Abstract
Background
According to several recently published studies, pediatric Corona virus infection is mostly mild. However, a severe COVID-19 illness could occur in children, resulting in grave outcomes. Unfortunately, the data regarding the major determinants of disease progression in the pediatric population is still limited. Here, we aimed to identify the most significant risk factors associated with severe COVID-19 infection in children to predict the patients at elevated risk for serious illness.
Results
This single-center, retrospective study enrolled eighty hospitalized children and adolescents under the age of 18 years with coronavirus type 2 infections, who were divided according to the level of clinical severity into severe and non-severe groups. Epidemiological data, clinical features, radiological findings, laboratory test results, and disease outcomes of the studied patients were collected and analyzed to demonstrate their relation to disease severity. Patients with severe illness tend to have more respiratory symptoms (97.8% vs. 79.4%, p = 0.007), cardiac affection (23 (50.0%) vs. 5 (14.7%), p = 0.001, and neurological involvement (13 (28.1%) vs. 1 (2.9%), p = 0.003). Furthermore, abnormal radiological findings and higher radiological scores were significantly more common among patients with severe disease compared to non-severe cases (p = 0.037, 0.013). In multivariable analysis, clinical scoring, abnormal coagulation function, and ICU admission were the most significant parameters for forecasting severe illness.
Conclusions
We identified the most remarkable parameters involved in the progression of severe disease in Egyptian children with COVID-19 infection, which may be implemented in anticipation of susceptible children for earlier prompt management and a better prognosis.
Similar content being viewed by others
Background
Coronavirus Disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first identified in Wuhan, China, in December 2019. It has rapidly spread across the world. The World Health Organization (WHO) had officially declared the disease a pandemic and a public health emergency [1]. Egypt reported the first cases of COVID-19 on February 14, 2020, with an initial daily increase in the number of confirmed cases. In Egypt, from January 3, 2020, to February 17, 2023, there have been 515,698 confirmed cases of COVID-19, with 24,809 deaths, reported to WHO [2].
The severity of symptoms among patients infected with COVID-19 varies considerably, from asymptomatic infection to critical illness with serious complications [3, 4]. According to international pediatric studies, children have lower rates of severe COVID-19 infection than adults [5, 6], which may be due to differences in immune system responses [7], and infected children typically have a good prognosis [8]. However, the higher prevalence of coronavirus 2 in Egypt increases the probability of having a severe pediatric illness. Although some researchers have suggested that there are several factors that might be responsible for the severity of COVID-19 infection among adults [9, 10], the potential factors responsible for severe cases in pediatrics have not been clearly identified.
In the medical literature, reports regarding the clinical features of children with COVID-19 have distinct results that may be peculiar to each population [11, 12]. Addressing the characteristics of the children with severe COVID-19 infection could assist healthcare providers in proper medical decision-making to improve the management of these vulnerable children and develop predictive tools to identify children at risk for clinical deterioration, in particular in countries with limited resources.
Therefore, this study was conducted to compare demographics, clinical characteristics, radiological findings, laboratory parameters, and treatment options among children and adolescents infected with severe and non-severe COVID-19 infection, to explore the potential risk factors associated with disease severity, and to examine the hypothesis that some potential risk factors may be attributed to severe COVID-19 illness in children. If this hypothesis is settled, we can further establish a risk profile model to facilitate categorization of the disease severity in pediatric patients.
Methods
Study settings
We carried out a retrospective study, including 80 COVID-19 confirmed cases, during the period from April 2021 through the end of June 2022. The study sample included all the consecutive patients admitted to Ain Shams University Children’s Hospital during the study period. The hospital is a tertiary care pediatric hospital, and it is one of the major referral hospitals in Cairo, Egypt. This hospital was designated as a pediatric COVID-19 center during the pandemic, and children with suspected or confirmed COVID-19 infection were transferred there. The treatment was administered according to the Egyptian National Guidelines for Clinical Management and Treatment of COVID-19 [13].
Study participants
The study enrolled hospitalized children under the age of 18 with confirmed COVID-19 infection using real-time reverse transcriptase-polymerase chain reaction (RT-PCR) [14], who were admitted to the hospital during the study period, including patients admitted for other illnesses such as malignancy, diabetic ketoacidosis, or polytrauma and developed COVID infection during their hospital stay. No exclusion criteria were applied. The studied patients were classified into severe and non-severe cases. A patient was diagnosed with a severe disease when any of the following criteria were met: 1) Adolescent COVID-19 patients with clinical signs of pneumonia (i.e., fever, cough, dyspnea, fast breathing) plus one of the following: respiratory rate > 30 breaths/minute, severe respiratory distress, or oxygen saturation < 90% on room air. 2) COVID-19 children and infants with clinical signs of pneumonia (i.e., cough or difficulty in breathing) plus at least one of the following: central cyanosis or oxygen saturation < 90%, severe respiratory distress (e.g., tachypnea according to age group, grunting, very severe chest indrawing), inability to breastfeed or drink, lethargy, unconsciousness, or convulsions. 3) respiratory failure requiring mechanical ventilation; 4) shock; or 5) other organ failure requiring intensive care [15]. A patient was discharged when all four criteria were met, including: 1) being afebrile for more than 3 days; 2) having respiratory symptoms that have remarkably improved; 3) improvement in the chest CT radiographic findings; and 4) having two consecutive negative RT-PCR for viral COVID-19 nucleic acid at least 24 h apart [15, 16].
Data collection
Data were collected from patients’ medical records and rechecked with the patients' caregivers by the study physician. The collected data included basic demographic information including age, sex, residence, and socioeconomic status using the El-Gilani score [17], exposure to passive smoking, history of any chronic illness), history of exposure to a confirmed case of COVID or household contact among the family members using laboratory confirmation of SARS-CoV-2, and history of travel to endemic countries in the last two weeks before the presentation. time of disease onset, duration of symptoms before presentation, duration of hospital stays, and the duration of illness, which was defined as time from the onset of the disease till outcome. In addition, the presenting symptoms and signs compatible with COVID infection, as reported in the literature [18, 19], were collected on admission, including fever (temperature ≥ 38 °C), cough, dyspnea, bony aches, sore throat, loss of taste or smell, vomiting, diarrhea, or abdominal pain, extreme fatigue and/or irritability, respiratory distress, which was identified by persistent tachypnea and/or use of accessory muscles documented during physical examination, symptoms suggestive of multi-system inflammatory syndrome in children (MIS-C), such as non-allergic conjunctivitis, skin rash, cracked lips, and changes in hands and feet, MIS-C was defined as a clinically severe illness requiring hospitalization with fever, elevated inflammatory markers, and multisystem organ dysfunction in the setting of current, recent, proven, or probable COVID-19 infection and in the absence of an alternative likely explanation [20]. In addition to the medications prescribed, other comorbidities such as chronic respiratory diseases, immunosuppression, or malignancy were documented. Disease severity and clinical course during admission were recorded.
Moreover, laboratory test results were recorded and analyzed, including complete blood cell counts along with a white blood cell count, with special focus on the presence of lymphopenia, which was defined as an absolute lymphocyte count (ALC) of less than 4500/ μL in infants under the age of 8 months or less than 1500/ μL in patients 8 months and older [21], neutrophil/lymphocyte ratio, platelet count, hemoglobin, the international normalized ratio, liver and renal function tests, and inflammatory markers such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), lactate dehydrogenase (LDH), serum ferritin, fibrinogen, and D-dimer levels. An electrocardiogram and an echocardiogram performed for patients with suspected cardiac involvement were also recorded. The results of imaging studies, including chest X-rays (CXR) and computed tomography (CT) of the chest, were interpreted in accordance with the recommendations of the Radiological Society of North America (RSNA) [22], the radiological findings were classified as normal or abnormal, including a detailed description of radiological abnormalities enclosing the presence of bilateral ground glass opacities, diffuse or focal consolidation, pleural effusion, numbers of lobes affected, and the largest opacity size (mm). Finally, the need for intensive care unit (ICU) admission and/or mechanical ventilation and patient outcomes, either discharged or dead, were also recorded.
Severity assessment
All the studied patients were evaluated for the risk of severe illness using the following severity scores:
-
1
CXR score: It is a radiological score aimed at evaluating COVID-19 manifestations in the lung. It considered the presence of interstitial and/or alveolar abnormalities, as described by Borghesi et al [23]. Mild and prevalent interstitial disease in different lung fields corresponded to a CXR score of 0–3, while multiple alveolar consolidations, suggesting bilateral or multi-lobar alveolar disease, corresponded to a CXR score ≥ 6.
-
2
Chest CT Score: In this score, from the apex to the bottom, the lung was divided into five levels: the suprasternal notch, the aortic arch, the tracheal carina, the intermediate bronchus, and the apex of the diaphragm. The left and right lungs were scored separately, and each of the 5 lung zones in each patient was assigned a score according to the distribution of affected parenchyma as previously described [24]. The chest CT density was also graded (0, normal attenuation; 1, frosted glass density; 2, ground-glass attenuation; and 3, consolidation). Then the lung parenchyma score was multiplied by the square of the CT density score and points from all zones and added for a final total cumulative score that ranged from 0 to 900.
-
3
CT severity score (CT-SS): The CT-SS is used to describe ground-glass opacity, interstitial opacity, and air trapping in SARS infection [25]. According to the anatomic structure, the 18 segments of both lungs were divided into 20 regions, in which the posterior apical segment of the left upper lobe was subdivided into apical and posterior segmental regions, whereas the anteromedial basal segment of the left lower lobe was subdivided into anterior and basal segmental regions. The lung opacities in all 20 lung regions were evaluated on chest CT images using scores of 0, 1, and 2 if parenchymal opacification involved 0%, less than 50%, or equal to or more than 50% of each region, respectively. The CT-SS was defined as the sum of the individual scores in the 20 lung segment regions, which may range from 0 to 40 points.
-
4
COVID-19 severity index: This score is used to assess COVID-19 severity using a set of selected clinical and laboratory variables [26]. The variables included age, male sex, respiratory rate, oxygen saturation, heart failure, diabetes, systolic blood pressure, temperature, pulse, D-dimer, dyspnea, lymphocytes, and platelet count. Patients were divided into four risk categories based on their score: low risk (0–2), moderate risk (3–5), high risk (6–7), Critical 8 or more (Supplementary Table 3).
Sample size calculation
The consecutive non-probability sample size technique has been used. Using the PASS11 program for sample size calculation and, based on a previous study [27], for the differentiation between the two study groups (severe versus non-severe), A sample size of at least 34 patients in each group achieved a study power of 80 percent to detect significance for the comparisons between the two groups, assuming an alpha error of 0.05 and a beta error of 0.2. The estimated study population frequency of the outcome factor is 61.1%, with a margin of error of ± 5. The design effect is 1 with a 95% confidence level.
Statistical analysis
All statistical calculations were done using IBM SPSS version 23. For quantitative data, mean standard deviation (SD), medians, and interquartile range (IQR) were used, while for qualitative data, frequencies (number of cases) and percentages were used. P values less than 0.05 were considered statistically significant, and P values less than 0.01 were considered highly significant. The kappa statistic was used to test the inter-rater reliability of the severity scores. A bivariate analysis was carried out comparing patient characteristics, radiological findings, comorbidities, and laboratory parameters between the severe and non-severe groups. This was conducted using nonparametric tests (the Mann–Whitney U test) or parametric tests (the Student's t-test) for continuous variables, as appropriate.
Results
Study Population
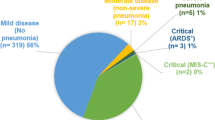
Between April 2021 and June 2022, 80 unique patients, whose ages ranged from 2 months to 16 years, who tested positive for SARS-CoV-2 infection were included in the current study. 34 (42.5%) were classified as non-severe, while 46 (57.5%) were classified as severe. The median ages of the studied patients were 5.5 (IQR: 2.5–10) and 6 (IQR: 1.5–10) years among the non-severe and severe groups, respectively. The majority of patients (70.6% and 60.9%, respectively) were in the scholar and preschooler age groups in both the non-severe and severe groups. The proportion of females was significantly higher among severe group 28 (60.9%) versus non-severe group 13 (38.2%) (p = 0.018). In addition, low socioeconomic status was significantly observed among severe group 18 (39.1%) compared to non-severe group 9 (26.5%; p = 0.049). The median time from the development of symptoms till admission was 2 days (IQR, 1–3) among the non-severe group and 2 days (IQR, 1–2) among the severe group. 62 of these 80 patients (77.5%) acquired SARS-CoV-2 in the community, while 10 (29.4%) and 8 (17.4%) patients developed health care-associated infections among the non-severe and severe groups, respectively. A history of direct exposure to a confirmed case of COVID-19 infection was reported among 18 (39.1%) of the severe group and 7 (20.6%) of the non-severe group. Comorbidities were observed among 21 (45.6%), 15 (44.1%) of severe, and non-severe groups sequentially (p = 0.890). Baseline characteristics of the recruited patients are presented by degree of severity in Table 1.
Clinical presentations
As shown in Table 2, the reported signs and symptoms of the study population were compared according to the level of severity. For the entire study population, fever, wheezes, and dyspnea were the most common symptoms upon admission. The patients with severe disease tended to have a higher number of upper respiratory tract symptoms: 45 (97.8%), fever 45 (97.8%), wheezes 34 (73.9%), dyspnea 32 (69.6%), and respiratory distress 32 (69.6%) (P < 0.001) compared with those without severe disease: 27 (79.4%), 28 (82.4%), 16 (47.1%), and 15 (44.1%) (p = 0.007, 0.015, 0.014, 0.022, 0.022) The percentage of patients with cardiac manifestations (including arrhythmia, myocarditis, and heart failure) and hypotension, was significantly higher among the severe group (P = 0.001, 0.002). Atypical presentations including neurological symptoms, seizures or conjunctivitis were significantly observed among severe group than non-severe group (p = 0.003, 0.017, 0.029). Moreover, the proportion of patients with COVID complications, and MIS-C were significantly more prevalent among severe group than non-severe groups (p = 0.002).
Radiographic findings
CXR and chest CT were performed for all the studied patients. Normal radiological findings were significantly higher in the non-severe group (19) (55.9%) than in the severe group (15) (32.6%) (P = 0.037). Abnormal findings, including consolidation, were significantly more common in group 20 (43.5%) than in group 3 (8.8%) (p = 0.001). Typical radiographic findings specific to COVID infection were reported in 7 (15.2%) of the severe group versus 0 (0.0%) in the non-severe group (p = 0.053). The patients in the severe group are more likely to have a higher frequency of lobe involvement 3 (0–4) and a larger opacity size (mm) 4 (0–5) than those in the non-severe group 0 (0–3), 0 (0–4) (P = 0.024). Regarding radiological severity, the median CXR score, chest CT severity score (CT-SS), chest CT score, and COVID-19 severity index were significantly higher among the severe group than the non-severe group (p = 0.019, 0.013, 0.013, 0.001). The radiological features were illustrated in Table 3 and Supplementary Fig. 1.
Laboratory data among the studied groups
On admission, the white blood cell counts were generally normal (median 8.95 (6.8–16.6) versus 12.35 (7.6–18.2) among the non-severe and severe groups, respectively. In addition, lymphopenia at admission did not differ significantly in patients with 18, (39.1%) and without severe disease (12, (35.3%). Moreover, patients with severe illness showed a significantly increased neutrophil/lymphocyte ratio, 3.51 (1.23–4.69) compared to those with non-severe disease 1.38 (1 – 3.72) (p = 0.039). Serum ferritin 592.65 (314–1150) and partial thromboplastin time 37.5 (34.3–43.3) were significantly higher at admission for patients with severe disease versus patients with non-severe disease (356 (172–495) and 33.35 (28.25–40.6), respectively (p = 0.013, 0.036). Table 4 displays the laboratory results for both patient groups.
Drug therapy and disease outcomes
Antibiotic therapy was the most commonly used drug overall (100%). Antiviral drugs were the second most common type of therapy administered for non-severe cases (15; 44.1%) and severe cases (18; (39.1%) respectively. 38 (82.6%) of the patients in the severe group received anticoagulant therapy, compared to 17 (50%) in the non-severe group (p = 0.054). A higher proportion of patients with severe conditions (primarily those with MIS-C) were treated with IVIG than those who were not (33 (71.7%) versus 10 (29.4%) (P = 0.001). Significantly more patients with severe disease were admitted to the intensive care unit (ICU): 42 (91.3%) versus 0 (0.0%), and received mechanical ventilation: 26 (56.5%) versus 0 (0.0%) in the non-severe group (p = 0.001 for all). In terms of patient outcomes, death was more significantly reported among severe patients: 12 (26.1%) versus 0 (0.0%) in the non-severe group (p = 0.001). Treatment measures and study outcomes for the studied patient groups are shown in Supplementary Table 1.
Risk factors for severe disease
We identified more than 19 risk factors in four categories (demographic characteristics, clinical data, radiological findings, and laboratory results), which were found to be significantly associated with disease severity relative to non-severe illness (specifically female sex, low socioeconomic status, respiratory symptoms, the presence of MIS-C, neurological affection, involvement of more than 3 lung lobes, CXR score > 3, chest CT score > 160, chest CT severity score > 9, COVID-19 severity index > 7, neutrophil/lymphocyte ratio > 1.54, partial thromboplastin time (sec) > 35.4, and serum ferritin (ng/ml) > 537.
According to the multivariate logistic regression analysis, the most important determinant factors associated with increased risk of severe illness among the studied patients were clinical manifestations, specifically the lower respiratory symptoms, with an OR (95% CI) of 31.359 (5.251–187.277) and p-value 0.001, followed by prolonged partial thromboplastin time (sec) > 35.4 with an OR (95% CI) of 18.763 (0.696- 505.502) and p-value = 0.012.All statistically significant factors in our univariate and multivariate analyses are summarized in Supplementary Table 2.
Discussion
COVID-19 is currently a major infectious disease, causing substantial morbidity and mortality worldwide [28]. Coronavirus diseases can manifest as mild, moderate, severe, or even fatal respiratory infections [11]. A proper understanding of the leading causes of severe illness may decrease the disease burden and fatality rates through early management to prevent serious complications. Moreover, it reduces unnecessary use of the healthcare system and reserves health care services for high-risk children who are really in need of more intensive care, thus reducing the economic burden during this pandemic.
In this work, we described the epidemiology, clinical characteristics, imaging findings, laboratory results, and disease outcomes of pediatric patients hospitalized with confirmed COVID-19 infection and compared these findings between severe and non-severe patient groups. The current study revealed that clinical severity, an abnormal coagulation profile, and the need for ICU admission were the most significant factors associated with COVID-19 severity among the studied population.
To the best of our knowledge, this is one of the few studies that looked at the characteristics of severe and non-severe children and adolescents infected with SARS-CoV-2 in our community.
In our study, the median age of the studied patients was 6 years (IQR: 2–10). Our findings are in line with a previous meta-analysis [29] including 203 children, which found that the mean age of the studied patients was 5.46 years.
The current study revealed no statistically significant difference between severe and non-severe groups regarding age, which was incongruent with a previous study from China, which reported that infants and younger children are more likely to develop severe clinical manifestations than older children, likely due to an immature immune system [30]. On the other hand, another recent study [31] revealed that infants had lower odds than older children of progressing to severe or critical disease. This difference between the studies may be due to the smaller percentage of infants and toddlers in our study population.
Our results revealed that 18 (22.5%) of the studied patients acquired COVID infection after hospitalization (Table 1), which necessitates the implementation of intensive infection control policies to reduce the risk of potential viral transmission and alleviate the burden on the healthcare system.
This work revealed the predominance of severe disease (57.5%) in comparison to non-severe disease (42.5%) among our pediatric population, which came against several published studies that stated that children have less serious diseases that necessitate hospitalization [12, 18]. Moreover, a meta-analysis [32] based on 52 case reports, including 203 children with COVID-19, revealed that only 15 (7.46%) were diagnosed as severe cases.
The higher percentage of severe cases among our study participants compared to the previous studies raises awareness about the high possibility of severe disease among children, especially in communities with a high prevalence of COVID infection, and the urgent need for prognostic tools for the early detection of high-risk children vulnerable to disease progression.
In the present study, the percentage of females was significantly higher among the severe group (p = 0.018), which disagrees with several published studies that showed that males were more likely to have severe disease than females [33,34,35] which was explained by the differences in infection susceptibility, inflammation, and immune response between females and males [36, 37].
Our study revealed that patients with severe COVID-19 infection had significantly lower socioeconomic status (SES) (p = 0.049) (Table 1), which agrees with what has been reported in the literature that communities with a high percentage of lower-income individuals are suffering from a greater rate of COVID-19 infection [38, 39]. In addition, Danielle et al., [40] found that 31% of patients hospitalized with SARS-CoV-2 were of low SES, where low earnings are associated with poor health outcomes and frequent ICU admissions.
The current study found that fever, respiratory symptoms, and gastrointestinal symptoms were the most common symptoms among the studied patients, followed by cardiac manifestations and atypical presentations (Table 2), which agrees with a recent study [19], which revealed that most COVID-19-infected children had fever (80%), upper and lower respiratory tract symptoms (64%), and atypical presentations including seizures or seizure-like activity (6%). Our findings were also consistent with the findings of Lechien et al. [41], lqahtani et al. [28] in adults.
In this study, 43 (53.75%) of the studied patients reported gastrointestinal symptoms, which were slightly higher among the severe group (63.0% versus 41.2%) (p = 0.052) (Table 2).
In agreement with our findings, A recent study [40] reported that up to half of the patients reported 1 of the gastrointestinal symptoms, whereas it was reported in less than one-quarter of hospitalized adults with COVID infection [42, 43].
Compared to our study, a previous pediatric study [19] reported that gastrointestinal tract symptoms were observed in only 7 patients (14%) with Corona virus infection.
The high percentage of gastrointestinal symptoms among our patients may be due to high prevalence of associated viral gastroenteritis in our tropical communities, and the predominance of severe cases among the studied population, therefore, their findings were close to adult patients.
Neurological presentation was significantly more common among patients with severe disease (p = 0.003) (Table 2), which is in line with a recently published study that suggested that neurological involvement was quite common in COVID-19, especially in severe cases [44]. In addition, neurologic manifestations have been documented in adults with COVID-19 infection [45].
The neurological ailment could be explained by the SARS-CoV-2 virus's ability to infect nerve cells via the olfactory, trans-synaptic, leukocytic, or haematogenic routes [46].
The cardiac involvement, especially MIS-C, was significantly higher among the severe group (p = 0.001, p = 0.002) (Table 2), which agrees with a recent study conducted by Li B et al., [47], who reported that the incidence of cardiac involvement in COVID-19 infection was nearly 13 times higher in severe patients than non-severe patients. Moreover, a recent adult study [48] stated that MIS-C was reported in combination with manifestations of severe pulmonary COVID-19 as evidenced by the respiratory symptoms along with the extensive pulmonary affection in the CT (CO-RADS 4 and 5).
The cardiac affection may be caused by viral replication, which may cause direct cardiac damage through downregulation of angiotensin-converting enzyme 2 (ACE2), or indirect damage, which may be mediated through the release of cytokines, coagulopathy, insulin resistance, or an immune inability to fight inflammatory pathogens as in MIS-C [49].
We observed that conjunctivitis was significantly seen only among patients with severe illness (p = 0.029) (Table 2), which is consistent with a study conducted by Wu et al. [50] who observed that ocular abnormalities occurred more frequently in patients with more severe COVID-19 illness. In addition, a recent study [40] reported that ocular and dermatologic findings were observed in 32% and 39% of MIS-C cases, which were rarely reported in adults.
Our study revealed that the most common radiological abnormalities were consolidation and ground glass opacities (GGOs), followed by mixed GGOs and consolidation in both severe and non-severe groups, which were consistent with previous reports [51]. However, consolidation was significantly higher among the severe group than the non-severe group (p = 0.001). In addition, disease severity was significantly associated with higher radiological and clinical severity scores (p = 0.019, 0.013, 0.013, 0.001) (Table 3; Supplementary Fig. 1).
Consistent with our report, previous studies declared that patients who had viral pneumonias with CT evident bilateral consolidations, had more severe clinical courses than those who presented with ground glass opacities [52, 53]. These abnormalities may be correlated with severe systemic inflammation and alveolar damage, which indirectly reflect the disease severity in the study participants.
Furthermore, a recent study [54] of 76 pediatric patients with confirmed COVID-19 found a significant relationship between chest CT score and COVID-19 severity, suggesting its use to assess disease severity.
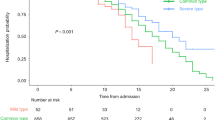
Our results revealed that the proportion of patients in need for ICU admission and mechanical ventilation was significantly higher among the severe cases compared to the non-severe group (p = 0.001) (Supplementary Table 1).
Similar to our findings, a previous cohort study [55] revealed that the severe/critical and mortality rates in pediatric COVID-19 cases were relatively high.
The findings of our study are in discordance with other published studies [8, 56] which reported that children have a better prognosis for COVID-19, when compared to adults, which was explained by the difference in the immune system response to viral infection [56, 57].
The difference between our findings and previous studies may be due to recent mutations in the virus strains and the appearance of new variants that may be more aggressive, increasing the susceptibility to severe disease in vulnerable children.
Our study revealed a high percentage of mortality (16.25%), which was predominately reported among the severe group (p = 0.001) (Supplementary Table 1). Our findings were remarkably higher than previous publications [28, 40], where the overall mortality rate was only 2%, or 0.44%, all in the severe group. This distinction emphasizes the significance of precise risk assessment of pediatric patients with COVID-19 infection in our community, as well as the need for early aggressive interventions for high-risk children to reduce mortality.
In the present study, patients with severe disease displayed significantly higher inflammatory markers, such as serum ferritin and the neutrophil/lymphocyte ratio, compared to those with non-severe disease (p = 0.013 and 0.039, respectively) (Table 4). Significantly increased inflammatory markers suggestive of an overactive immune response and a hyperinflammatory state were mostly seen in patients with severe disease, as described in adults [58].
This was in conformity with previous studies that reported marked elevations of serum ferritin in patients with severe COVID-19 infection [59], which can be explained by the fact that serum ferritin is an acute-phase reactant that rises during the inflammatory process of Corona virus infection.
Our findings are also consistent with a previous study [60], which found that patients infected with COVID-19 who had an elevated neutrophil/lymphocyte ratio were more likely to develop critical illness, and it may be a predictor of disease severity; this was also established in other multiple studies [61,62,63].
This finding may be explained by the negative effect of COVID-19 infection on T lymphocytes counts [64], in addition to an elevated total leucocytic count, which is mediated by infection-induced proinflammatory cytokines produced during severe illness [65].
The present study revealed that the most significant determinants of disease severity were symptom severity, coagulation dysfunction based on prolonged activated partial thromboplastin time (aPTT), and ICU admission. (P 0.001, 0.012, 0.001), which have been mostly associated with poor prognosis (Supplementary Table 2).
Our results are consistent with a recent meta-analysis of research [66], which found 11 factors to be significantly associated with the risk of severe illness, especially for dyspnea and tachypnea, an abnormal chest X-ray, age, comorbidity, cough, and LDH.
On the same hand, Tan et al. [67] and Bowles et al. [68] reported that patients with severe COVID-19 had mostly mildly prolonged aPTT clotting times. While previous studies [69, 70], reported that the levels of aPTT differed negligibly between severe and mild patients, Our finding may be due to the presence of lupus anticoagulants among COVID-19 patients, as reported by previous publications [68, 71, 72].
On the other hand, a subset of COVID-19 patients can have an abnormally short aPTT [73], which is often related to elevated factor VIII [74], which acts as an acute-phase reactant to a severe COVID-19 induced inflammatory response [75].
In contrast to our findings, a recent study [40] reported that obesity, increasing white blood cell count, younger age, hypoxia, and bilateral infiltrates on chest radiographs at admission were independent predictors of severe disease.
Another pediatric registry [76] conducted in Asia revealed that the risk factors for severe disease in children were age younger than 12 months, presence of comorbidities, and cough at presentation.
On the other hand, a national prospective surveillance study [77] conducted in Canada reported that severe COVID-19 was detected across all age groups and in children with and without comorbid conditions.
This difference between our study and the previous studies may be due to the variability in the age groups, clinical presentations, and methods of severity assessment among the studied populations.
Study limitations
This study has some limitations. First, the study was performed in a single center, so our findings may not be generalizable to the whole pediatric population with COVID-19 infection. Second, some of the data may have been missed in this retrospective study, so further multicentric longitudinal studies applied to larger numbers of patients should be done to confirm our results. However, it is important to note that the findings in the present study have not been previously reported.
Conclusions
The percentage of severe illness among children and adolescents with COVID-19 infection was higher in this study than previously reported. In addition, atypical presentations were displayed in our study with a high prevalence of cardiac involvement. The study identified the major determinants of pediatric COVID-19 severity that should be used in clinical practice to identify children at high risk for disease progression and provide prompt management to reduce future complications and mortality while conserving medical resources, particularly in low-income countries.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ICU:
-
Intensive Care unit
- COVID-19:
-
Coronavirus Disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- WHO:
-
World Health Organization
- RT-PCR:
-
Real time reverse transcriptase-polymerase chain reaction
- MIS-C:
-
Multi-system inflammatory syndrome in children
- ALC:
-
Absolute lymphocyte count
- CRP:
-
C- reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- LDH:
-
Lactate dehydrogenase
- CXR:
-
Chest X-ray
- CT:
-
Computed tomography
- RSNA:
-
Radiological society of North America
- CT-SS:
-
CT severity score
- SPSS:
-
Statistical Package for the Social Science
References
Organization WH (2020) World Health Organization coronavirus disease (COVID-19) dashboard. Published online, World Heal Organ.
World Health Organization (2023) Therapeutics and COVID-19: Living Guideline, 13 January 2023; No. WHO/2019-nCoV/therapeutics/2023.1. World Health Organization, Geneva
Zhang J, Wu S, Xu L (2020) Asymptomatic carriers of COVID-19 as a concern for disease prevention and control: more testing, more follow-up. Biosci Trends 14(3):206–208.
Armin S, Mirkarimi M, Pourmoghaddas Z, Tariverdi M, Shamsizadeh A, Alisamir M, et al (2022) Evidence-Based Prediction of COVID-19 Severity in Hospitalized Children. Int J Clin Pract 2022:1918177.
Henry BM, Aggarwal G, Wong J et al (2020) Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med 38(9):1722–1726.
Bai G-H, Shih P-Y, Chen S-Y et al (2022) Clinical features and characteristics of pediatric patients with COVID-19 infection: Experiences in a Tertiary Taiwan Hospital. Medicine (Baltimore) 101(35):e30157.
Chen Z-M, Fu J-F, Shu Q (2020) New coronavirus: new challenges for pediatricians. World J Pediatr 16(3):222.
Castagnoli R, Votto M, Licari A et al (2020) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr 174(9):882–889.
Berlin I, Thomas D, Le Faou A-L, Cornuz J (2020) COVID-19 and smoking. Nicotine Tob Res 22(9):1650–1652.
Subramanian A, Nirantharakumar K, Hughes S et al (2022) Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med 28(8):1706–1714.
Maniu I, Maniu G, Totan M (2022) Clinical and laboratory characteristics of pediatric COVID-19 population—a bibliometric analysis. J Clin Med 11(20):5987.
Wong JJM, Gan CS, Kaushal SH et al (2022) Pediatric COVID-19 Risk Factors in Southeast Asia-Singapore and Malaysia: A Test-Negative Case-Control Study. Am J Trop Med Hyg 106(4):1113.
Mostafa AS, Abdalbaky A, Fouda EM et al (2020) Practical approach to COVID-19: an Egyptian pediatric consensus. Egypt Pediatr Assoc Gaz 68(1):1–8.
Corman VM, Landt O, Kaiser M et al (2020) Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 25(3):2000045
World Health Organization (2020) Clinical management of COVID-19 interim guidance [Internet]. Geneva: World Health Organization. Available from https://www.who.int/publications-detail/clinical-management-ofsevere-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
Medicine NHC& NA of TC. (2020) Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7). Chin Med J (Engl) 133(09):1087–1095.
El-Gilany A, El-Wehady A, El-Wasify M (2012) Updating and validation of the socioeconomic status scale for health research in Egypt. East Mediterr Heal J 18(9):962–968. https://doi.org/10.26719/2012.18.9.962
Eastin C, Eastin T (2020) Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China: Dong Y, Mo X, Hu Y, et al. Pediatrics. 2020. J Emerg Med 58(4):712–713.
Zachariah P, Johnson CL, Halabi KC et al (2020) Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children’s hospital in New York City. New York JAMA Pediatr 174(10):e202430–e202430.
Jain MK, Sahu SK, Behera JR, Patnaik S (2021) Multisystem inflammatory syndrome in children associated with COVID 19 treated with oral steroid. Indian J Pediatr 88(1):106.
Régent A, Kluger N, Bérezné A, Lassoued K, Mouthon L (2012) Lymphocytopenia: aetiology and diagnosis, when to think about idiopathic CD4 (+) lymphocytopenia? La Rev Med interne 33(11):628–634.
Simpson S, Kay FU, Abbara S et al (2020) Radiological society of north America expert consensus document on reporting chest CT findings related to COVID-19: endorsed by the society of thoracic Radiology, the American college of Radiology, and RSNA. Radiol Cardiothorac Imaging 2(2):e200152.
Borghesi A, Maroldi R (2020) COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Med 125(5):509–513.
Zhang J, Meng G, Li W et al (2020) Relationship of chest CT score with clinical characteristics of 108 patients hospitalized with COVID-19 in Wuhan. China Respir Res 21(1):1–11.
Yang R, Li X, Liu H et al (2020) Chest CT severity score: an imaging tool for assessing severe COVID-19. Radiol Cardiothorac Imaging 2(2):e200047.
Huespe I, Bisso IC, Di Stefano S, Terrasa S, Gemelli NA, Las HM (2020) COVID-19 Severity Index: A predictive score for hospitalized patients. Published online, Med Intensiva.
Fisler G, Izard SM, Shah S et al (2020) Characteristics and risk factors associated with critical illness in pediatric COVID-19. Ann Intensive Care 10(1):1–8.
Alqahtani AM, AlMalki ZS, Alalweet RM et al (2020) Assessing the Severity of Illness in Patients With Coronavirus Disease in Saudi Arabia: A Retrospective Descriptive Cross-Sectional Study. Front public Heal 8:775.
Amenomori M, Mukae H, Sakamoto N et al (2010) HSP47 in lung fibroblasts is a predictor of survival in fibrotic nonspecific interstitial pneumonia. Respir Med 104(6):895–901.
Dong Y, Mo X, Hu Y et al (2020) Epidemiology of COVID-19 among children in China. Pediatrics 145(6):e20200702. https://doi.org/10.1542
Merckx J, Morris SK, Bitnun A et al (2022) Infants hospitalized for acute COVID-19: disease severity in a multicenter cohort study. Eur J Pediatr 181(6):2535–2539.
Lippi G, Henry BM (2020) Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir Med 167:105941.
Bai Y, Yao L, Wei T et al (2020) Presumed asymptomatic carrier transmission of COVID-19. JAMA 323(14):1406–1407.
Wan S, Xiang YI, Fang W et al (2020) Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol 92(7):797–806.
Zhang Q, Wang Z, Lv Y et al (2021) Clinical features and prognostic factors of patients with COVID-19 in Henan Province. China Hum Cell 34(2):419–435.
Conti P, Younes A (2020) Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents 34(2):339–343.
Sama IE, Ravera A, Santema BT et al (2020) Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin–angiotensin–aldosterone inhibitors. Eur Heart J 41(19):1810–1817.
Hawkins D (2020) Social determinants of COVID-19 in Massachusetts, United States: an ecological study. J Prev Med Public Heal 53(4):220.
Maroko AR, Nash D, Pavilonis BT (2020) COVID-19 and inequity: a comparative spatial analysis of New York City and Chicago hot spots. J Urban Heal 97(4):461–470.
Fernandes DM, Oliveira CR, Guerguis S et al (2021) Severe acute respiratory syndrome coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J Pediatr 230:23–31.
Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Mat Cabaraux P, Q, Cantarella G, (2020) Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Int Med 288(3):335–44
Cheung KS, Hung IFN, Chan PPY et al (2020) Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology 159(1):81–95.
Goyal P, Choi JJ, Pinheiro LC et al (2020) Clinical characteristics of Covid-19 in New York city. N Engl J Med 382(24):2372–2374.
Berlit P, Bösel J, Gahn G et al (2020) “Neurological manifestations of COVID-19”-guideline of the German society of neurology. Neurol Res Pract 2(1):1–14.
Mao L, Jin H, Wang M et al (2020) Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan. China JAMA Neurol 77(6):683–690. https://doi.org/10.1001/jamaneurol.2020.1127
Leven Y, Bösel J (2021) Neurological manifestations of COVID-19–an approach to categories of pathology. Neurol Res Pract 3(1):1–12.
Li B, Yang J, Zhao F et al (2020) Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 109(5):531–538.
Hamed T, Sarhan DT (2021) Combined presentation of severe pulmonary COVID-19 and multisystem inflammatory syndrome in children (MIS-C): two reported Egyptian pediatric cases. Egypt Pediatr Assoc Gaz 69(1):1–8.
Chang W-T, Toh HS, Liao C-T, Yu W-L (2021) Cardiac involvement of COVID-19: a comprehensive review. Am J Med Sci 361(1):14–22.
Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, Wu K (2020) Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province. China. JAMA Ophthalmol 138(5):575–8
Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D (2020) Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol 55(5):1169–1174
Gattinoni L, Caironi P, Cressoni M et al (2006) Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354(17):1775–1786
Marchiori E, Zanetti G, Fontes CAP et al (2011) Influenza A (H1N1) virus-associated pneumonia: High-resolution computed tomography–pathologic correlation. Eur J Radiol 80(3):e500–e504
Çetin C, Karaaslan A, Akın Y, Arifoglu M, Rona G, Demirhan R (2022) Relationship of chest computed tomography score with disease severity and laboratory values in children with COVID-19. J Paediatr Child Health 58(5):802–808
Nguyen PNT, Thuc TT, Hung NT et al (2022) Risk factors for disease severity and mortality of children with Covid-19: A study at a Vietnamese Children’s hospital. J Infect Chemother 28(10):1380–1386
Ludvigsson JF (2020) Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 109(6):1088–1095.
Henry BM, Lippi G, Plebani M (2020) Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med 58(7):1135–1138.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062
Kappert K, Jahić A, Tauber R (2020) Assessment of serum ferritin as a biomarker in COVID-19: bystander or participant? Insights by comparison with other infectious and non-infectious diseases. Biomarkers 25(8):616–625.
Liu J, Liu Y, Xiang P et al (2020) Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. Published online, MedRxiv.
Grifoni E, Valoriani A, Cei F et al (2020) Interleukin-6 as prognosticator in patients with COVID-19. J Infect 81(3):452–482.
Cao Y, Wei J, Zou L et al (2020) Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol 146(1):137–146.
Qin C, Zhou L, Hu Z et al (2020) Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan. China Clin Infect Dis 71(15):762–768.
Liu WJ, Zhao M, Liu K et al (2017) T-cell immunity of SARS-CoV: Implications for vaccine development against MERS-CoV. Antiviral Res 137:82–92.
Shi Y, Wang Y, Shao C et al (2020) COVID-19 infection: the perspectives on immune responses. Cell Death Differ 27(5):1451–1454.
Zhou B, Yuan Y, Wang S, Zhang Z, Yang M, Deng X, Niu W (2021) Risk profiles of severe illness in children with COVID-19: a meta-analysis of individual patients. Pediatr Res 90(2):347–352
Tan CW, Low JGH, Wong WH, Chua YY, Goh SL, Ng HJ (2020) Critically ill COVID-19 infected patients exhibit increased clot waveform analysis parameters consistent with hypercoagulability. Published online, Am J Hematol.
Bowles L, Platton S, Yartey N et al (2020) Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med 383(3):288–290.
Gao Y, Li T, Han M et al (2020) Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol 92(7):791–796.
Gong J, Ou J, Qiu X et al (2020) A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong. China Clin Infect Dis 71(15):833–840.
Connell NT, Battinelli EM, Connors JM (2020) Coagulopathy of COVID-19 and antiphospholipid antibodies. J Thromb Haemostasis 18(9):E1–E2. https://doi.org/10.1111/jth.14893
Sharathkumar AA, Faustino EVS, Takemoto CM (2021) How we approach thrombosis risk in children with COVID-19 infection and MIS-C. Pediatr Blood Cancer 68(7):e29049.
Spyropoulos AC, Levy JH, Ageno W et al (2020) Subcommittee on perioperative, critical care thrombosis, haemostasis of the scientific, standardization committee of the international society on thrombosis and haemostasis. Scientific and standardization committee communication: clinical guidance on the. J Thromb Haemost 18(8):1859–1865.
Panigada M, Bottino N, Tagliabue P et al (2020) Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 18(7):1738–1742.
Connors JM, Levy JH (2020) COVID-19 and its implications for thrombosis and anticoagulation. Blood 135(23):2033–2040.
Wong JJM, Abbas Q, Chuah SL et al (2021) Comparative analysis of pediatric COVID-19 infection in Southeast Asia, south Asia, Japan, and China. Am J Trop Med Hyg 105(2):413.
Farrar DS, Drouin O, Hepburn CM et al (2022) Risk factors for severe COVID-19 in hospitalized children in Canada: A national prospective study from March 2020–May 2021. Lancet Reg Heal 15:100337.
Acknowledgements
The author deeply appreciated the help of our patients to complete this work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
The author revised and approved the manuscript and agree to publish it in the Egyptian Pediatric Association Gazette (EPAG). The author made substantial contributions to the conception and design, acquisition of data, and/or analysis and interpretation of data. HA:designed the study, followed the patients, analyzed the data, drafted, writing and revised the manuscript. The author approved the manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Research Ethics Committee of the Faculty of Medicine, Ain Shams University, approved the protocol. Written consent was obtained from the patients’ guardians for access of the patients’ medical records prior to the inclusion in the study.
Consent for publication
Not applicable.
Competing interests
The author declares that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table 1.
Lines of treatment and outcomes among the studied patients’ groups. Table 2. Univariate and multivariate logistic regression analysis for factors associated with severe COVID-19 infection. Table 3. Agreement between WHO classification and COVID-19 severity index.
Additional file 2. Figure 1.
The relation between COVID 19 severity with chest CT severity score (1A), and COVID 19 severity index (1B).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, H.A. Major determinant factors of pediatric COVID-19 severity; a single center study. Egypt Pediatric Association Gaz 71, 22 (2023). https://doi.org/10.1186/s43054-023-00161-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43054-023-00161-2