Abstract
Background
Febrile neutropenia (FN) is a life-threatening complication of cancer therapy. Appropriate antibiotic treatment improves the clinical outcome in these patients; however, the increasing rate of anti-microbial resistance makes its therapy particularly challenging.
Aim
This study aims to investigate the microbial spectrum and antimicrobial resistance pattern in cancer patients with FN at King Abdullah University Hospital, Jordan.
Method
Blood cultures of 261 FN patients pre-diagnosed with malignancy (age 1–18 years) were enrolled in this study.
Results
The most common isolated microorganisms were gram-positive bacteria (50.2℅). Gram-infections with coagulase-negative Staphylococcus (CONS) are the most prevalent pathogens, followed by gram-negative infections with Klebseilla pneumonia and fungal infections with nonalbicans strains. All CONS, Methicillin-resistant Staphylococcus aureus (MRSA), and enterococcus species were sensitive to Vancomycin and Teicoplanin. Ten percent of the gram-negative organisms were Extended-spectrum beta-lactamase (ESBL) and all were sensitive to carbapenems. 66.7% of pseudomonas aeruginosa blood cultures were sensitive to Piperacillin-Tazobactam and 83.4% were sensitive to carbapenems. All Enterobacter species were sensitive to Carbapenems.
Conclusion
Isolates showed various antibiotic sensitivity and resistance patterns; therefore, a judicious management plan is essential to establish an appropriate and effective institutional policy for the use of empirical antibiotics in patients of FN.
Similar content being viewed by others
Background
Febrile neutropenia (FN) is known as the most common serious complication of cancer therapy [10]. It is encountered in the majority of pediatric patients undergoing chemotherapy [13, 27]. Previous studies reported that the mortality rate of febrile neutropenic pediatric patients is approximately 1–6% ([13, 17, 22]; María E [33]) and can be as high as 11–12% in some groups [27, 34]. Documented infections include mostly bacterial infections, but fungal and viral infections are also recognized. The management of FN is considered an oncologic emergency. Prompt antibiotic therapy greatly influences and improves the clinical outcome; however, the increasing rate of anti-microbial resistance makes FN particularly challenging with subsequent increases in morbidity and mortality rates ([10]; M E [32]).
FN is defined as a single oral temperature greater than or equal to 38.3 °C (101 F) or a temperature greater than 38 °C (100.4 F) persisting for at least an hour in a patient who has an absolute neutrophilic count (ANC) < 1500 cells/mm3 [39]. The Common Toxicity Criteria established by the US National Cancer Institute grades neutropenia based on ANC include grade 0: ≥ 2000 cells/mm3; grade 1: ≥ 1500 – < 2000 cells/mm3; grade 2: ≥ 1000–< 1500 cells/mm3; grade 3: ≥ 500–< 1000 cells/mm3; grade 4: < 500 cells/mm3. However, the term neutropenia is clinically used for ANC < 1500 cells/mm3 (grades 2 or greater) [7].
Despite the substantial decline in infection-related mortality in recent years, infections are still the main reason for morbidity and mortality in cancer patients with fever and neutropenia [38]. The appropriate strategy of clinical vigilance and immediate treatment is considered a key factor in managing febrile neutropenic patients [10, 15]. Several guidelines of empiric antibiotic therapy were developed in specific sites and regions in the world; however, some recommendations may not be as applicable in other areas where there are considerable differences in the microbial spectrum, the incidence of resistant organisms, availability of antibiotics, and/or healthcare-associated economic conditions [10].
While the pattern of bacterial pathogens causing infection in neutropenic patients has shown a significant shift from gram-negative to gram-positive bacteria over the past decades [21, 38, 41], there are still remarkable sites and region-specific differences in the microbial spectrum and resistance patterns of the pathogen [10]. In addition, despite bacteria being the most common pathogens in febrile neutropenic pediatric patients, fungal infections are an emerging concern and deserve early intervention [15]. Therefore, the need for ongoing updates on the initial choice of empiric antibiotic therapy is becoming essential. The aim of this study is to investigate the microbial spectrum and antimicrobial resistance pattern in cancer patients with FN at King Abdullah University Hospital (KAUH), a tertiary hospital in the north of Jordan that offers advanced health care for patients in the Middle East region.
Methods
This study aims to investigate the microbial spectrum and antimicrobial resistance pattern in cancer patients. This is a hospital-based retrospective study conducted at King Abdullah University Hospital (KAUH), a tertiary hospital in the north of Jordan that offers advanced health care for patients in Jordan. This study included all episodes of chemotherapy-induced FN with positive blood culture in children admitted to the hospital from January 2008 to December 2021. The study included patients with ages ranging from 1 to 18 years old, at diagnosis, and followed for hematological malignancies or solid tumors who were admitted for chemotherapy-induced FN and positive blood culture. Patients who have received cancer treatment other than chemotherapy were excluded from this study.
The standard practice at KAUH for microbiological testing is to obtain at least two sets of blood cultures at the time of admission before starting antibiotic treatment. Samples were cultured (inoculated onto Chocolate agar, 5% sheep Blood agar, MacConkey agar, and Sabouraud dextrose agar plates) and bacterial identification and antibiotic susceptibility were determined by using the Vitek 2 Compact system (Automated instrument for ID/AST testing) (bioMérieux, Marcy l’Etoile, France). VITEK-2 identification cards were used for gram-positive, and gram-negative bacteria and yeast. VITEK-2 AST-cards were used for performing antimicrobial susceptibility (antibiotics and antifungal susceptibility).
The standard approaches for FN in KAUH are to manage the infection with empiric therapy, initiated immediately after blood culture sampling and before conducting any other investigations. Empirical therapy choices are based on patients’ history availability and updated information regarding the local antimicrobial susceptibility pattern. The efficient laboratory diagnoses and workflows are of great importance in narrowing the antimicrobial spectrum as definitive therapy as soon as possible to match the antimicrobial stewardship program principles.
The study was approved by the Institutional Review Board at Jordan University of Science and Technology (IRB 893-2020). Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS, version 16.0) software.
Results
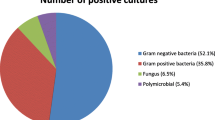
As shown in Table 1, this study analyzed the blood culture results of 261 patients pre-diagnosed with malignancy (146 male and 115 female patients). The majority of underlying malignancies were hematological malignancies, in 158 patients (60.5%) compared to solid tumors in 103 patients (39.5%). Acute lymphoblastic leukemia was the most common underlying hematological diagnosis (104 of 158; 65.8%), followed by lymphomas (33 of 158; 20.8%; Non-Hodgkin lymphoma 17; 10.7%, Hodgkin lymphoma 16;10.1%), and acute myeloid leukemia (AML) (21 of 158; 13.4%). The number of patients classified with low ANC (grades 2, 3, and 4) was 125 patients, the average ANC during admission for these patients was 830 cells/microliter. The percentage of patients with ANC less than 500 cells/microliter (grade 4) at the time of admission was 39.2% in hematological malignancy, compared to 21.3% seen in solid tumors. The most common isolated microorganisms from FN cancer patients were gram-positive bacteria (142/283: 50.2℅), followed by gram-negative bacteria (109/283; 38.5%), and fungal infection (32/283; 11.3%) (Fig. 1). Blood cultures with gram-positive organisms were higher in hematological malignancy (83; 59.3%) compared to solid tumors (59; 40.7%). Similarly, blood cultures with gram-negative organisms were also higher in hematological vs. solid tumors (74; 67.9% vs 35; 32.1%). Conversely, fungal infections were shown to be higher in solid tumors (18; 56.3%) compared to hematological malignancies (14; 43.7%). It should be noted that 10.3% of all patients had polymicrobial bacteremia (gram-positive and gram-negative).
CONS was the most frequently isolated pathogen from gram-positive cultures (75 %), followed by MRSA (7.7℅), Enterococcus fecalis and Methicillin-sensitive Staphylococcus aureus (MSSA) (5.6 %) (Table 2). ESBL-negative Klebseilla pneumonia was the most common gram-negative organism (21%), followed by ESBL-positive Escherichia coli (15 %) and Acinetobacter baumanni (12%) (Table 3). It should be mentioned that 107 patients had CONS sepsis, 60% were seen in hematological malignancy and 40% were seen in solid tumors. Twenty-six of 107 patients were on prophylactic antibiotics (24 patients on Bactrim and 2 patients on Ciprofloxacin) with 4 of 26 patients coexisting gram-negative organisms. As seen in Table 4, candida species represent 90% of fungal blood cultures, mostly Candida parapsilosis (47%) followed by candida albicans (19%). 11 of 261 patients were on a prophylactic antifungal (fluconazole or voricnazole) where only 1 patient developed fungus fusarium positive culture.
All CONS, MRSA, and enterococcus species were sensitive to vancomycin and teicoplanin. One blood culture of streptococcus viridians was resistant to vancomycin and teicoplanin and sensitive to rifampicin (Table 5). Ten percent of the gram-negative organisms were ESBL and all were sensitive to carbapenems. 66.7% of pseudomonas aeruginosa blood cultures were sensitive to Piperacillin-Tazobactam and 83.4% were sensitive to carbapenems. Two patients with extensively drug-resistant (XDR) pseudomonas aeruginosa were resistant to all antibiotics (shown in Table 6); therefore, so they were treated with colistin. Fifty percent of Acinetobacter calcoaceticus baumannii organisms showed sensitivity to Cefepime, Piperacillin-Tazobactam, Carbapenems, and Aminoglycosides. Two patients with multidrug-resistant (MDR) acinetobacter baumannii were resistant to all antibiotics (shown in Table 6) and were treated with colistin. All Enterobacter species were sensitive to Carbapenems, 70% to Aminoglycosides, and 80% 4th generation Cephalosporin (Table 6). Finally, All Candida parapsilosis cultures were sensitive to voricnazole and amphotericin B, 50% to Echinocandins and 64% to fluconazole (Table 7).
Discussion
FN is a life-threatening complication associated with chemo/radiotherapy-treated cancer in pediatric patients (M E [32]). More than half of pediatric cancer patients undergoing chemotherapy have been shown to develop infection [7, 39]. Delayed management of these neutropenic patients can increase morbidity and mortality rates [20, 23]. Although the risk assessment and management criteria of patients with FN have been widely carried out in adults, these models of assessment have shown that they are not consistent/compatible with pediatric patients ([4]; María E [33]). In addition, the prevalence and evolution of antimicrobial resistance, due to the empirically unjustified use of broad-spectrum antibiotics, have shown to be variable among different care centers in the world [9, 37]. Therefore, it is essential to continuously check the prevalence and sensitivity patterns of different microorganisms to be able to carefully revise the institutional policies for the use of appropriate empirical antibiotics in patients of FN. The present study represents the second report of the microbial spectrum and antimicrobial susceptibility profile from febrile neutropenic cancer patients in King Abdullah University Hospital (KAUH), a tertiary-care center in northern Jordan, Irbid.
In this study, we found that gram-positive bacteria were the most prevalent pathogens (50.2 ℅) followed by gram-negative bacteria (38.5%). Our results are consistent with previous data analysis on febrile neutropenic pediatric patients conducted in the same care center (KAUH) where gram-positive bacteria were the most prevalent pathogens [2]. This shift in the microbiological pattern of infection from gram-negative to gram-positive has been reported by previous studies performed in developing and developed countries [3, 5, 11, 17, 25, 26, 29, 41]. However, while other studies reported an increasing proportion of gram-positive bacterial infection, gram-negative bacteria were still the most prevalent pathogens [16, 18, 24, 35, 37]. In consistent with earlier studies [30, 31] Staphylococcus species were found as the most prevalent gram-positive pathogen in this study, but in contrast to a recent report from Palestine reported that Enterococcus spp. was the most prevalent gram-positive pathogen [17]. 50% of Staphylococcus aureus (MRSA and MSSA) cultures were found in patients who developed Grade 4 ANC. In agreement with previous reports [12, 35, 37] Klebseilla pneumonia and Escherichia coli were found to be the most common isolated gram-negative organisms in this study. Twenty-five percent of Klebseilla pneumonia and 50% of Escherichia coli and pseudomonas aeruginosa cultures were found in patients who developed grade 4 ANC.
The shift in the microbiological pattern of infection from gram-negative to gram-positive can be attributed to secondary factors; for instance, the potential oro-intestinal mucosa damage, caused by the intensive courses of chemotherapy, which can be considered a predisposing factor for the infection with gram-positive pathogens. In addition, the use of prophylactic oral antibiotics during the course of chemotherapy might selectively diminish the intestinal infection with gram-negative pathogens. Finally, the frequent use of indwelling catheters in cancer patients can lead to the development of skin-derived gram-positive infections as previously stated in former studies [2, 26, 28]. This shift in the bacterial spectrum emphasizes the need to alter treatment schedules for proper patient care. Even though gram-positive bacteria have become more prevalent pathogens, gram-negative bacteria still make up 40% of the pathogens found in patients with FN. In addition, gram-negative bacteria are associated with higher morbidity and mortality compared to gram-positive bacteria [40]. Therefore, guidelines for the empirical treatment should also cover these organisms specifically. However, it should be noted that other factors such as the local resistance patterns, previous culture data, patient’s symptoms, and grade neutropenia should be considered when selecting empirical antibiotic treatments, as the most appropriate treatment approach may vary in various local settings.
In the present study, fungal infections were shown to be the third most commonly isolated pathogen (11.3%) in the blood cultures of febrile neutropenic patients. Unlike gram-positive and gram-negative microorganisms, fungal infections are shown to be higher in solid tumors (56.3%) compared to hematological malignancies (43.7%). The nonalbicans strains have shown to be the most frequently isolated pathogen from the blood cultures of fungal infections, results that are consistent with previous reports [2, 3, 19]. Forty-three percent of fungal infections were in grades 3 and 4 neutropenia. The infection rate and severity were inversely related to the ANC [6, 8].
The antimicrobial susceptibility profile of microorganisms was also evaluated in this study in order to select the appropriate antimicrobial agent [1]. All staphylococcus aureus isolates were sensitive to vancomycin (100%) and teicoplanin (100%). MSSA was 100% sensitive to clindamycin, whereas MRSA was 80% sensitive to clindamycin. These findings also match with previous studies reporting that 100% staphylococcus aureus strains were sensitive to vancomycin [26, 35]. On the other hand, our findings contradict findings from a study conducted in Iran [37], where all staphylococcus aureus isolates were resistant to vancomycin, however, the results of the same study were similar to our findings regarding the majority of staphylococcus aureus stains that were sensitive to clindamycin. In our study, all Klebseilla pneumonia isolates were highly sensitive (100℅) to carbapenems. In addition, the majority of isolated Klebseilla pneumonia was ESBL negative and all were sensitive to cephalosporin. These results are also consistent with other studies that reported susceptibility ranging from 90 to 100% for carbapenems and cephalosporins [36, 37]. E coli resistance to fluoroquinolones is known as a common phenomenon in many care centers around the world [14] which was also found to be the case in our study where only 30–40% of E coli isolates were sensitive to fluoroquinolones. The majority of E. coli were ESBL positive (65%) and all were resistant to cephalosporins, which is contrary to other studies that reported the majority E. coli were susceptible to cephalosporins [37]. Regarding the susceptibility to carbapenems, we found that E coli isolates were 100% sensitive to carbapenems, compared to only 65% susceptibility reported in other studies [35, 37]. Pseudmanous aurgenosa showed 66% sensitivity to tazocin and 100% to amikacin, which comes in partially agreement with previous study reporting 100% sensitivity to both tazocin and amikacin [35]. Therefore, based on the results of antimicrobial susceptibility profile in this study, the implementation of multidisciplinary antimicrobial stewardship policies for patients with high-risk FN in our center was feasible and well accepted. For example, the significant decrease in the carbapenem and glycopeptide consumptions based our local guidelines has been reflected on antimicrobial sensitivity profile as shown above.
The results of this study provided valuable information about the microbial spectrum and antimicrobial susceptibility profile to develop treatment guidelines in febrile neutropenic cancer patients. However, this study has some limitations. First, our data were collected from a single care center. The pattern of causative pathogens and their antibiotic susceptibilities are influenced by the antibiotic resistance status which can be varied between each care center and community. Second, there were some limitations in data collection since our study is retrospective. Finally, the sample size was relatively small.
Conclusion
Gram-positive infections with coagulase-negative Staphylococci are the most prevalent pathogens in febrile neutropenic pediatric patients, followed by gram-negative infections with Klebseilla pneumonia and fungal infections with nonalbicans strains. Pathogen isolates show various antibiotic sensitivity and resistance patterns; therefore, a judicious management plan is essential to establish an appropriate and effective institutional policy for the use of empirical antibiotics in patients of FN.
Availability of data and materials
The data supporting the findings listed are available from the corresponding author upon request.
Abbreviations
- FN:
-
Febrile neutropenia
- ANC:
-
Absolute neutrophilic count
- KAUH:
-
King Abdullah University Hospital
- CLSI:
-
Clinical and Laboratory Standard Institute
References
Almaziny MA-I (2014) Isolation, identification, and profile of antibiotic resistance of bacteria inchildhood febrile neutropenia patients. European. J Exp Biol 4
Al-Sweedan S, Hayajneh W, Al-Ostath A (2012) Patterns of bacteremia in cancer patient with febrile neutropenia at King Abdullah University Hospital – Jordan 2003 - 2008. J Pediatr Infect Dis 7:15–20. https://doi.org/10.3233/JPI-2012-0343
Al-Tawfiq JA, Hinedi K, Khairallah H, Saadeh B, Abbasi S, Noureen M, Raza S, Alkhatti A (2019) Epidemiology and source of infection in patients with febrile neutropenia: A ten-year longitudinal study. J Infect Public Health 12(3):364–366. https://doi.org/10.1016/j.jiph.2018.12.006
Ariffin H, Navaratnam P, Lin HP (2002) Surveillance study of bacteraemic episodes in febrile neutropenic children. Int J Clin Pract 56(4):237–240
Aslan S, Citak EC, Yis R, Degirmenci S, Arman D (2012) Bacterial spectrum and antimicrobial susceptibility pattern of bloodstream infections in children with febrile neutropenia: experience of single center in southeast of Turkey. Indian J Microbiol 52(2):203–208. https://doi.org/10.1007/s12088-011-0210-6
Bodey GP, Buckley M, Sathe YS, Freireich EJ (1966) Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 64(2):328–340. https://doi.org/10.7326/0003-4819-64-2-328
Cherif H, Johansson E, Björkholm M, Kalin M (2006) The feasibility of early hospital discharge with oral antimicrobial therapy in low risk patients with febrile neutropenia following chemotherapy for hematologic malignancies. Haematologica 91(2):215–222
Crawford J, Dale DC, Lyman GH (2004) Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer 100(2):228–237. https://doi.org/10.1002/cncr.11882
de la Court JR, Heijmans J, Huynh J, Sieswerda E, de Jonge NA, van Dijk K, Sigaloff KCE, Schade RP (2022) Guidance of empirical antimicrobial therapy by surveillance cultures in high-risk neutropenic patients: a retrospective cohort study. Antimicrob Resist Infect Control 11(1):160. https://doi.org/10.1186/s13756-022-01198-5
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young J-AH, Wingard JR (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious Diseases Society of America. Clin Infect Dis 52(4):e56–e93. https://doi.org/10.1093/cid/cir073
González-Barca E, Fernández-Sevilla A, Carratalá J, Grañena A, Gudiol F (1996) Prospective study of 288 episodes of bacteremia in neutropenic cancer patients in a single institution. Eur J Clin Microbiol Infect Dis 15(4):291–296. https://doi.org/10.1007/BF01695660
Greenberg D, Moser A, Yagupsky P, Peled N, Hofman Y, Kapelushnik J, Leibovitz E (2005) Microbiological spectrum and susceptibility patterns of pathogens causing bacteraemia in paediatric febrile neutropenic oncology patients: comparison between two consecutive time periods with use of different antibiotic treatment protocols. Int J Antimicrob Agents 25(6):469–473. https://doi.org/10.1016/j.ijantimicag.2005.01.020
Hakim H, Flynn PM, Knapp KM, Srivastava DK, Gaur AH (2009) Etiology and clinical course of febrile neutropenia in children with cancer. J Pediatr Hematol Oncol 31(9):623–629. https://doi.org/10.1097/MPH.0b013e3181b1edc6
Huang K-P, Wang T-F, Chu S-C, Wu Y-F, Wang R-Y, Kao R-H (2011) Analysis of pathogens and susceptibility in cancer patients with febrile neutropenia and bacteremia: Experience in a single institution in eastern Taiwan. Tzu Chi Med J 23(4):115–118. https://doi.org/10.1016/j.tcmj.2011.09.002
Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, Feld R, Pizzo PA, Rolston KVI, Shenep JL, Young LS (2002) 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 34(6):730–751. https://doi.org/10.1086/339215
Jamal A, Fatima N, Shaikh S, Kaleem B, Rizvi QA, Zaidi U, Borhany M, Shamsi T (2019) Pattern of antimicrobial sensitivity in microbiologically documented infections in neutropenic patients with haematological malignancies: a single center study. Indian J Microbiol 59(2):188–192. https://doi.org/10.1007/s12088-019-00789-y
Joudeh N, Sawafta E, Abu Taha A, Hamed Allah M, Amer R, Odeh RY, Salameh H, Sabateen A, Aiesh BM, Zyoud SH (2023) Epidemiology and source of infection in cancer patients with febrile neutropenia: an experience from a developing country. BMC Infect Dis 23(1):106. https://doi.org/10.1186/s12879-023-08058-6
Jungrungrueng T, Anugulruengkitt S, Lauhasurayotin S, Chiengthong K, Poparn H, Sosothikul D, Techavichit P (2021) The pattern of microorganisms and drug susceptibility in pediatric oncologic patients with febrile neutropenia. J Pathogens 2021:6692827. https://doi.org/10.1155/2021/6692827
Klastersky J, Ameye L, Maertens J, Georgala A, Muanza F, Aoun M, Ferrant A, Rapoport B, Rolston K, Paesmans M (2007) Bacteraemia in febrile neutropenic cancer patients. Int J Antimicrob Agents 30(Suppl 1):S51–S59. https://doi.org/10.1016/j.ijantimicag.2007.06.012
Klastersky J, Paesmans M, Rubenstein EB, Boyer M, Elting L, Feld R, Gallagher J, Herrstedt J, Rapoport B, Rolston K, Talcott J (2000) The Multinational Association for Supportive Care in Cancer risk index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol 18(16):3038–3051. https://doi.org/10.1200/JCO.2000.18.16.3038
Koll BS, Brown AE (1993) The changing epidemiology of infections at cancer hospitals. Clin Infect Dis 17(Suppl 2):S322–S328. https://doi.org/10.1093/clinids/17.supplement_2.s322
Lam JC, Chai JY, Wong YL, Tan NW, Ha CT, Chan MY, Tan AM (2015) Causative pathogens of febrile neutropaenia in children treated for acute lymphoblastic leukaemia. Ann Acad Med Singap 44(11):530–534
Lanzkowsky P (2005) Manual Of Pediatric Hematology And Oncology 4th Ed. Elsevier Inc.
Lee D-G, Kim S-H, Kim SY, Kim C-J, Park WB, Song YG, Choi J-H (2011) Evidence-based guidelines for empirical therapy of neutropenic fever in Korea. Korean J Internal Med 26(2):220–252. https://doi.org/10.3904/kjim.2011.26.2.220
Lehrnbecher T, Varwig D, Kaiser J, Reinhardt D, Klingebiel T, Creutzig U (2004) Leukemia 18(1):72–77. https://doi.org/10.1038/sj.leu.2403188
Meidani M, Bagheri A, Khorvash F (2013) A Population-Based Study of Bacterial Spectrum in Febrile Neutropenic Patients. Jundishapur J Microbiol 6:150–156
Moon H, Choi YJ, Sim SH (2018) Validation of the clinical index of stable febrile neutropenia (CISNE) model in febrile neutropenia patients visiting the emergency department. Can it guide emergency physicians to a reasonable decision on outpatient vs. inpatient treatment? PloS One 13(12):e0210019. https://doi.org/10.1371/journal.pone.0210019
Oude Nijhuis CSM, Daenen SMGJ, Vellenga E, van der Graaf WTA, Gietema JA, Groen HJM, Kamps WA, de Bont ESJM (2002) Fever and neutropenia in cancer patients: the diagnostic role of cytokines in risk assessment strategies. Crit Rev Oncol Hematol 44(2):163–174. https://doi.org/10.1016/s1040-8428(01)00220-7
Özdemir ZC, Koç A, Ayçiçek A (2016) Microorganisms isolated from cultures and infection focus and antibiotic treatments in febrile neutropenic children from Şanlıurfa, Turkey. Turk J Pediatr 58(1):47–53. https://doi.org/10.24953/turkjped.2016.01.007
Parodi RL, Lagrutta M, Tortolo M, Navall E, Rodríguez MS, Sasia GF, De Candia LF, Gruvman MA, Bottasso O, Greca AA (2019) A multicenter prospective study of 515 febrile neutropenia episodes in Argentina during a 5-year period. PLoS One 14(10):e0224299. https://doi.org/10.1371/journal.pone.0224299
Rabayah R, Alsayed RB, Taha AA, Salameh H, Amer R, Sabateen A, Aiesh BM, Zyoud SH (2022) Microbial spectrum and drug resistance profile in solid malignancies in a large tertiary hospital from Palestine. BMC Infect Dis 22(1):385. https://doi.org/10.1186/s12879-022-07375-6
Santolaya ME, Alvarez AM, Becker A, Cofré J, Enríquez N, O’Ryan M, Payá E, Pilorget J, Salgado C, Tordecilla J, Varas M, Villarroel M, Viviani T, Zubieta M (2001) Prospective, multicenter evaluation of risk factors associated with invasive bacterial infection in children with cancer, neutropenia, and fever. J Clin Oncol 19(14):3415–3421. https://doi.org/10.1200/JCO.2001.19.14.3415
Santolaya ME, Alvarez AM, Avilés CL, Becker A, Mosso C, O’Ryan M, Payá E, Salgado C, Silva P, Topelberg S, Tordecilla J, Varas M, Villarroel M, Viviani T, Zubieta M (2007) Admission clinical and laboratory factors associated with death in children with cancer during a febrile neutropenic episode. Pediatr Infect Dis J 26(9):794–798. https://doi.org/10.1097/INF.0b013e318124aa44
Sereeaphinan C, Kanchanasuwan S, Julamanee J (2021) Mortality-associated clinical risk factors in patients with febrile neutropenia: a retrospective study. IJID Regions 1:5–11. https://doi.org/10.1016/j.ijregi.2021.09.002
Taj M, Farzana T, Shah T, Maqsood S, Ahmed SS, Shamsi TS (2015) Clinical and microbiological profile of pathogens in febrile neutropenia in hematological malignancies: a single center prospective analysis. J Oncol 2015:596504. https://doi.org/10.1155/2015/596504
Trecarichi EM, Tumbarello M (2014) Antimicrobial-resistant Gram-negative bacteria in febrile neutropenic patients with cancer: current epidemiology and clinical impact. Curr Opin Infect Dis 27(2):200–210. https://doi.org/10.1097/QCO.0000000000000038
Vahedian-Ardakani HA, Moghimi M, Shayestehpour M, Doosti M, Amid N (2019) Bacterial spectrum and antimicrobial resistance pattern in cancer patients with febrile neutropenia. Asian Pacific J Cancer Prevent 20(5):1471–1474. https://doi.org/10.31557/APJCP.2019.20.5.1471
Viscoli C, Varnier O, Machetti M (2005) Infections in patients with febrile neutropenia: epidemiology, microbiology, and risk stratification. Clin Infect Dis 40(Suppl 4):S240–S245. https://doi.org/10.1086/427329
Yoo J-H, Choi SM, Lee D-G, Choi J-H, Shin W-S, Min W-S, Kim C-C (2005) Prognostic factors influencing infection-related mortality in patients with acute leukemia in Korea. J Korean Med Sci 20(1):31–35. https://doi.org/10.3346/jkms.2005.20.1.31
Zimmer AJ, Freifeld AG (2019) Optimal management of neutropenic fever in patients with cancer. J Oncol Pract 15(1):19–24. https://doi.org/10.1200/JOP.18.00269
Zinner SH (1999) Changing epidemiology of infections in patients with neutropenia and cancer: emphasis on gram-positive and resistant bacteria. Clin Infect Dis 29(3):490–494. https://doi.org/10.1086/598620
Acknowledgements
Not applicable
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
DA, HO, DG, OA, and OM performed data collection, DA, DS, and BD performed the statistical analysis. DA, GTA, and SA analyzed and interpreted the data. DA and SA prepared the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted after receiving approval from the Ethics Review Committee of the Institutional Review Board at Jordan University of Science and Technology (IRB 893-2020).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alzu’bi, D., Obeidat, H., Ghabashineh, D. et al. The microbial spectrum and antimicrobial resistance pattern in pediatric cancer patients with febrile neutropenia at King Abdullah University Hospital, Jordan. Egypt Pediatric Association Gaz 72, 12 (2024). https://doi.org/10.1186/s43054-024-00249-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43054-024-00249-3