without associated mass: a preliminary analysis. Eur Radiol 26(4):1082–1089. https://doi.org/10.1007/s00330-015-3904-z
Li L, Roth R, Germaine P, Ren S, Lee M, Hunter K, Tinney E, Liao L (2017) Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): a retrospective comparison in 66 breast lesions. Diagn Interv Imaging 98(2):113–123. https://doi.org/10.1016/j.diii.2016.08.013
Cheung Y-C, Juan Y-H, Lin Y-C, Lo Y-F, Tsai H-P, Ueng S-H, Chen S-C (2016) Dual-energy contrast-enhanced spectral mammography: enhancement analysis on BI-RADS 4 non-mass microcalcifications in screened women. PLoS One 11(9):e0162740. https://doi.org/10.1371/journal.pone.0162740
Ghaderi KF, Phillips J, Perry H, Lotfi P, Mehta TS (2019) Contrast-enhanced mammography: current applications and future directions. Radiographics 39(7):1907–1920. https://doi.org/10.1148/rg.2019190079
Sung JS, Lebron L, Keating D, D’Alessio D, Comstock CE, Lee CH, Pike MC, Ayhan M, Moskowitz CS, Morris EA, Jochelson MS (2019) Performance of dual-energy contrast-enhanced digital mammography for screening women at increased risk of breast cancer. Radiology 293(1):81–88. https://doi.org/10.1148/radiol.2019182660
Dromain C, Vietti-Violi N, Meuwly JY (2019) Angiomammography: a review of current evidences. Diagn Interv Imaging 100(10):593–605. https://doi.org/10.1016/j.diii.2019.01.011
Mariscotti G, Belli P, Bernardi D, Brancato B, Calabrese M, Carbonaro LA, Cavallo-Marincola B, Caumo F, Clauser P, Martinchich L, Montemezzi S, Panizza P, Pediconi F, Tagliafico A, Trimboli RM, Zuiani C, Sardanelli F (2016) Mammography and MRI for screening women who underwent chest radiation therapy (lymphoma survivors): recommendations for surveillance from the Italian College of Breast Radiologists by SIRM. Radiol Med 121(11):834–837. https://doi.org/10.1007/s11547-016-0667-9
Cozzi A, Schiaffino S, Sardanelli F (2019) The emerging role of contrast-enhanced mammography. Quant Imaging Med Surg 9(12):2012–2018. https://doi.org/10.21037/qims.2019.11.09
Edwards SD, Lipson JA, Ikeda DM, Lee JM (2013) Updates and revisions to the BI-RADS magnetic resonance imaging lexicon. Magn Reson Imaging Clin N Am 21(3):483–493. https://doi.org/10.1016/j.mric.2013.02.005
Hoon Tan P, Ellis I, Allison K, Brogi E, Fox SB, Lakhani S, Lazar AJ, Morris EA, Sahin A, Salgado R, Sapino A, Sasano H, Schnitt S, Sotiriou C, van Diest P, White VA, Lokuhetty D, Cree IA (2020) The 2019 WHO classification of tumours of the breast. Histopathology. 77(2):181–185. https://doi.org/10.1111/his.14091
Tagliafico AS, Bignotti B, Rossi F, Signori A, Sormani MP, Vadora F, Calabrese M, Houssami N (2016) Diagnostic performance of contrast-enhanced spectral mammography: systematic review and meta-analysis. Breast 28:13–19. https://doi.org/10.1016/j.breast.2016.04.008
Houben IPL, Vanwetswinkel S, Kalia V, Thywissen T, Nelemans PJ, Heuts EM, Smidt ML, Meyer-Baese A, Wildberger JE, Lobbes MBI (2019) Contrast-enhanced spectral mammography in the evaluation of breast suspicious calcifications: diagnostic accuracy and impact on surgical management. Acta Radiol 60(9):1110–1117. https://doi.org/10.1177/0284185118822639
Acknowledgements
This research was carried out at Baheya Charity Women’s Cancer Hospital which is fully equipped with modern machines for breast cancer diagnosis. We want to thank our colleagues who helped us to do such research work.
Funding
No funding sources.
Author information
Authors and Affiliations
Contributions
AM wrote the manuscript. AM is responsible for correspondence to journal. MG and MF collected patient data and contributed to image processing and collection of patients’ images. OM participated in the design of the study and performed the statistical analysis. GM and SZ were responsible for pathology data. MG and OM conceived of the study, participated in its design and coordination, and helped to draft the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethical committee of Baheya center for Early Detection and Treatment of Breast Cancer with ethical committee approval number 20190105.3. An informed written consent was taken from all subjects.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shetat, O.M.M., Moustafa, A.F.I., Zaitoon, S. et al. Added value of contrast-enhanced spectral mammogram in assessment of suspicious microcalcification and grading of DCIS. Egypt J Radiol Nucl Med 52, 186 (2021). https://doi.org/10.1186/s43055-021-00554-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-021-00554-6