Abstract
Background
This work aims at elucidating the role of adding complementary T2 mapping to the routine 1.5 Tesla MRI protocol in the articular knee cartilage assessment for early detection of osteoarthritis, and also, comparing the articular cartilage thickness and T2 relaxation times between the case and control groups regarding knee compartments affection.
Results
Both sensitivities and specificities were 73.3% and 100%, respectively, for the standard MR protocol alone and 96.7% and 90% after adding the T2 mapping to the standard MR protocol that leads to significant sensitivity improvement. A comparison between patients and controls as regards T2 values showed a highly statistically significant difference (independent T test, p <0.001).
Conclusion
A combination of both morphological and T2 mapping MRI, together with clinical evaluation represents a desirable multimodal approach to the diagnosis of osteoarthritis. In the early detection of osteoarthritis, adding T2 mapping sequence to the standard MR protocol at 1.5 Tesla improved sensitivity from 73.3 to 96.7%.
Similar content being viewed by others
Background
Knee osteoarthritis is characterized by progressive loss of articular cartilage. It is considered to be the most common joint disease and the leading cause of long-term disability, placing tremendous financial burdens at the individual and societal levels [1].
It is important clinically to diagnose osteoarthritis in its early stages to prevent its inevitable progression. With advances in joint preservation strategies that are intended to alter the course of osteoarthritis and potentially slow the joint degeneration rate of early intervention and development of many promising disease-modifying osteoarthritis drugs [2], there is a rising demand in developing accurate and reliable quantitative MRI techniques that are sensitive to early structural degeneration in articular cartilage. Altering early osteoarthritis is indeed much promising than altering its advanced stages. Thus, effective treatment of osteoarthritic patients necessitates the determination of early degeneration of knee cartilage [3].
Routine MR imaging sequences used in the identification of knee osteoarthritis can assess articular cartilage morphology, but despite its accuracy for identifying deep hyaline cartilage defects which are considered irreversible cartilage degeneration with limited treatment options, it lacks sufficient sensitivity for early cartilage changes and low-grade chondral lesions which are the reversible stages for the potential alteration of the osteoarthritis progression [4].
To promote earlier and more precise depiction of articular cartilage changes, quantitative imaging techniques of knee cartilage such as T2 mapping sequence have been utilized which are considered compositional assessment techniques that can reveal the collagen network and water changes and the cartilage three-dimensional ultrastructure [5].
T2 mapping as a biochemically sensitive MRI technique can add robust biomarkers for disease onset and progression, and therefore, could be a meaningful assessment tool for the diagnosis and follow-up of cartilage abnormalities [3].
It has been demonstrated that the T2 mapping sequence is sensitive to T2 prolongation which occurs due to cartilage degeneration in knee osteoarthritis [6]; however, it has been shown in other studies that T2 values are not correlated with radiographic late stages of osteoarthritis [7]. Thus, this study was accomplished to decide whether adding the quantitative T2 mapping sequence to the routine MRI protocol could add a diagnostic value in early detection of initial degeneration of the knee articular cartilage at 1.5 Tesla.
Methods
This is a case-control study, conducted between July 2017 and November 2019, and included fifty (50) subjects, divided into two groups, 30 osteoarthritic patients (symptomatic group: 23 males and 7 females) ranging from 20 to 75 years old with clinical evidence of osteoarthritis, whose symptom severity was graded using the Western Ontario and McMaster University (WOMAC) osteoarthritis index “Likert version,” and 20 normal control group (asymptomatic group: 13 males and 7 females) ranging from 19 to 40 years old.
Our study was approved by the Ethical Committee of Scientific Research in our institute. Inclusion criteria included any patient with clinically evident knee osteoarthritis. The control group inclusion criteria were clinically normal individuals with no knee symptoms. The exclusion criteria of both osteoarthritic patients and the control group were trauma, history of previous knee joint operation, acute arthropathy, and any MRI contraindication.
To avoid cartilage T2 variations by physical activity, loading of the knee, temperature, and diurnal alteration, all individuals had a resting time about 30 min and we performed all exams between 4 and 6 pm, with the same room temperature (17 °C).
Instructions to the patients and clinical scoring
We used the self-administered WOMAC osteoarthritis index which takes around 5 min to complete. We asked the patients to answer questions regarding the pain, stiffness, and physical function at the time of presentation. The simple Likert version is used which includes 5 options ranging from “none” to “extreme.” An option of “none” is scored as 0, “mild” as 1, “moderate” as 2, “severe” as 3, and “extreme” as 4. Total scores for each question are gathered and summed to form pain, stiffness, and physical function subscale scores. Then, we transformed total WOMAC score to a 0–100 scale for ease of correlation with other values.
MRI technical aspects
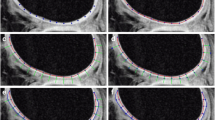
We use a 1.5 Tesla MRI scanner (Signa; GE Healthcare, USA). Routine MRI sequences included sagittal T2-WI (repetition time/echo time, 3000/81 ms, duration 2 min 58 s), axial T2-weighted images (3000/87 ms, duration 2 min 19 s), sagittal T1-weighted images (435/10 ms, duration 2 min 55 s), sagittal proton density-WI with fat saturation (2440/40 ms, duration 2 min 38 s), coronal proton density-weighted images with fat saturation (3890/40 ms, duration 2 min 25 s), and axial merge (750/16ms, duration 2 min 27 s) with a knee coil. Field of view, 10 × 10 mm; matrix, 320 × 224; slice gap, 4 mm and slice thickness, 4 mm. Three axial data sets through the knee were evaluated to get T2 mapping by applying FSE with a repetition time of 1000 and eight echo times (8.4 ms, 16.8 ms, 25.2 ms, 33.5 ms, 41.9 ms, 50.3 ms, 58.7 ms, and 67.1 ms). FOV 90-130 mm; matrix, 256 × 256; slice gap, 3.5 mm; slice thickness, 3 mm. The T2 mapping total acquisition time was 4-7 min. A T2 colored-map is created using the default software settings and functions. The default parameters of the T2 intensity are 11–89 ms, with a calculated cut-off point of 50 ms (Fig. 1). The color scale ranges from red to blue colors in which green or blue color corresponds to high T2 values on the color-coded scale on at least two consecutive slices. The MR images were transferred to a workstation for the off-line quantification of T2 values and cartilage thickness in each case. The average T2 value was calculated by manually created elliptical ROI which is inspected on the sequences. Regions of interest were created considering a margin of 0.5 to 1 mm from the bone surface to prevent subchondral bone inclusion. Standard MRI and a corresponding T2 map were visualized side by side, using a multi-planar localization key on the PACS.
Statistical analysis
T2 values were assessed by two independent musculoskeletal radiologists. All the readings of both the OA patients and the control group were collected and compared individually. The unpaired sample t test was used to find the significant difference between the bivariate samples in the independent groups. To find the significance in the categorical data, the chi-square test or ANOVA test was used according to the number of categories. T2 values were described in terms of mean ± standard deviation. All statistical analyses were performed using the SPSS commercial software. P values < 0.05 were considered significant statistically.
Results
In this study, we included 50 individuals (30 in the osteoarthritic patients’ group and 20 in the control group). Osteoarthritic patients included 23 males and 7 females with a mean age of 42.3 ± 16.6 years (range from 20 to 75 years). The control group involved 13 males and 7 females with a mean age of 34.8 ± 10.3 years (range from 19 to 55 years) (Table 1). The control group had lower median T2 values than that of the osteoarthritis patients: 36 ms [33.25-38.75 ms IQR] and 64ms [56.75-72 ms IQR]. The osteoarthritic patients had mean T2 values which tended to be higher (63.9 ± 9.1 ms) (Figs. 2, 3, and 4) than that of the control group (39.3 ± 10 ms) (Fig. 5) with a significant p value < 0.05 (Table 2). Both sensitivities and specificities were 73.3% and 100%, respectively, for the standard MRI protocol alone and 96.7% and 90% after adding the T2 mapping that leads to significant sensitivity improvement (Table 3). The mean cartilage thickness tended to be lower in patients with OA (1.6 ± 0.5 ms) than in the control group (2 ± 0.6 ms) with a significant p value < 0.05.
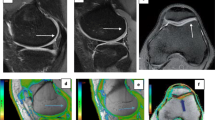
a Sagittal fat-suppressed proton density-weighted shows full-thickness defects of articular cartilage of medial tibiofemoral condyle and exposed subchondral bone (blue arrow). b and c Corresponding T2 map shows elevated cartilage T2 relaxation time (ROI). [b: 0 ms ≤ T2 ≤ 39 ms; c: T2 = 74 ms (>50 ms)]
a Sagittal fat-suppressed proton density-weighted image shows a preserved signal intensity of the articular cartilage of the anterior aspect of medial femoral condyle with uniform cartilage thickness (blue arrow). b and c The corresponding T2 map shows increased cartilage T2 relaxation time (ROI). b: 0 ms ≤ T2 ≤ 39 ms; c: T2 = 63 ms)
a Sagittal fat-suppressed proton density-weighted image shows a preserved signal intensity of the articular cartilage of the posterior aspect of the medial femoral condyle with uniform cartilage thickness (blue arrow). b and c Corresponding sagittal T2 map shows a region of increased T2 relaxation time [false positive] (ROI). b: 0 ms ≤ T2 ≤ 39 ms; c: T2 = 66 ms)
a Sagittal fat-suppressed proton density-weighted shows preserved signal intensity of the cartilage covering the medial femoral articular cartilage with uniform cartilage thickness (blue arrow). b and c Corresponding T2 map shows normal cartilage T2 relaxation time (ROI). b: 0 ms ≤ T2 ≤ 39 ms; c: T2 ≤ 40 ms)
After comparing mean T2 values between different knee compartments (medial, lateral, and anterior) in OA patients, we found no statistically significant difference between them with a p value > 0.05 (Table 1). Also, we found a moderate negative correlation (r = −0.43) between T2 values and cartilage thickness (p = 0.001). Regarding age, our study showed a significant medium positive correlation (r = 0.32) between age and T2 values (p = 0.02) (Fig. 6).
Regarding WOMAC index, for the 30 studied cases, pain scores ranged from 2.1 to 7.2, stiffness scores ranged from 0 to 3.2, physical function ranged from 3.1 to 21.9, and the total score ranged from 5.2 to 32.3, with mean value = 15.5 ± 7.6 (Table 4). On correlation of WOMAC score with T2 values, we found a strong positive correlation (r = 0.9) between them (p = 0.001), while cartilage thickness showed moderately negative correlation (r = −0.4) with WOMAC score (p = 0.04) (Fig. 7).
Discussion
Detection of knee osteoarthritis, which represents more than 80% of osteoarthritis total burden [8], in an early stage is a must to treat it effectively [9]. We can only differentiate early alterations in cartilage morphology from the normal articular cartilage surface using a spatial resolution of 0.3 mm, which is considered beyond the sequences used for morphological cartilage imaging [10]. T2 mapping sequence is not dependent on a spatial resolution to detect early alterations in articular cartilage but rather depicts areas of increased water content and collagen matrix ultrastructure alteration in degenerative cartilage [11,12,13,14,15]. So, T2 mapping helps detect early and potentially reversible cartilage damage before the onset of symptoms and morphological alterations, allowing early action by disease-modifying agents [16].
In our study, adding a T2 mapping sequence to a standard MRI protocol significantly improves the sensitivity for cartilage lesion detection, especially the early ones, within the knee joint from 73.3 to 96.7% (p=0.001). This agrees with Liebl et al. [4] who suggested, in a case-control study using 130 subjects that knees with incident tibiofemoral osteoarthritis had significantly higher mean T2 values in each compartment compared with controls similar to Dunn et al. [17] case/control study of 55 subjects that showed significantly (p <0.05) increases in T2 relaxation times between diseased (34.4-41.0 ms) and healthy (32.1-35.0 ms) knees. Also, Kijowski et al. [18], prospectively studied 150 subjects, found that increased T2 values in cartilages corresponded to cartilage lesions arthroscopically, and stated that increased articular cartilage T2 value could be used as an indicator of cartilage degeneration in OA.
The relative lower sensitivity of the standard MRI protocol of knee cartilage for identifying early cartilage degeneration is close to the previous studies’ findings, which have reported sensitivities for detecting cartilage abnormalities and defects ranging from 9-62% at both 1.5 T and 3.0 Tesla [19,20,21,22,23,24].
On the other hand, our study reported that the T2 mapping sequence showed a decrease in specificity for detecting knee cartilage lesions from 100% by routine MRI to 90% by adding T2 mapping. This decrease may be from the magic angle effect leading to a false increase in T2 relaxation time or early asymptomatic cartilage degeneration.
While all these studies, including our study, showed an association between increased T2 values and cartilage degeneration, Hirose et al. study [25] reported no significant change in T2 values with cartilage degeneration relative to normal cartilage does exist in the literature.
Our study results show that, as reported by Crema et al. [5] and Dautry et al. [26], T2 mapping imaging may add a robust value to MR imaging ability to identify focal knee chondral lesions when T2 mapping abnormalities and pain location are correlated. We found a correspondence between pain location and cartilage with T2 prolongation time in our study. T2 mapping focal abnormalities in the symptomatic compartments of 29 out of 30 patients (5 out of 5 symptomatic anterior compartments, 11 out of 11 symptomatic medial compartments, and 3 out of 4 symptomatic lateral compartments) are found.
Concerning T2 values in different knee joint compartments, our study results—similar to the Dautry et al. [26] study—showed no significant difference (p = 0.55) between the mean T2 values of medial, lateral femorotibial cartilage, and anterior patellofemoral cartilage of OA patients. Contrariwise, another study by Mosher et al. [27], involving persons with and without radiographic knee OA, showed a positive correlation with higher T2 values in the medial compartment cartilage and a higher degree of knee pain.
In agreement with the cross-sectional CT arthrography retrospective study by Omoumi et al. [28] carried on 535 consecutive knees showing that cartilage of the posterior aspect of the medial condyle was statistically thicker in osteoarthritic knees compared to non-osteoarthritic ones (p<0.001), our study results showed that there was a significant difference between cartilage thickness in OA patients and controls (p = 0.03). On the contrary, Mittal et al. [29] reported a lack of difference in average articular cartilage thickness between OA patients and controls (p>0.05). Also, Li et al. [30] reported that there was no significant difference in the average cartilage thickness in OA patients and control subjects (p = 0.37). They suggested that there is no significant correlation found between T2 values and cartilage thickness (p > 0.05), different from our study results that observed a significant moderate negative correlation (r = −0.43) between T2 values and cartilage thickness (p = 0.001).
Few studies in literature inspect the effect of physical activity on T2 mapping values. For example; one study by Mosher et al. [31], tested the effect of running/training on T2 mapping values eliciting the so-called functional cartilage MRI T2 mapping. Our study, however, has eliminated the effect of physical activity by necessitating an average of 30-45 min of rest for all patients prior to the examination. The aim of that was to cancel the effect of knee loading in an attempt to study the degenerative effect solely without the interference of other factors.
Regarding correlation with the gold standard techniques, one study in literature by Bazaldua et al. [32] correlated T2 mapping values with arthroscopic results and found that the T2 mapping sequence could identify 88 (92.6%) of the total number of lesions found with arthroscopy, while the conventional sequence protocol (conventional T2 sequence), evaluated by certified radiologists, was able to find 83 lesions (87.3%).
However, in our study, patients were referred for initial MR assessment in the context of a conservative treatment plan rather than arthroscopy (the gold standard) which was not a near plan. Accordingly, we resorted to correlation with clinical severity scoring of pain, stiffness, and physical disability as a reference and a good alternative to the non-applicable gold standard in addition to using a case-control study pattern. Based on that, we were able to find a credible correlation between the T2 values and WOMAC scores.
According to the results of our study, there was a highly significant positive correlation between total WOMAC score and T2 values of osteoarthritic patients (p>0.001), supporting the reliability of T2 mapping (i.e., the higher the T2 value; the higher the WOMAC score). While cartilage thickness of osteoarthritic patients showed a moderately negative correlation with WOMAC score (p = 0.04) (i.e., the smaller the cartilage thickness, the higher the WOMAC score).
Regarding age, our study showed a significant medium positive correlation (r = 0.32) between age and T2 values (p = 0.02) that agrees with ÇAĞLAR et al. study [33], which demonstrated that T2 values elevation correlated with age in all compartments measured in the subgroups of both patient and control groups.
Regarding gender, our study showed that there is no significant difference between T2 values of males and females (p = 0.5) that agrees with Mosher et al. study 19 [34], which comparing differences in T2 values between healthy men and women found no differences between genders. Also, ÇAĞLAR et al. study [33] found no statistically significant difference between the male and female cartilage T2 relaxation times in the subgroups with no knee joint pathology and the total group in all three compartments.
Regarding the literature [5,6,7], the “T2 mapping” sequence is a sensitive technique for hyaline cartilage lesions. However, it has several limitations. T2 value of the cartilage is dependent on field strength [35], moderately dependent on temperature [36], and more remarkably on physical activity, loading of the knee [37], and age [38]. Therefore, in our study, MR imaging was done on the same MRI field strength, at the same temperature, and after 30 min resting time.
Our study had several limitations; the most important was the lack of confirmatory arthroscopic procedure, as it would be inadequate for arthroscopy to assess for early or subtle intrinsic cartilage abnormalities shown on T2 maps. The second limitation was that T2 mapping is susceptible to the magic angle effect; therefore, we avoid the measurement of cartilage oriented at 55° to the main magnetic field. The third limitation was the non-recording of the body mass index of the patients and controls.
Conclusion
Our study has demonstrated that adding up T2 mapping, as a biochemically sensitive MRI sequence, to the routine MRI protocol of the knee improves sensitivity to detect early structural degeneration with both qualitative (color-map) and quantitative (average T2 values) diagnostic information in the knee articular cartilage helping for early therapeutic planning, to reduce or prevent the morbidity of knee osteoarthritis and its sequential burden.
Availability of data and materials
All data and material of this article are available from the corresponding author on reasonable request.
Abbreviations
- MRI:
-
Magnetic resonance imaging
- MR:
-
Magnetic resonance
- FOV:
-
Field of view
- ROI:
-
Region of interest
- PACS:
-
Picture archiving and communication system
- ANOVA:
-
Analysis of variance
- IQR:
-
Interquartile range
- CT:
-
Computed tomography
- T:
-
Tesla
References
Mobasheri A, Batt M (2016) An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med 59(5-6):333–339
Anandacoomarasamy A, March L (2010) Current evidence for osteoarthritis treatments. Ther Adv Musculoskelet Dis 2(1):17–28
Hesper T, Hosalkar HS, Bittersohl D, Welsch GH, Krauspe R, Zilkens C, Bittersohl B (2014) T2* mapping for articular cartilage assessment: principles, current applications, and future prospects. Skeletal Radiol 43:1429–1445
Liebl H, Joseph G, Nevitt MC, Singh N, Heilmeier U, Subburaj K, Jungmann PM, McCulloch CE, Lynch JA, Lane NE, Link TM (2015) Early T2 changes predict onset of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Ann Rheum Dis 74(7):1353–1359
Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, Baum T, Mosher TJ, Carrino JA, Guermazi A (2011) Articular cartilage in the knee: current MRI techniques and applications in clinical practice and research. Radiographics 31(1):37–61
Li X, Benjamin MC, Link TM et al (2007) In vivo T1 [rho] and T2 mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthr Cartil 15:789–797
Koff MF, Amrami KK, Kaufman KR (2007) Clinical evaluation of T2 values of patellar cartilage in patients with osteoarthritis. Osteoarthr Cartil 15:198–204
Vos T et al (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet 380:2163–2196
Favero M, Ramonda R, Goldring MB, Goldring SR, Punzi L (2015) Early knee osteoarthritis. RMD Open 1(Suppl 1):e000062
Rubenstein JD, Li JG, Majumdar S, Henkelman RM (1997) Image resolution and signal-to-noise ratio requirements for MR imaging of degenerative cartilage. AJR Am J Roentgenol 169(4):1089–1096
Fragonas E, Mlynárik V, Jellús V et al (1998) Correlation between biochemical composition and magnetic resonance appearance of articular cartilage. Osteoarthr Cartil 6(1):24–32
Nieminen MT, Rieppo J, Töyräs J et al (2001) T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med 46(3):487–493
Xia Y, Moody JB, Alhadlaq H (2002) Orientational dependence of T2 relaxation in articular cartilage: a microscopic MRI (micro MRI) study. Magn Reson Med 48(3):460–469
Liess C, Lüsse S, Karger N, Heller M, Glüer CC (2002) Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthr Cartil 10(12):907–913
Goodwin DW, Wadghiri YZ, Zhu H, Vinton CJ, Smith ED, Dunn JF (2004) Macroscopic structure of articular cartilage of the tibial plateau: influence of a characteristic matrix architecture on MRI appearance. AJR Am J Roentgenol 182(2):311–318
Guermazi A, Alizai H, Crema MD, Trattnig S, Regatte RR, Roemer FW (2015) Compositional MRI techniques for evaluation of cartilage degeneration in osteoarthritis. Osteoarthr Cartil 23(10):1639–1653
Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S (2004) T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology 232(2):592–598
Kijowski R, Blankenbaker DG, Munoz Del Rio A, Baer GS, Graf BK (2013) Evaluation of the articular cartilage of the knee joint: value of adding a T2 mapping sequence to a routine MR imaging protocol. Radiology 267(2):503–513
Kijowski R, Davis KW, Woods MA et al (2009) Knee joint: comprehensive assessment with 3D isotropic resolution fast spin-echo MR imaging—diagnostic performance compared with that of conventional MR imaging at 3.0 T. Radiology 252(2):486–495
Kijowski R, Blankenbaker DG, Klaers JL, Shinki K, De Smet AA, Block WF (2009) Vastly undersampled isotropic projection steady-state free precession imaging of the knee: diagnostic performance compared with conventional MR. Radiology 251(1):185–194
Kijowski R, Blankenbaker DG, Davis KW, Shinki K, Kaplan LD, De Smet AA (2009) Comparison of 1.5- and 3.0-T MR imaging for evaluating the articular cartilage of the knee joint. Radiology 250(3):839–848
Kijowski R, Blankenbaker DG, Woods MA, Shinki K, De Smet AA, Reeder SB (2010) 3.0-T evaluation of knee cartilage by using three dimensional IDEAL GRASS imaging: comparison with fast spin-echo imaging. Radiology 255(1):117–127
von Engelhardt LV, Schmitz A, Pennekamp PH, Schild HH, Wirtz DC, von Falkenhausen F (2008) Diagnostics of degenerative meniscal tears at 3-tesla MRI compared to arthroscopy as reference standard. Arch Orthop Trauma Surg 128(5):451–456
von Engelhardt LV, Kraft CN, Pennekamp PH, Schild HH, Schmitz A, von Falkenhausen M (2007) The evaluation of articular cartilage lesions of the knee with a 3-tesla magnet. Arthroscopy 23(5):496–502
Hirose J, Nishioka H, Nakamura E, Oniki Y, Yamashita Y, Mizuta H (2012) T1rho and T2 mapping of the proximal tibiofibular joint in relation to aging and cartilage degeneration. Eur J Radiol 81:2776–2782
Dautry R, Bousson V, Manelfe J, Perozziello A, Boyer P, Loriaut P, Koch P, Silvestre A, Schouman-Claeys E, Laredo JD, Dallaudière B (2014) Correlation of MRI T2 mapping sequence with knee pain location in young patients with normal standard MRI. JBR-BTR 97(1):11–16
Mosher TJ, Smith H, Dardzinski BJ, Schmithorst VJ, Smith MB (2001) MR imaging and T2 mapping of femoral cartilage: in vivo determination of the magic angle effect. AJR Am J Roentgenol 177:665–669
Omoumi P, Michoux N, Roemer FW, Thienpont E, Vande Berg BC (2015) Cartilage thickness at the posterior medial femoral condyle is increased in femorotibial knee osteoarthritis: a cross-sectional CT arthrography study (part 2). Osteoarthr Cartil 23(2):224–231
Mittal S, Pradhan G, Singh S, Batra R (2019) T1 and T2 mapping of articular cartilage and menisci in early osteoarthritis of the knee using 3-tesla magnetic resonance imaging. Pol J Radiol 84:e549–e564
Li X, Benjamin Ma C, Link TM, Castillo DD, Blumen Krantz G, Lozano J, Carballido-Gamio J, Ries M, Majumdar S (2007) In vivo T1rho and T2 mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthr Cartil 15(7):789–797
Mosher TJ, Liu Y, Torok CM (2010) Functional cartilage MRI T2 mapping: evaluating the effect of age and training on knee cartilage response to running. Osteoarthr Cartil 18(3):358–364
Bazaldua-Cheda HM, Onofre-Castillo JJ, Torres-Gómez E (2019) Evaluation of articular cartilage of the knee using T2 mapping sequence in magnetic resonance. Anales de Radiología México 18:99–107
Çağlar E, Şahn G, Oğur T, Aktaş E (2014) Quantitative evaluation of hyaline articular cartilage T2 maps of knee and determine the relationship of cartilage T2 values with age, gender, articular changes. Eur Rev Med Pharmacol Sci 18(22):3386–3393
Mosher TJ, Collins CM, Smith HE, Moser LE, Sivarajah RT, Dardzinski BJ et al (2004) Effect of gender on in vivo cartilage magnetic resonance imaging T2 mapping. J Magn Reson Imaging 19:323–328
Gold GE, Han E, Stainsby J et al (2004) Musculoskeletal MRI at 3.0 T: relaxation times and image contrast. AJR 183:343–351
Nelson TR, Tung SM (1987) Temperature dependence of proton relaxation times in vitro. Magn Reson Imaging 5:189–199
Mosher TJ, Dardzinski BJ, Smith MB (2000) Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2-preliminary findings at 3 T. Radiology 214:259–266
Apprich S, Welsch GH, Mamisch TC, Szomolanyi P, Mayerhoefer M, Pinker K, Trattnig S (2010) Detection of degenerative cartilage disease: comparison of high-resolution morphological MR and quantitative T2 mapping at 3.0 tesla. Osteoarthr Cartil 18(9):1211–1217
Acknowledgements
Not applicable.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
MAIA evaluated MR images and measurements of the exact T2 value for all patients included in the study; also, participating in the statistical analysis and writing the manuscript. KAAS assisted in the evaluation of MR images; also, he assisted in data collection and result analysis and in writing the manuscript. RTK performed measurements of cartilage thickness for all patients included in the study and assisted in the evaluation of MR images; also, she assisted in data collection and analysis. All authors discussed the results and contributed to the final manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This prospective case-control study was approved by the Ethical Committee of Scientific Research of the Faculty of Medicine, Ain Shams University together with departmental approval from the ethics committee of the Radiology Department of Ain Shams University.
The ethics committee reference number is not applicable in this study as the protocol of this study was prepared by 2017 and we started patient enrollment and data collection by 2018–2019 which at such time the ethics committee reference number system was not established in our institute.
Also, we did not make any changes in the protocol or methodology throughout the period of the study from the time of ethics committee approval and this technique is a well-established technique worldwide. All procedures performed in the study involving human participants were following the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
The whole procedure is explained in detail to the patients, and they received detailed written information that explains the entire procedure and any possible risks that may happen. All patients included in this study gave written informed consent to participate in this research.
Consent for publication
All patients included in this research gave informed written consent to publish the data contained within this study.
Competing interests
None
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alsayyad, M.A.I., Ali Shehata, K.A. & Khattab, R.T. Role of adding T2 mapping sequence to the routine MR imaging protocol in the assessment of articular knee cartilage in osteoarthritis. Egypt J Radiol Nucl Med 52, 78 (2021). https://doi.org/10.1186/s43055-021-00453-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-021-00453-w