Abstract
Background
Urinary bladder cancer is the second most common neoplasm of the urinary tract. Tumor staging and regional disease spread are the strongest predictors of treatment outcome. Our study aims to compare the diagnostic performance of conventional and diffusion-weighted magnetic resonance imaging (DW-MRI), using a 3T MR scanner, in grading and staging of urinary bladder cancer with histopathologic correlation.
Results
The sensitivity, specificity, and accuracy of combined use of HR T2WI and DWI were better than using HR T2WI only to differentiate T1 stage from further stages (95%, 100%, and 98%, respectively). Differentiating organ confined bladder cancer (T1 and T2 stages) from tumors with extravesical extension was better when using both HR T2WI and DWI than using HR T2WI (accuracy 100% and 93%, respectively). Kappa agreement was better when using both HR T2WI and DWI than HR T2WI only (0.963 and 0.496 respectively). Grade III lesions showed statistically significant lower ADC values than grades I and II with ADC cut off value ≤ 0.95 × 10−3 mm2/s. No statistically significant difference was found in ADC values between transitional cell carcinoma (TSC) and other cell types.
Conclusion
Combined use of DWI and HR T2WI provides more accurate urinary bladder cancer staging and prediction of aggressiveness of certain histologic grade (GIII lesions) using ADC values. 3 T scanners have the potential to achieve higher diagnostic capability in evaluation of urinary bladder cancer.
Similar content being viewed by others
Background
Urinary bladder cancer is the second most common neoplasm of the urinary tract after cancer prostate with male-to-female ratio of 3:1 and age peak between 50 and 70 years [1]. Bladder cancer is a heterogeneous disease, with 70% of patients presenting with superficial tumors, which tend to recur, and 30% presenting as muscle-invasive disease associated with a high risk of death from distant metastases [2]. Tumor staging and regional disease spread are the strongest predictors of treatment outcome in urinary bladder cancer [3]. The management and prognosis of bladder cancer are based on T staging, pathologic grading of the tumor, and the presence or absence of metastatic disease [4]. Magnetic resonance imaging (MRI) is currently the best imaging technique for loco-regional staging for several malignancies because of its superior soft tissue contrast resolution with the advantage of avoiding exposure to ionizing radiation [5].
There are few reports in literature that have compared anatomic and functional MRI sequences on 3 Tesla (3T) scanners, in predicting aggressiveness and stage of urinary bladder cancer [6, 7]. Our study aims to compare the diagnostic performance of conventional and DW-MRI, using a 3T MR scanner, in grading and staging of urinary bladder cancer with histopathologic correlation.
Methods
Patients
This prospective study was conducted at the radiology department of Specialized University Center between April 2018 and April 2020 on 86 patients. An MRI was done after confirming a bladder lesion on flexible cystoscopy at an outpatient clinic. Patients included had a presumed diagnosis of urinary bladder cancer either: clinically; by urine cytology, digital rectal examination, and flexible cystoscopy or by other radiologic investigations including ultrasonography and/or multi-detector row computed tomography. Exclusion criteria comprised of patients who had general MRI contraindications as pacemakers or metallic prosthesis and those who had claustrophobia. The study was conducted in full accordance with the guidelines for Good Clinical Practice and the Declaration of Helsinki and has been approved by our Institutional Research Board (IRB). Data for patients were collected only after obtaining patients’ informed consents.
MRI protocol
The procedure was conducted using 3 Tesla scanner (Ingenia, Philips medical systems, Veenpluis, the Netherlands) in the radiology department, Urology and Nephrology center-Mansoura University. Each patient will be subjected to conventional, mainly high-resolution (HR) T2-weighted sequences and diffusion-weighted (DW) MRI study. Before starting the examination, patients were checked if they had previous operations, especially pacemakers or any contraindication to MRI exam. Patients were informed about procedure steps and were trained how to hold breath and how to listen and follow the instructions. Patients who failed to hold their breath were instructed to breath as shallow as possible during the acquisition. Patients were asked to be calm and tolerate the procedure.
Patients were scanned in supine position with the hands beside the body. Body coil was well fitted on the pelvis. The region of interest was taken from the urinary bladder to the end of the prostate. Scanning sequences included HR T2WI and DWI. The parameters that were used in HR T2WI are field of view (FOV) 200 mm × 200 mm, TR (repetition time) 5000 ms, TE (echo time) 110 ms, slice thickness 3 mm, and slice interval 0.3 mm. Regarding (DWIs), the parameters were TE 85 ms, TR 7255 ms, FOV 350 mm, and slice thickness 3 mm. Regarding the b values taken, we used low and high b values (b0 and b1400). The region of interests (ROI) was placed on lesion to measure ADC values. ADC maps were obtained from DW images at b0 and b1400 s/mm2 gradients.
Tumor staging
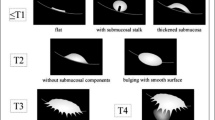
Bladder cancer staging comprises four major categories according to American Joint Committee on Cancer: T1, T2, T3, and T4 [8]. On T2 images, intact bladder wall should appear as a low SI line while being of intermediate SI on DWI images with no disruption [9]. Tables 1 and 2 and Fig. 1 show bladder cancer staging criteria using T2WI and DWI.
Schematic diagram that shows diagnostic criteria for using diffusion-weighted imaging for staging bladder cancer. Cancer component, muscle layer, and submucosa show high, intermediate, and low-signal intensity, respectively. Submucosal stalk or thickened submucosa indicates T1 or lower stage; smooth tumor margin without submucosal components, T2; irregular margin toward the perivesical fat tissue, T3; and extension into adjacent organs, T4 [11]
ADC maps
ADC maps were generated automatically by the MRI device. The urinary bladder lesions showed hypointense signal. We drew the region of interest (ROI) within the mass lesion at the most hypointense area. The ADC values were measured to estimate the degree of diffusion.
Image analysis
Images reviewed by two independent radiologists with 10 years’ experience in MRI, the first interpreted HR T2 images only while the second interpreted both HR T2 and DWI images. Both readers were blinded to histopathological data. Image sets were reviewed for tumor location, morphology, extensions, stage, as well as prediction of grade and cell type using ADC values.
Histopathology
The histopathological results were provided from the transurethral cystoscopy resection or radical cystectomy and were considered the standard reference of our study. Histopathological data of interest included tumor cell type, T stage of the urinary bladder cancer, and the histologic grade. The T stage of the tumor was classified into four stages as aforementioned. Histologic grades were G1, the least level of anaplasia; G2, intermediate anaplasia; and G3, severe degree of anaplasia.
Statistical analysis
Data were entered and analyzed using IBM-SPSS software (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0). Qualitative data were expressed as absolute frequency [N] and percentage [%]. Quantitative data were initially tested for normality using Shapiro-Wilk’s test with data being normally distributed if p > 0.050. Examination for the presence of outliers (extreme values) was done by inspecting boxplots. Quantitative data were expressed as mean ± standard deviation (SD) if normally distributed with no significant outliers or median and interquartile range (IQR) if not. Inter-observer reliability reflects the variation between 2 MR imaging sequences that measure the same group of subjects. Cohen’s kappa (κ) agreement was used to compare between results of HR T2 and HR T2 plus DWI to histopathological results where histopathological results were considered the gold standard in our study. For qualitative data, Chi-square test (or Fisher’s exact test) was used. Z-test was used with Bonferroni method to adjust p values when comparing column proportions while in quantitative data for two groups, we used independent-samples t test for normally distributed data and the non-parametric alternative; Mann-Whitney U test was used if not. For any of the used tests, results were considered as statistically significant if p value ≤ 0.050.
Results
This study included 86 patients, 5 of them had pathologically proved non-malignant bladder lesions (2 benign and 3 inflammatory) and they were excluded from statistical analysis. The remaining 81 patients had malignant bladder lesions. Age and sex frequency distribution are shown in Table 3. The most common complaint was hematuria (64 patients, 79%). Other complaints include irritative lower urinary tract symptoms (LUTS) (8 patients, 9.9%) and obstructive LUTS (5 patients, 6.2%) (8 patients of the previous groups had more than one symptom). Twelve patients (14.8%) were incidentally discovered. Among the patients, there were 21 patients (25.9%) who had multicentric lesions, in which the lesion with highest stage was chosen to be included in statistical analysis, the rest of patients had a single lesion (60 patients, 74.1%). Posterior urinary bladder wall was the most common location of lesions (23 patients, 28.4%). Regarding lesion morphology, polypoidal lesions were the most common (40 patients, 49.4%) (Table 3).
The use of HR T2WI only for tumor staging showed that 16 patients (19.8%) were T1, 36 patients (44.4%) were T2, 26 patients (32.1%) were T3, and 3 patients (3.7%) were T4. While the use of HR T2WI plus DWI for tumor staging revealed that 35 patients (43.2%) were T1, 23 patients (28.4%) were T2, 21 patients (25.9%) were T3, and 2 were patients (2.5%) T4. ADC values median was 0.8 × 10−3 mm2/s. with IQR (0.7–1.0 × 10−3 mm2/s) with minimum 0.1 × 10−3 mm2/s and maximum 1.4 × 10−3 mm2/s (Figs. 2, 3, 4, 5, and 6).
Multiple nodular growths are seen arising from posterior and right lateral wall with no evidence of abnormal signals in overlying muscle layer. a Axial HR T2 image, b sagittal HR T2 image, c axial DWI image (b 1400), and d ADC map with ADC measured within lesion (1.1 × 10−3 mm2/s). Pathologically proved urothelial carcinoma T1, GI
An intravesical soft tissue mass is seen arising from anterior wall with infiltration of the muscle layer. a Axial HR T2 image, b coronal HR T2 image, c sagittal HR T2 image, d axial DWI (b 1400) image, e coronal DWI image (b 1400), and f axial ADC map with ADC measured within lesion (0.62 × 10−3 mm2/s). Pathologically proved urothelial carcinoma, T2, GIII
A vesical soft tissue mass is seen arising from superior part of posterior wall with muscular invasion and presumably perivesical invasion on HR T2 images, yet it seems confined to the wall on DWI images. a Axial HR T2 image, b coronal HR T2 image, c sagittal HR T2 image, d axial DWI image (b 1400), e coronal DWI image (b 1400), and f axial ADC map with ADC measured within lesion (0.93 × 10−3 mm2/s). Pathologically proved adenocarcinoma, T2, GII
An intravesical hypointense soft tissue mass is seen arising from anterior wall, dome and both lateral walls with infiltration of perivesical fat. a Axial HR T2 image, b coronal HR T2 image, c sagittal HR T2 image, d axial DWI (b 1400) image, e coronal DWI image (b 1400), and f axial ADC map with ADC measured within lesion (0.23 × 10−3 mm2/s). Pathologically proved small cell carcinoma T3, GIII
An intravesical soft tissue mass is seen arising from anterior wall with infiltration of anterior peritoneal reflection and anterior abdominal wall on T2 WI, seems separable from anterior abdominal wall muscles on DWI. a axial HR T2 image, b coronal HR T2 image, c sagittal HR T2 image, d axial DWI (b 1400) image, and e Axial ADC map with ADC measured within lesion (0.71 × 10−3 mm2/s). Pathologically proved urothelial cancer, T4, GIII
Histopathological data revealed that 37 tumors (45.7%), 21 tumors (25.9%), 21 tumors (25.9%), and 2 tumors (2.5%) were T1, T2, T3, and T4, respectively. Regarding histopathological grade, grade I was found in 5 cancers (6.2%), grade II in 23 cancers (28.4%), and grade III in 53 patients (65.4%). For cell type, urothelial type was the most common (72 patients, 88.9%). Non-urothelial cell types were found in 9 patients (11.1%) including adenocarcinoma (4 patients, 4.9%), squamous cell carcinoma (3 patients, 3.7%), small cell carcinoma (1 patient, 1.2%), and inflammatory myofibroblastic tumor (1 patient, 1.2%).
The results of T2WI only and T2WI plus DWI were compared to histopathological results. Using HR T2WI only to differentiate non muscle invasive bladder cancer (T1) from muscle invasive bladder cancer (T2, T3, and T4) showed sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of 41%, 98%, 94%, 66%, and 72% respectively. But when using T2WI plus DWI, we found that sensitivity, specificity, PPV, NPV, and accuracy in differentiating non muscle invasive bladder cancer (T1) from muscle invasive bladder cancer (T2, T3, and T4) were 95%, 100%, 100%, 96%, and 98% respectively (Table 4).
When HR T2WI only was used to differentiate tumors confined to urinary bladder (T1 and T2 stages) from those extending beyond the bladder wall (T3 and T4 stages), the sensitivity, specificity, PPV, NPV, and accuracy were 90%, 100%,100%, 79%, and 93%, respectively, while combined use of HR T2WI and DWI to differentiate tumors limited to urinary bladder (T1 and T2) from tumors with extravesical extension (T3 and T4) showed sensitivity, specificity, PPV, NPV, and accuracy of 100%, 100%, 100%, 100%, and 100%, respectively (Table 5).
The Kappa agreement was of moderate strength between HR T2 and histopathological results (Kappa = 0.496) while being of very good strength between HR T2 plus DWI and histopathological results (Kappa = 0.963).
ADC values when compared to different tumor grades showed statistically significant negative correlation of moderate strength between ADC value and tumor grades being lower in grade III lesions than in grades I and II. ADC cut off value ≤ 0.95 × 10−3 mm2/s with AUC, sensitivity, specificity, PPV, and NPV of 0.7, 83%, 54%, 77%, and 63%, respectively, discriminated grade III lesions from lesions of grades I and II (Table 6) (Figs. 7, 8, 9, and 10).
No statistically significant difference was found in ADC values between urothelial and non-urothelial cell types (Table 7).
Discussion
Accurate staging is critical, as prognosis and management of patients with bladder cancer largely depends on the local tumor stage as well as lymph node (LN) or distant metastases [12]. However, there is a substantial discrepancy between preoperative clinical staging (combined bimanual examination, TUR, and conventional imaging) and the final pathologic staging based on radical cystectomy and LN dissection with an inaccuracy rate of 23–50%, mainly due to understaging of both the depth of local invasion and LN metastatic involvement [13]. Superficial tumors are treated with transurethral resection (TUR) with or without adjuvant intravesical chemotherapy or photodynamic therapy, whereas invasive tumors are treated with radical cystectomy, radiation therapy, chemotherapy, or a combination [10].
Our study was done to compare between conventional and functional MRI (i.e., HR T2WI and DWI, respectively) on 3 T scanner to achieve proper staging and grade prediction. Our essential results showed more accurate staging when using both HR T2WI and DWI than using HR T2WI only. Also, we found that using ADC values to predict tumor grade provided better discrimination of highly aggressive tumor grade (GIII) than earlier grades (GI and GII).
Several studies assessed the combined use of HR T2WI and DWI in predicting stage, grade, and possibly cell type of bladder cancer but usually a 1.5 T scanner was used. In a study done by Barsoum et al. [8] on a 1.5 T scanner, they found that sensitivity, specificity, and accuracy of T2WI in differentiating T1 or less from T2 to T4 tumors were 97, 64, and 88% respectively, while combined DWI and T2WI showed sensitivity, specificity, and accuracy of 100%, 86%, and 96% respectively. Our study revealed that using only T2WI to differentiate T1 or less from T2 to T4 tumors had sensitivity, specificity, and accuracy were 41%, 98% and 72% respectively. Combined use of T2WI and DWI raised the degree of concordance with histopathological results in our study when used to differentiate T1 or less from T2 to T4, where sensitivity, specificity, and accuracy were 95%, 100%, and 98% respectively. There is some difference between our study and that of Barsoum et al. [8], which may be due to the difference in number of patients considering T2WI only for stage assessment. Tekes et al. [14] reported that 81% of bladder tumors showed SI similar to bladder wall muscle and overstaging was the most common error. This may explain relatively decreased accuracy of T2WI in our study when evaluating muscular invasion. In a study done by El-Assmy et al. [15]—on a 1.5 T scanner—they found that overall staging accuracy was statistically significantly higher in DW compared with T2W MRI (P < 0.001). They showed that T2W MRI could not reliably separate superficial tumors (T1) from those invading muscle layers (T2) (accuracy 6%) while DWI could diagnose accurately superficial tumors T1 and differentiate them from T2 to T4 tumors (P < 0.001, accuracy 63.6%). Our study showed an accuracy of 72% for T2WI when differentiating T1 from T2–T4 tumors while combined use of HR T2WI and DWI showed accuracy of 98%. Higher accuracy staging in our study than that found in El-Assmy et al.’s study [15] may be due to the use of 3 T scanner in our study rather than 1.5 T used in El-Assmy et al.’s study [15]. Another multiobserver study done on 3 T scanner by WU et al. [7] showed greater accuracy for T2WI and DWI than for T2WI alone when differentiating T1 or less from T2 to T4 stages (observer 1, 98% vs. 83% [P < .001]; observer 2, 96% vs. 77% [P < .001]; observer 3, 92% vs. 74% [P < .001]). Specificity of T2WI and DWI (observer 1,100%; observer 2, 98%; observer 3, 93%) was greater than that of T2WI (observer 1, 79%; observer 2, 73%; observer 3, 71%) while sensitivity was not improved even when T2WI plus DWI were used. This agrees with our study in terms of specificity and accuracy when using HR T2WI plus DWI to differentiate the previously mentioned staging categories (specificity 100% and accuracy 98%), while using T2WI only showed specificity and accuracy of 98% and 72% respectively. Meanwhile, sensitivity in our study was significantly improved when adding DWI to T2WI unlike the study done by WU et al. [7]. Our study showed T2WI sensitivity of 41% to differentiate T1 or less from T2 to T4 stages, while T2WI plus DWI raised sensitivity to 95% in differentiating T1 or less from T2 to T4 stages.
Barsoum et al. [8] found that using T2WI only to differentiate stages T2 or less from T3 to T4 tumors showed sensitivity, specificity, and accuracy of 92%, 92 %, and 94% while adding DWI to T2WI showed sensitivity, specificity, and accuracy of 100%, 100%, and 100% respectively. This in strong concordance with our study that showed HR T2WI sensitivity, specificity, and accuracy of 90%, 100%, and 93% respectively, but adding DWI to HR T2WI showed sensitivity, specificity, and accuracy of 100%, 100%, and 100%, which matched Barsoum et al.’s results [8]. While in El-Assmy et al.’s study [15], when the stages were grouped as T1–T2 and T3–T4, staging accuracy for organ-confined disease (≤ T2) was significantly higher in DW compared with T2 MRI (69.7% and 15.1%, respectively; P < 0.001). In non-organ confined disease (> pT2), staging accuracy was 92.5% and 80.1% for DW and T2W MRI, respectively (P > 0.05).
From the previous comparisons between our results and previous studies, we can explain the significant increase in accuracy levels especially when differentiating non muscle invasive (T1) from muscle invasive (T2–T4) tumors by the use of 3 T instead of 1.5 T scanners as well as the combined use of HR T2WI and DWI instead of HR T2WI or DWI alone. This agrees with the meta-analysis study done by Huang et al. [16], which revealed that studies that used a 3.0-T device and DWI pulse sequence had the highest sensitivity and specificity for bladder cancer T staging in all studies and also agrees with Abou El-Ghar et al.’s study [17], which addressed that addition of DWI improves tumor staging by MRI.
Several authors had reported decreased ADC value among malignant lesions due to dense cellularity and large cellular size [18]. In the study done by Abd elsalam and Abdelbary [10], the mean ADC of G1 tumors was significantly higher than that of G2 and G3 tumors. Meanwhile, there was an inverse relationship between the mean ADC values and the histological grade of the tumor. This agrees with results in our study that showed statistically significant negative correlation of moderate strength between ADC value and tumor grades, where GIII lesions showed lower ADC values than in GI and GII. However, in our study, there was no statistically significant difference in ADC values that could differentiate between GI and GII lesions. This may be due to relatively small proportion of GI and GII patients in our study (28 patients) while 53 patients were GIII lesions.
Dynamic contrast-enhanced MRI is an imaging modality used in the staging of bladder cancer via assessment of tumor vascular behavior and angiogenesis that can affect therapy planning with antiangiogenic therapy [19].
Multiparametric MRI (mPMRI) is also considered an important tool to assess response to chemotherapy and radiotherapy for bladder cancer patients [20]. mpMRI includes T2WIs, DWI, and dynamic contrast-enhanced study [12] and its findings are used to provide guidelines for detection of muscle invasion through Vesical Imaging-Reporting and Data System (VI-RADS) [20].
DWI showed many advantages regarding acquisition such as being a sequence of short duration, non-invasive, and of course does not use ionizing radiation. Also, it provided much data regarding staging and grade of bladder cancer without the need to administer contrast medium, particularly useful in patients with renal impairment. The acquisition was done either breath hold or with shallow respiration, which is helpful to severely ill or obese patient.
Our study had some limitations in aspects of a relatively small number of G1 and GII patients. In addition, the use of non-contrast studies only did not evaluate the role of dynamic contrast studies in comparison to HR T2WI and DWI as we focused on the role of these two entities representing conventional and functional sequences, respectively.
Conclusion
3T scanners have the potential to achieve higher capability in evaluation of urinary bladder cancer. Combined use of DWI and HR T2WI provides more accurate urinary bladder cancer staging and prediction of aggressiveness of certain histologic grade (GIII) lesions using ADC values.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DWI:
-
Diffusion-weighted imaging
- ADC:
-
Apparent diffusion coefficient
- HR T2WI:
-
High-resolution T2-weighted imaging
- TSC:
-
Transitional cell carcinoma
- LN:
-
Lymph node
- LUTS:
-
Lower urinary tract symptoms
- TUR:
-
Transurethral resection
- VIRADS:
-
Vesical imaging-reporting and data system
- mP MRI:
-
Multiparametric MRI
References
Afifi AH, Maksoud TASA, EL-noueam KI, Ataa MA, Abdallah DM. (2017) Multiparametric-MRI as a comprehensive study in evaluation, characterization & local staging of urinary bladder carcinomas. Egypt J Radiol Nucl Med 48(2):493–507
Kaufman DS, Shipley WU, Feldman AS (2009) Bladder cancer. Lancet 374(9685):239–249
de Haas RJ, Steyvers MJ, Fütterer JJ (2014) Multiparametric MRI of the bladder: ready for clinical routine? Am J Roentgenol 202(6):1187–1195
Lin W-C, Chen J-H (2015) Pitfalls and limitations of diffusion-weighted magnetic resonance imaging in the diagnosis of urinary bladder cancer. Transl Oncol 8(3):217–230
Panebianco V, Barchetti F, de Haas RJ, Pearson RA, Kennish SJ, Giannarini G et al (2016) Improving staging in bladder cancer: the increasing role of multiparametric magnetic resonance imaging. Eur Urol Focus 2(2):113–121
Gandrup KL, Nordling J, Thomsen HS (2014) MRI of the bladder in patients suspected of bladder tumors. Open J Radiol 4:207–214
Wu L-M, Chen X-X, Xu J-R, Zhang X-F, Suo S-T, Yao Q-Y et al (2013) Clinical value of T2-weighted imaging combined with diffusion-weighted imaging in preoperative T staging of urinary bladder cancer: a large-scale, multiobserver prospective study on 3.0-T MRI. Acad Radiol 20(8):939–946
Barsoum N, Talaat M, Saraya S (2017) Can diffusion-weighted MRI predict the histological grade of urinary bladder carcinoma? Kasr Al Ainy Med J 23(2):86
Al Johi RS, Seifeldein GS, Moeen AM, Aboulhagag NA, Moussa EM, Hameed DA et al (2018) Diffusion weighted magnetic resonance imaging in bladder cancer, is it time to replace biopsy? Cent Eur J Urol 71(1):31
Abd elsalam SM, Abdelbary AM (2020) Accuracy of diffusion-weighted magnetic resonance imaging in evaluation of muscle invasion and histologic grading of the urinary bladder carcinoma. Egypt J Radiol Nucl Med 51:1–9
Takeuchi M, Sasaki S, Ito M, Okada S, Takahashi S, Kawai T et al (2009) Urinary bladder cancer: diffusion-weighted MR imaging—accuracy for diagnosing T stage and estimating histologic grade. Radiology 251(1):112–121
Panebianco V, Narumi Y, Altun E, Bochner BH, Efstathiou JA, Hafeez S et al (2018) Multiparametric magnetic resonance imaging for bladder cancer: development of VI-RADS (Vesical Imaging-Reporting And Data System). Europ Urol 74(3):294–306
Caglic I, Panebianco V, Vargas HA, Bura V, Woo S, Pecoraro M et al (2020) MRI of bladder cancer: local and nodal staging. J Magn Reson Imaging. 52(3):649-67.
Tekes A, Kamel IR, Chan TY, Schoenberg MP, Bluemke DA (2003) MR imaging features of non-transitional cell carcinoma of the urinary bladder with pathologic correlation. Am J Roentgenol 180(3):779–784
El-Assmy A, Abou-El-Ghar ME, Mosbah A, El-Nahas AR, Refaie HF, Hekal IA et al (2009) Bladder tumour staging: comparison of diffusion- and T2-weighted MR imaging. Eur Radiol 19(7):1575–1581
Huang L, Kong Q, Liu Z, Wang J, Kang Z, Zhu Y (2018) The diagnostic value of MR imaging in differentiating T staging of bladder cancer: a meta-analysis. Radiology 286(2):502–511
Abou El-Ghar ME, Badawy MA, El-Diasty TA (2018) Bladder and Upper Urinary Tract Urothelial Cancer. In: Akata D, Papanikolaou N (eds) Diffusion weighted imaging of the genitourinary system. Springer International Publishing, Cham, pp 73–104
Woodhams R, Matsunaga K, Kan S, Hata H, Ozaki M, Iwabuchi K et al (2005) ADC mapping of benign and malignant breast tumors. Magn Reson Med Sci 4(1):35–42
Hassanien OA, Abouelkheir RT, Abou El-Ghar MI, Badawy ME (2019) El Gamal SA-h, El-Hamid MA. Dynamic contrast-enhanced magnetic resonance imaging as a diagnostic tool in the assessment of tumour angiogenesis in urinary bladder cancer. Can Assoc Radiol J. 70(3):254–263
Thoeny HC, Bellin M-F, Comperat E-M, Thalmann G (2018) Vesical imaging-reporting and data system (VI-RADS): added value for management of bladder cancer patients? Europ Urol 74(3):307–308
Acknowledgements
Not applicable.
Funding
This study had no funding from any resource.
Author information
Authors and Affiliations
Contributions
MMS gave idea and collected the patients' data and analysed them. DMS put study design and followed the patients postoperatively. SME, TAE, and RTA wrote and revised the paper. They all approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The current study was approved by the Institutional Research Board (IRB), Faculty of Medicine, Mansoura University (Approval Code: MD.18.04.35.R1). All procedures performed in the current study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. Additionally, all potential hazards were explained to the participants and their families. All participants gave their written informed consent before the start of the study. For patients with decreased conscious, level written informed consent was obtained by their legal guardian before the start of the study.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study. If the patient was deceased, or unconscious when consent for publication was requested, written informed consent for the publication of this data was given by their parent or legal guardian.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Settein, M.M., Sobh, D.M., Eteba, S.M. et al. Comparison between conventional and diffusion-weighted magnetic resonance imaging in predicting grade and stage of urinary bladder cancer. Egypt J Radiol Nucl Med 52, 16 (2021). https://doi.org/10.1186/s43055-020-00365-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-020-00365-1