Abstract
Background
Diffusion tensor imaging (DTI) is a non-invasive MR modality that provides an evaluation of brain tissue microstructure and architecture in vivo. We aimed to assess the diagnostic value of DTI parameters in evaluating cerebral white matter integrity in patients of severe chronic obstructive pulmonary disease (COPD) and correlate these parameters with smoking index (SI) and the number of exacerbations in the last year.
This prospective study included 30 COPD male past smoker patients and 15 age- and sex-matched nonsmoker controls. Staging of COPD, SI and number of exacerbations in the last year were obtained. Routine brain MRI and DTI were done in all subjects. The selected white matter tracts’ fractional anisotropy (FA), and mean diffusivity (MD) were calculated in the region of interest in axial slices.
Results
The mean FA and MD values of all selected white matter tracts showed a high significant difference (p < 0.001) between patients and control group. The correlation between FA, SI and exacerbation frequency was not significant in the majority of white matter tracts (p > 0.05). The correlation between MD, SI and exacerbation frequency was significant for the majority of tracts (p < 0.05).
Conclusion
DTI metrics are valuable non-invasive tools in evaluating the white matter abnormalities in COPD patients. Smoking index and frequency of exacerbations have possible relation to extra-pulmonary cerebral manifestations of COPD.
Similar content being viewed by others
Background
Chronic obstructive pulmonary disease (COPD) remains a major cause of morbidity and mortality. It is increasingly recognized to have different systemic effects that extend beyond the lung. Hypoxemia and hypercapnia usually develop as a result of irreversible airflow limitation caused by COPD, which could later on result in a decrease in oxygen transport to the brain. Hypoxia during COPD has been previously established to induce cerebral perfusion decline and metabolic changes. Furthermore, systematic inflammation may also induce neuronal damages in the brain of COPD patients [1,2,3,4].
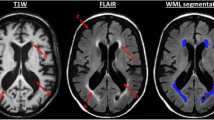
Conventional magnetic resonance imaging (MRI) has been used for detection of structural brain changes seen in cerebral small vessel disease which is common in elderly and in those with impaired pulmonary function. It has been found that COPD is linked to cerebral small vessel disease through increased prevalence of white matter lesions, and cerebral microbleeds. Early cerebral microstructural changes at the cellular level still are not within the scope of the conventional MRI spatial resolution [5, 6].
Diffusion tensor imaging (DTI) is an intrinsically T2-weighted method that provides an evaluation of brain tissue microstructure and architecture in vivo through studying diffusion properties of water molecules within tissues. Moreover, it provides non-invasive quantitative parameters as fractional anisotropy (FA) which captures the directionality of diffusion inside the tissue and mean diffusivity (MD) which reflects the tissue microstructure, correlated with subtle tissue damage that is not detectable in T2 and fluid attenuation inversion recovery (FLAIR) imaging [7,8,9,10,11].
In cerebral small vessel disease, there is an affection of microvessels supplying the white matter leading to axon demyelination or degradation that will be captured by DTI metrics in the form of reduced restrictions, increased free diffusion of water and accordingly decreased anisotropy [12].
Several studies have demonstrated the effect of smoking and nicotine addiction on white matter integrity and revealed decreased FA in the corpus callosum and some other regions [13,14,15,16].
This work aims to assess the diagnostic value of DTI parameters in evaluating the changes and microstructural abnormalities of white matter tracts in patients of severe chronic obstructive pulmonary disease and correlate these parameters with smoking index and the number of exacerbations in the last year.
Methods
This is a prospective case-control study that was performed after the approval from our institutional review board and informed consents were obtained from all patients before scanning. From January 2017 to February 2018, 30 male past smoker patients diagnosed as severe COPD (aged from 45 years to 70 with a mean age of 59.2 years) were enrolled in our study. Another 15 age- and sex-matched nonsmoker controls referred to the MRI unit for varying different brain investigations were included in the study (aged from 48–68 years with a mean age of 56.3).
The inclusion criteria were patients clinically diagnosed as severe COPD, according to GOLD guidelines 2015 [17]. All patients were past smokers meaning that the patient was a smoker 1 year or more ago, but who has not smoked for at least 1 year; pack-year smoking index was determined by multiplication of the number of cigarette packs a person smokes a day (where a standard pack contains 20 cigarettes) times the number of years the patient smoked [18]. The number of acute exacerbations (i.e. sudden worsening of COPD symptoms as shortness of breath, quantity and colour of sputum that typically last for several days and may be triggered by an infection or by environmental pollutants [19]) within the last year were obtained. All subjects with a history of associated pulmonary, cerebrovascular, neurological and any other chronic medical disease that may have a cerebral effect were excluded. All subjects underwent routine brain MR imaging and DTI.
MR imaging
MR imaging was performed using a 1.5 Tesla scanner (Ingenia, Philips) with head circular polarization surface coil and obtained parameters include T1 sequence (TR/TE, 475/15 ms); T2 sequence (TR/TE, 3607/100 ms); matrix size 80 × 80; slice thickness 3 mm; and interslice gap 1.8 mm.
DTI
This consisted of a single-shot echo-planar imaging sequence in an axial plane designed to acquire diffusion data in a total scan duration of about 7–8 min. The included parameters were TR/TE, 2131/86 ms; field of view (FOV) 224 × 156 mm2; data matrix size 64 × 60; slice thickness of 2.5 mm; and interslice gap 2 mm; diffusion gradients were applied along 32 axes, using two diffusion weightings, a b-value of 0 and 1000 s/mm2 and voxel dimensions (3.5 × 3.71 × 2.5 mm3). Parallel imaging (SENSitivity Encoding [SENSE] reduction factor P 2) was used.
Image analysis
The DTI row data were sent to the workstation (extended MR Workspace 2.6.3.5, Philips medical systems Nederland B.V) supplied by the vendor for further processing. Firstly, automated registration of the DTI data was run to correct eddy current distortions, then a diffusion tensor model was set, following that calculation of each voxel direction and magnitude of diffusion yields fractional anisotropy (FA) and mean diffusivity (MD) images for each subject (Fig. 1). In the identification of selected white matter tracts (superior longitudinal fasciculus (SLF), inferior occipitofrontal fasciculus (IOFF), uncinate fasciculus (UF), cingulate gyrus (CG), optic radiation (OR) in each hemisphere, genu of corpus callosum (GCC) and splenium of corpus callosum (SCC)) (Fig. 2) then careful positioning of polygonal region of interest (ROI) with electronic cursor in selected tracts, for each analysed tract, three different ROIs were placed on each tract, and the mean FA and MD values were calculated (Fig. 3).
Identification of selected white matter tracts: a paired cingula just cephalad to corpus callosum (star), superior longitudinal fasciculus (white arrow), b inferior occipitofrontal fasciculus (white arrow), c Uncinate fasciculus (white arrow), d Genu and splenium of corpus callosum (GCC,SCC), optic radiation (open arrow)
Statistical analysis
Statistical analyses were performed using commercially available Software Package for Social Sciences version 21 (SPSS, Chicago, IL). The normality of data was first tested with Shapiro test. Quantitative data were presented as the mean ± standard deviation (SD). We compared normally distributed FA and MD metrics of selected white matter tracts between the two major groups using the Student t test. Pearson correlation was used to correlate continuous data while sensitivity and specificity, positive predictive value (PPV) and negative predictive value (NPV) at different cutoff points were tested by ROC curve. For all statistical tests done, the threshold of significance is fixed at 5% level (p value). The results were considered significant when the probability of error is less than 5% (p < 0.05) and highly significant when the probability of error is less than 0.1% (p < 0.001). The smaller the p value obtained, the more significant are the result.
Results
The study included 30 past smoker patients with severe COPD and 15 age- and gender-matched nonsmoker controls, no significant difference in age distribution between patients and controls (p = 0.195). Pack-year smoking index ranged from 15 to 70 pack-year, mean 39.23 packs, and included (1–7) acute exacerbations in the last year, mean 3.5.
The mean FA values of selected white matter tracts showed a highly significant difference (p < 0.001) between patients and control group (Table 1). ROC curve of FA is shown in Fig. 4. AUC of FA of the cingulate gyrus and optic radiation in patients and controls were the highest 0.912 and 0.897, respectively (Table 2). Selection of 0.344 and 0.399 as cutoff points of FA to differentiate between the two groups revealed accuracy of 80%, sensitivity of 83.3%, specificity of 73.3%, PPV of 86.2% and NPV of 68.7% for both tracts (Table 2).
The mean MD values of selected white matter tracts showed a highly significant difference (p < 0.001 between patients and control group (Table 1). ROC curve of MD has been shown in (Fig. 4). AUC of MD of superior longitudinal fasciculus (SLF) and cingulate gyrus of patients and controls was the highest (0.991and 0.996). Selection of 0.894 × 10−3 mm2/s and 0.914 × 10−3 mm2/s as cut off points to differentiate between the two groups revealed an accuracy of 93.3% and 95.5%, a sensitivity of 96.7% and 100%, a specificity of 86.7% and 86.7%, PPV of 93.5% and 93.7% and NPV of 92.8% and 100%, respectively (Table 2).
The correlation between the smoking index (SI) and FA values of white matter tracts showed a significant negative correlation with genu and splenium of corpus callosum, r = (− 0.370, − 0.370), p = (0.044, 0.013), respectively. However, a significant positive correlation between SI and MD values was seen in all selected white matter tracts, p < 0.05 (Table 3).
The correlation between the number of exacerbations and FA values of white matter tracts was not significant, while a significant positive correlation between the number of exacerbations and MD values was seen in the majority of selected white matter tracts, p < 0.05, sparing the optic radiation and genu of corpus callosum (Table 4).
Discussion
Chronic obstructive pulmonary disease (COPD) increases the risk of neuronal damage and cognitive dysfunction that increases the mortality and morbidity of the disease [11]. Neuronal damage could be due to chronic hypoperfusion, chronic systemic inflammation, which is observed with repeated acute exacerbations, or associated comorbidities as vascular disease and smoking [1, 12, 20].
Conventional MRI images helped in the illustration of the diffuse white matter changes associated with COPD. Diffusion-weighted magnetic resonance imaging (DWI or DW-MRI) can give details about tissue architecture, either diseased or normal through detection of water molecule diffusion patterns. Recently, a more advanced type of DWI has been available, which is DTI that played a crucial role in the assessment of the integrity of white matter tracts [21].
Fractional anisotropy and MD are DTI measures. FA is a measure of tissue integrity while MD is an index for tissue ultrastructure. Thus, FA will decrease, and MD will increase with tissue microstructural damage [ 22 ] .
The FA is a mathematical value ranging from “0” to “1” and corresponds to the directionality and anisotropy per voxel; it is higher in more organized, cylindrical anisotropic media like corpus callosum approaching “1,” while decreases in less organized and isotropic media as grey matter and approaches “0” in the CSF [23,24,25,26].
The MD value is an average combination of the three eigenvectors, and it reflects the general diffusivity and the overall water motion without any directionality. It is an index to the amount of water in the extracellular space, so it is increased in vasogenic edema, axonal and myelin loss [9, 22, 24].
Many studies performed on structural brain damage in COPD patients found a decrease in FA and increase in MD metrics throughout brain regions, mainly in cingulate gyrus, uncinate fasciculus and superior longitudinal fasciculus, which play a major role in cognitive performance [11, 21, 22]. Patients have been found to have abnormalities in cognition and daily activities [27,28,29]; this signifies that impaired brain microstructure and function related to cognition are recognized complications of COPD.
This study investigated the white matter tracts integrity in COPD patients using DTI measures and revealed a highly significant difference between the FA and MD of white matter tracts of patients and the control group (p < 0.001). DTI matrices increased diagnostic performance of MR imaging in assessing the microstructural changes occurring in the cerebral white matter of severely affected COPD patients. The decrease in FA values was not significantly correlated with increased smoking index and increased exacerbation frequency, while MD values increased with increased smoking index and increased number of exacerbations significantly.
In our study, FA values were typically decreased, and the MD values increased in a broad range of white matter regions with a high significant difference in comparison with healthy controls mainly in uncinate fasciculus and cingulate gyrus. Decreased FA is attributed to compromised myelin structure and white matter microstructure damage, while the increase in MD owed to myelin loss, axonal degeneration and increase in the extracellular amount of water leading to increased diffusivity.
In this work, the FA values were decreased with increasing the SI, but without significant difference, and this can suggest that impaired white matter integrity is not depending on smoking only; other factors may have a role. A previous study reported the lack of significance of reduced white matter integrity in relation to smoking [21].
The correlation between the number of exacerbations and FA values of white matter tracts was not significant; this may suggest the improper self-reported exacerbation frequency [30].
In this study, MD had a significant positive correlation with SI and the number of exacerbations in the majority of selected white matter tracts. This may be attributed to increased sensitivity to excess extracellular fluid edema and myelin loss. FA is sensitive to the number of the dominant fibre directions, fibre’s size and organization of the tissue membranes within each voxel and can be influenced by partial volume effects from neighbouring grey matter, but less specific for the type of microstructure change [10, 31].
Limitations
There are a few limitations of this study. First, there is a relatively small number of patients, still comparable; further studies upon a large number of patients may alter DTI metrics. Second, further work needs to be done to correlate cerebral integrity metrics with other evaluations of cerebral function related to cognition. Third, this study was done on a 1.5 Tesla scanner; further studies will be done on a higher-tesla scanner with the application of advanced post-processing of the DTI such as kurtosis imaging. Assessment of changes in cerebral blood flow could be improved by application of multi-parametric imaging with diffusion tensor magnetic resonance imaging, dynamic contrast magnetic resonance imaging and arterial spin labelling at higher 3 Tesla scanners that may help for novel methods of therapy to improve cerebral compensation in these patients [32].
Conclusion
We concluded that DTI parameters can be used in evaluating the changes and microstructural abnormalities of cerebral white matter in COPD patients and can help in the assessment of the impact of other underlying factors as smoking and exacerbation frequency.
Availability of data and materials
The corresponding author is responsible for sending the used data and materials upon request.
Abbreviations
- DTI:
-
Diffusion tensor imaging
- COPD:
-
Chronic obstructive pulmonary disease
- FA:
-
Fractional anisotropy
- MD:
-
Mean diffusivity
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- SI:
-
Smoking index
References
Barnes PJ (2010) Chronic obstructive pulmonary disease: effects beyond the lungs. PLoS Med 7:e1000220
Sinha S, Kumar V, Jagannathan NR et al (2009) Proton magnetic resonance spectroscopy of brain to study the cerebral metabolic abnormalities in COPD patients: a case-control study in north India. Indian J Chest Dis Allied Sci 51:15–19
Borson S, Scanlan JM, Friedman S et al (2008) Modeling the impact of COPD on the brain. Int J Chron Obstruct Pulmon Dis 3:429–434
Zangrando KTL, Trimer R, de Carvalho Jr LCS, Arêas GPT, Caruso FCR, Cabiddu R, Roscani MG, et al (2018). Chronic obstructive pulmonary disease severity and its association with obstructive sleep apnea syndrome: impact on cardiac autonomic modulation and functional capacity. International Journal of COPD13:1343–1351
Mori S, Zhang J (2006) Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51:527–539
Lahousse L, Tiemeier H, Ikram MA, Brusselle GG (2015) Chronic obstructive pulmonary disease and cerebrovascular disease: a comprehensive review. Respir Med 109:1371–1380
Rovaris M, Agosta F, Pagani E, Fillipi M (2009) Diffusion tensor MR imaging. Neuroimaging Clin N Am 19:37–43
Oudeman J, Nederveen AJ, Strijkers GJ, Maas M, Luijten PR, Froeling M (2016) Techniques and applications of skeletal muscle diffusion tensor imaging: a review. J Magn Reson Imaging 43(4):773–788
Hooijmans MT, Damon BM, Froeling M, Versluis MJ, Burakiewicz J, Verschuuren JJGM et al (2015) Evaluation of skeletal muscle DTI in patients with Duchenne muscular dystrophy. NMR Biomed 28(11):1589–1597
O’Donnell LJ, Westin C (2011) An introduction to diffusion tensor image analysis. Neurosurg Clin N Am 22(2):185–196
Um M, Lee S, Kim WJ, Cho SW, Yoon H, Lim M, Pyun S, Tae W, Kim S (2017) Alterations of white matter integrity in patients with chronic obstructive pulmonary disease: tract-based analysis using tracts constrained by underlying anatomy. J Korean Soc Radiol 77(3):148–156
Papma JM, Groot MD, Koning ID, Mattace-Raso FU, Lugt AVD, Vernooij M et al (2014) Cerebral small vessel disease affects white matter microstructure in mild cognitive impairment. Human Brain Mapping 35:2836–2851
Liao Y, Tang J, Liu T, Chen X, Hao W (2012) Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addict Biol 17:977–980
Weiland BJ, Sabbineni A, Calhoun VD, Welsh RC, Hutchison KE (2015) Reduced executive and default network functional connectivity in cigarette smokers. Hum. Brain Mapp 36:872–882
Yu D, Yuan K, Zhang B, Liu J, Dong M, Jin C et al (2016) White matter integrity in young smokers: a tract-based spatial statistics study. Addict Biol 21:679–687
Huang P, Shen Z, Wang C, Qian W, Zhang H, Yang Y, Zhang M (2017) Altered white matter integrity in smokers is associated with smoking cessation outcomes. Front Hum Neurosci 11:438. https://doi.org/10.3389/fnhum.2017.00438
Global Initiative for Chronic Obstructive Lung Disease [webpage on the Internet] Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015.pdf. Accessed 7 May 2015.
Singh N, Aggarwal AN, Gupta D et al (2011) Prevalence of low body mass index among newly diagnosed lung cancer patients in North India and its association with smoking status. Thoracic Cancer 2:27–31
Van Geffen WH, Slebos DJ, Kerstjens HA (2015) Hyperinflation in COPD exacerbations. Lancet Respir Med 3(12):e43–e44
Barnes PJ, Celli BR (2009) Systemic manifestations and comorbidities of COPD. Eur Respir J 33:1165–1185
Dodd JW, Chung AW, van den Broek MD, Barrick TR, Charlton RA, Jones PW (2012) Brain structure and function in chronic obstructive pulmonary disease: a multimodal cranial magnetic resonance imaging study, Am. J. Respir. Crit. Care Med 186:240–245
Zhang H, Wang X, Lin J, Sun Y, Huang Y, Yang T et al (2012) Grey and white matter abnormalities in chronic obstructive pulmonary disease: a Case-control study. BMJ Open 2:e000844. https://doi.org/10.1136/bmjopen-2012-000844
Schlaffke L, Rehmann R, Froeling M, Kley R, Tegenthoff M, Vorgerd M et al (2017) Diffusion tensor imaging of the human calf: variation of inter-and intramuscle-specific diffusion parameters. J Magn Reson Imaging 46:1137–1148
Sbardella E, Tona F, Petsas N, and Pantano P (2013). DTI measurements in multiple sclerosis: evaluation of brain damage and clinical implications. Multiple Sclerosis International doi.org/https://doi.org/10.1155/2013/671730.
Santis SD, Drakesmith M, Bells S, Assaf Y, Jones DK (2014) Why diffusion tensor MRI does well only some of the time: variance and covariance of white matter tissue microstructure attribute in the living human brain. Neuro Image 89:35–44
Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N et al (2001) Diffusion tensor imaging: concepts and applications. J Magnetic Resonance Imaging 13:534–546
Li J, Fei GH (2013) The unique alterations of hippocampus and cognitive impairment in chronic obstructive pulmonary disease. Respir Res 14:140
Spilling CA, Jones PW, Dodd JW, Barrick TR (2017) White matter lesions characterise brain involvement in moderate to severe chronic obstructive pulmonary disease, but cerebral atrophy does not. BMC Pulmonary Med 17:92–114
Dodd JW, Getov SV, Jones PW (2010) Cognitive function in COPD. Eur Respir J 35:913–922
Frei A, Siebeling L, Wolters C, Held L, Muggensturm P, Strassmann A et al (2016) The inaccuracy of patient recall for COPD exacerbation rate estimation and its implications: results from central adjudication. CHEST J 150:860–868
Alexander AL, Hurley SA, Samsonov AA, Adluru N, Hosseinbor AP, Mossahebi P, et al (2012). Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connectivity doi.org/https://doi.org/10.1089/brain.2011.0071
Razek AAKA, El-Serougy L, Abdelsalam M, Gaballa G, Talaat M (2018) Differentiation of residual/recurrent gliomas from post-radiation necrosis with arterial spin labeling and diffusion tensor magnetic resonance imaging-derived metrics. Neuroradiology 60:169–177
Acknowledgment
Not applicable
Funding
No source of funding.
Author information
Authors and Affiliations
Contributions
HEM is the guarantor of integrity of the entire study. HEM and EAR contributed to the study concepts and design, SAA and AS contributed to the literature research. HEM, AS, and EAR contributed to the clinical and experimental studies. SAA and AS contributed to the data analysis. HEM and EAR contributed to the statistical analysis. HEM, SAA, and EAR contributed to the manuscript preparation and editing. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethical committee of the Chest Medicine and the Radiology Departments of an academic highly specialized multidisciplinary hospital, and an informed written consent was taken from all the control and case groups of patients that were included in the study.
The ethics committee’s reference number is: R.18.08.252.R1.R2
Consent for publication
All authors approved the manuscript. All patients included in this research were legible. They gave written informed consent to publish the data contained within this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Helmy, E.M., Sakrana, A.A., Abdel-Fattah, S. et al. Diffusion tensor imaging of white matter integrity in relation to smoking index and exacerbations in severe chronic obstructive pulmonary disease. Egypt J Radiol Nucl Med 50, 70 (2019). https://doi.org/10.1186/s43055-019-0082-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-019-0082-z