Abstract
Background
Brain is one of the most sensitive organs to hypoxia during resuscitation. NIRS-measured cerebral oxygenation is an evolving brain monitoring tool for neonatal resuscitation.
Methods
We undertook a prospective observational study for monitoring of cerebral oxygenation and peripheral preductal saturation at 1, 5, and 10 min after birth. Fractional tissue oxygen extraction was calculated as well. In addition to studying factors affecting cerebral oxygenation at those points in time. For the current cohort, cerebral oxygenation centile charts were provided at those time points. This work was registered in the cinicaltrial.gov NCT05158881.
Results
Sixty healthy term neonates were enrolled to define reference ranges and centile charts of cerebral oxygenation at 1, 5, and 10 min after birth. The strongest correlations between cerebral oxygenation metrics and peripheral preductal saturation were at 5 min after birth with r value = 0.628. Using logistic regression analysis to determine the most significant factors affecting NIRS-measured crSO2 in healthy full-term neonates, we found that the mode of delivery was the most significant factor with OR = 4.000 (1.367–11.703) at 5 min and 8.726 (2.517–30.25) at 10 min.
Conclusions
Normal values for NIRS may help to identify infants with cerebral oxygenation below a certain value indicating regional hypoxia at certain time points and can guide neonatal resuscitation interventions. Caesarean section is the most significant factor affecting cerebral oxygenation during the transition of healthy full-term neonates.
Similar content being viewed by others
Background
Fetal to neonatal transition is a very complex physiological adaptation [1]. Any disturbances occurring during transition have potentiality for longer term harm [2]. Clinical assessment of neonates using Apgar score has high inter-observer variability [3, 4].
First 10 min ranges of SpO2 neither provide information about oxygen tissue delivery nor reflect cerebral oxygenation [5, 6]. Monitoring of brain oxygenation may influence interventions which can affect survival, as well as, short- and long-term neurodevelopment outcomes [7, 8].
Cerebral regional oxygen saturation (crSO2) measured via near infrared spectroscopy (NIRS) represents mixed tissue saturation value, thus enabling information about the balance of cerebral oxygen delivery and oxygen consumption [7, 9].
Aim of the study was to establish normal cerebral oxygenation measures, crSO2, and FTOE in healthy full-term neonates with gestational age 38 weeks or more using centile charts. In addition, we studied different factors affecting those metrics and compared the cerebral oxygenation metrics of infants born by C-section and those born vaginally.
Methods
The present study was conducted in the delivery rooms of the Alexandria University Maternity Hospital (AUMH) from December 2020 till August 2021. Sixty-nine healthy full-term neonates with gestational age (GA) ≥ 38 weeks and normal pregnancy were included in this study. Healthy neonates had no maternal history or risk factors that would have affected their transition like severe pregnancy-induced hypertension (PIH), antepartum hemorrhage, and cord prolapse. They received only routine care or initial steps in resuscitation. They did not have respiratory distress after resuscitation. Patients with mothers with risk factors such as vaginitis, urinary tract infection (UTI), and premature rupture of membranes (PROM) should have at least 2 negative C-reactive protein (CRP), 12 h apart. Patients had no obvious anomalies antenatally or postnatally diagnosed.
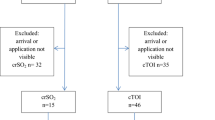
Thirty patients were born through cesarean section and the other 30 were born vaginally. Nine cases were excluded in the first 10 min; four required oxygen, three needed respiratory support after resuscitation, and two required positive airway pressure during resuscitation (Fig. 1).
This study excluded patients who had perinatal asphyxia, intrauterine growth restriction, birth complications (e.g., vacuum extraction or forceps application), suspected or known brain and/or cardiac malformations, or required respiratory support or oxygen therapy within the first 10 min of birth. Measurements of crSO2 were recorded using NIRS (INVOS™ 5100C Cerebral/Somatic Oximeter Monitor; Covidien Troy, MI, USA) by placing a neonatal brain sensor on the left fronto-parietal area of the newborn’s head for 10 min and crSO2 values were obtained at the end of 1, 5, and 10 min after birth.
The FTOE were calculated during the first 10 min of life with the following formula: FTOE = (SpO2-crSO2)/SpO2. At the same time, SpO2 were measured using Nellcor N-395 pulse oximeter (Tyco/Nellcor, Pleasanton, CA USA) or Masimo Set Radical 8 pulse-oximeter (Masimo Corp., Irvine, CA, USA) by applying a preductal pulse oximetry sensor to the right wrist. Venus blood gas samples were obtained from umbilical cord blood of all neonates and clinical status was assessed by Apgar score at 1 and 5 min of life. Informed parental consents were obtained for all enrolled newborns and the criteria for the ethical committee of the faculty of medicine at Alexandria University were strictly applied.
Data were fed to the computer and analyzed with IBM SPSS software package version 20.0. The Kolmogorov–Smirnov test was conducted to verify the normality of distribution. Qualitative data were described using numbers and percent. Quantitative data were described using range (minimum and maximum), mean, standard deviation, median, interquartile range (IQR), and percentiles. The centiles (10th, 25th, 50th, 75th, and 90th) for the crSO2 and FTOE were calculated by the least mean square (LMS) method proposed by Cole and Green. For categorical variables, chi-squared and Fisher’s exact tests were conducted to compare between different groups. Student’s t test and Mann–Whitney test were applied to both normally and abnormally distributed quantitative variables, respectively. Univariate and multivariate binary logistic regression analyses were constructed to identify independent risk factors (predictors) for low and high crSO2 norms (below and above 50th centile values). Pearson and Spearman correlation coefficients were used to correlate normally and abnormally distributed quantitative variables, respectively. Significance of the obtained results was judged at the 5% level [10].
Results
Table 1 shows descriptive analysis of the studied cases according to demographic data (sex, GA, and weight) mode of delivery, resuscitation needs, APGAR score, venous cord blood gases and clinical parameters (heart rate at 10 min, capillary refill time (CRT), preductal oxygen saturation at 1, 5, and 10 min).
There are significant positive correlations between cerebral tissue oxygenation and preductal oxygen saturation (pulse oximeter) at 1, 5, and 10 min with r = 0.532, r = 0.73, and r = 0.34, respectively, as shown in Fig. 2. Whereas, there are significant negative correlations between FTOE and preductal SPO2 at 1, 5, and 10 min with r = 0.532, r = 0.73, and r = 0.34, respectively.
Table 2 and Fig. 1 show centile (5th, 25th, 50th, 75th, 90th) charts for crSO2 and FTOE at 1, 5, and 10 min after birth in healthy full-term neonates. Fiftieth centile was used to classify patients into low norms and high norms.
Univariate logistic regression analysis for the predictors of low normal and high normal cerebral tissue oxygenation at 1, 5, and 10 min, as shown in Tables 3, 4, and 5. The most significant factor affecting cerebral oxygenation at 5 and 10 min is the mode of delivery. Infants born by C-section have significantly higher SPO2 and crSO2, and significantly lower FTOE at 5 and 10 min after birth. In addition to mode of delivery, PROM was significantly related to crSO2 at 5 min. Despite no significant difference in cerebral oxygenation metrics and spO2 between infants born by C-section or vaginally in the first 1 min after birth, the rate of rise from 1st to 5th minutes was significantly higher in infants born vaginally (Table 6).
Discussion
The NIRS technology has been around for about 45 years and is increasingly being used in newborns’ first minutes [2, 11]. It is possible to prevent hypoxic and hyperoxic brain injury [12, 13].
Here in our work, we introduce new charts for cerebral oxygenation metrics in the first 10 min of life. As far as our knowledge is concerned, they are the only charts from Africa and lower middle-income countries (LMICs) where birth asphyxia is relatively common, thus regional charts are essential [10, 14]. There may be differences in regional perfusion, skin color, and clinical practice between high-income country (HIC), LMICs, and African populations; more caesarean deliveries, different practices in maternal anesthesia [15]. Racial differences in regional perfusion of the brain might have gene determinants or environmental factors. Pulse oximeter readings as well as the regional oxygenation metrics might have racial differences [16, 17]. The concept of normality might be different according to the region.
Cerebral regional oxygen saturation measures were obtained within the first minute of life, and they gradually increased through the first 10 min. During this period cerebral oxygenation metrics should be kept between 10 and 90th centile and the use of medical support should aim at keeping the patient in these zones.
In contrast to cerebral fractional oxygen extraction readings, they gradually declined during the first 10 min after birth. Median readings (10th–90th centiles) for crSO2 are 44.5% (19.6–64) at 1 min, 72.5% (57–82.9) at 5 min, and 83% (72–89) at 10 min. While median values (10th–90th centiles) for FTOE measurements are 0.44(0.18–0.73) at 1 min, at 5 min 0.18(0.1–0.35), and at 10 min 0.13(0.07–0.25).
In Tables 3, 4, and 5, FTOE centiles are highest in the first minute, reflecting oxygen consumption of the brain. They then decrease to their third or half values by 5 min and remain relatively constant from 5 to 10 min. For example, the 50th centile value of FTOE is 0.45 at 1 min and then drops sharply to 0.18 and 0.13 at 5 and 10 min, respectively. This means that cerebral oxygen consumption is higher at 1 min than at 5 and 10 min.
In short, the discrepancy between crSO2 and SPO2 values, as well as the high FTOE, may also be caused by changes in cerebral autoregulation at 1 min relative to 5 and 10 min. Day1-FTOE is the earliest and most sensitive predictor for both clinical seizures and abnormal MRI [18].
Table 7 shows cerebral oxygenation centiles in the two NIRS devices at different time points that included full-term neonates in their studies. Current work, Nastase et al. and Pichler et al. used INVOS 5100, and Baik et al. used NIRO200NX device [7, 11, 19]. Current work and Nastase et al. recorded cerebral oxygenation at 1 min, while Baik et al. and Pichler et al. recorded cerebral oxygenation starting from 2 min after birth.
NIRO200NX device shows lower 90th centile values of cerebral oxygenation measures than INVOS 5100 at 5 and 10 min. It also displays the lowest 10th, 50th, and 90th centile values at 10 min [19]. The current work shows higher centile values of cerebral oxygenation at different time points in comparison to other studies.
Pichler et al. (excluded infants with oxygen support, device; IVNOS5100) and Fuchs et al. (infants receiving oxygen support and/or respiratory support, device; FORE-SIGHT) had centile charts for preterm infants in their cohorts. Their cerebral oxygenation measures were similar at 5 min, despite they were measured by different devices and oxygen supplements were different in both studies [7, 20]. At 10 min, the metrics were lower in Fuchs et al. chart, nevertheless, they used oxygen in resuscitation.
In Pichler et al.’s study, the centile values were higher in preterm infants in comparison to full-term infants in the same study whether born by C-section or NVD at 10 min. At 5 min preterm centiles were similar to those of term infants born by NVD and higher than infants delivered by C-section. The ineffective cerebral autoregulation of preterm infants is expected to result in higher cerebral oxygenation values.
Based on Table 7, the current study’s higher crSO2 values were closer to those of Pichler et al., who used common charts for both preterm (with relatively higher crSO2) and full-term infants.
In the Nastase L’s study (2017), the same NIRS device (INVOS 5100) was used with the same criteria, resulting in median crSO2 readings of 35% (15–58.2) at 1 min, 64% (46.2–85) at 5 min, and 76% (67.6–87.4) at 10 min. The higher 1 min crSO2 readings in our study might have been attributed to maternal exposure to 40% oxygen through a face mask during cesarean section, an anaesthiologist's practice, which was not mentioned in Nastase L. [11].
A high risk for brain injury is still present in infants who suffer mild encephalopathy within the first 6 h of life [18, 21,22,23]. According to a recent systematic review published in 2018, a significant proportion of infants with mild (subclinical) hypoxic ischemic encephalopathy (HIE) have abnormal outcomes at follow-up [24]. This finding contrasts with historical observations that infants with mild encephalopathy had normal neurodevelopmental outcomes [25]. The cerebral oxygenation metrics during transition not only provide guidance during resuscitation, but also might be an early indicator of mild HIE, where there are no obvious encephalopathy signs.
Neonatal encephalopathy progresses differently depending on the time of the insult. Despite the fact that the timing of the insult is predominantly perinatal, it is not always evident and can occur prenatally, acutely, and chronically. Infants who had a severe antenatal event may recover by the time of birth at which time the stage of encephalopathy is perceived as mild. In contrast, an infant with a more acute insult can have only mild abnormalities on neurological examination in the first 6 h of age. These abnormalities can then evolve to moderate or severe abnormalities after the first day of life. Furthermore, confounding variables such as maternal sedation, anesthesia, or tocolytics (e.g., magnesium sulfate) may affect the accuracy of determining the stage of HIE during a neurological examination immediately following birth [26].
A tool capable of guiding the clinician to early detection of perfusion disturbances is therefore urgently needed. Clinical diagnosis of mild encephalopathy might be improved by using NIRS for measuring cerebral oxygenation [8, 19]. Furthermore, there is strong correlation between cerebral oxygenation measures and initial Thompson score, and cerebral oxygenation measures are early predictors of HIE grades [18].
There are several factors that influence crSO2 ranges, including the NIRS device used, gestational age, and the need for respiratory support. Different NIRS devices calculate cerebral oxygenation values using different algorithms, so the calculated values vary between vendors. According to Lucia Gabriella (2009), preterm infants have higher brain oxygenation metrics due to impaired brain autoregulation as well as a reduced ability of brain tissue to extract oxygen [27]. Schwaberger B (2014) found that neonates who require respiratory support have lower arterial oxygen saturation levels and limited perfusion than those without disturbed transition [28].
In the current study, those factors will not affect readings since only healthy full-term neonates were monitored using the same device. We divided patients into high and low norms based on their 50th centile values in order to discover other factors that might influence cerebral oxygenation. In order to determine which predictors were significant for low and high norms, we constructed logistic regression models. The only significant factor that affected cerebral oxygenation at 5 and 10 min was the mode of delivery. Therefore, we divided the patients into C-section and NVD groups and compared them as regards different clinical and oxygenation parameters.
Preductal SpO2 measures were compared at 1, 5, and 10 min of life in patients delivered vaginally or by C-section as presented in Table 7. Oxygen saturation at the first minute showed no statistically significant difference between the 2 studied groups (p = 0.146). This might be attributed to maternal oxygen administration through a face mask with FIO2 (fraction of inspired oxygen) 40% which was reported in all cases during cesarean delivery. Additionally, the normal vaginal and C-section groups showed statistically significant differences in SpO2 at 5 and 10 min (p = 0.001 and p = 0.031, respectively). The reason for this might be a delayed clearance of fetal lung fluid after cesarean delivery [29].
NIRS measurements of crSO2 at 1, 5, and 10 min of life were compared between CS and NVD patients in Table 7. In a number of studies, crSO2 was affected differently by delivery mode. As opposed to Nastase L et al. (2017) study, there was no statistically significant difference (p = 0.213) between patients born vaginally and those born by C-section regarding crSO2 at the first minute. As a result of maternal oxygen administration through face masks during C-section, fetal oxygen saturation at birth may consequently be elevated. crSO2 measures at 5 and 10 min showed significant difference between the patients delivered vaginally or by C-section. The normal vaginal group averaged 74.67% crSO2 at 5 min compared with 66.37% in the cesarean group (p = 0.001), while the NVD group averaged 84.13% crSO2 at 10 min compared to 78.37% in the CS group (p = 0.001). Nastase L et al. (2017) found the same result, in contrast with Gerhard Pichler et al. (2013) and Berndt Urlesberge et al. (2011) [11, 29]. The rate of rise from 1 to 5 min of life was statistically significantly higher among the vaginal group (36%) than the cesarean group (22%) with p < 0.001.
FTOE indicates an increase in oxygen consumption due to increased cerebral metabolic rate, decreased oxygen delivery or both [14]. FTOE at 1, 5, and 10 min were calculated and compared between patients delivered vaginally or by C-section. Although crSO2 was not different between the 2 groups, FTOE at 1 min had a tendency to show a statistically significant difference between the 2 groups with median values (0.38) in the C-section group and 0.52 in the normal vaginal group (p = 0.07). FTOE decreased from 5 to 10 min and showed statistically significant differences between infants born by NVD and C-section at 5 and 10 min (p = 0.005 and p = 0.004 respectively), following the Nastase L study (2017) [11].
Higher brain oxygenation metrics and peripheral oxygen saturation were found to be significantly higher in the vaginal group. This is likely due to the raised level of carbon dioxide which is the most potent cerebral vasodilator [29]. According to Kenichi Isobe et al. (2002), vaginally delivered newborns had significantly higher catecholamine levels at birth in comparison to those born by cesarean section. Released catecholamines act to constrict peripheral blood vessels, thereby increasing cerebral blood flow. Additionally, fetal lung fluids are cleared more rapidly during vaginal delivery [5].
It is logical that SPO2 and cerebral oxygenation parameters (crSO2 and FTOE) should be correlated. At 1, 5, and 10 min, however, their relationship is not constant. At 5 min, SPO2 and cerebral oxygenation parameters (crSO2 and FTOE) showed the highest correlation (r = 0.6 and r = 0.4, respectively).
Many factors can explain the discrepancy in correlation strength at those time points. Firstly, crSO2 reaches a plateau faster than peripheral arterial saturation (SpO2), and oxygen is delivered to the brain more efficiently in the first minutes of life. Second, after 5 min, the cerebral vascular bed constricts to protect the brain from excessive oxygen exposure following the postnatal rise in blood oxygen levels. The third hypothesis proposed by Kehrer M et al. (2005) is that an increase in left ventricular output could result in a decrease in cerebral blood flow as a result of an increased left-to-right shunt through the patent ductus arteriosus [30,31,32].
The main limitation of this work is the small sample size, but the cost of the sensor was the main restraint.
Conclusions
The use of NIRS to measure crSO2 in conjunction with centile charts allows resuscitation teams to monitor and quantify cerebral oxygenation in a rapid and non-invasive manner during resuscitation. It might influence interventions needed during resuscitation and might be a predictor of short- and long-term outcomes. NIRS-measured crSO2 in healthy full-term neonates is significantly affected by delivery mode.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
American Academy of Pediatrics Committee on Fetus Newborn (2015) American College of Obstetricians Gynecologists Committee on Obstetric Practice. The APGAR Score Pediatrics 136(4):819–822
Bruckner M, Pichler G, Urlesberger B (2020) NIRS in the fetal to neonatal transition and immediate postnatal period. Semin Fetal Neonatal Med 25(2):101079
Casey BM, McIntire DD, Leveno KJ (2001) The continuing value of the Apgar score for the assessment of newborn infants. N Engl J Med 344(7):467–471
O’Donnell CP, Kamlin COF, Davis PG, Carlin JB, Morley CJ (2006) Interobserver variability of the 5-minute Apgar score. J Pediatr 149(4):486–489
Rabi Y, Yee W, Chen SY, Singhal N (2006) Oxygen saturation trends immediately after birth. J Pediatr 148(5):590–594
Pichler G, Schmölzer GM, Urlesberger B (2017) Cerebral tissue oxygenation during immediate neonatal transition and resuscitation. Front Pediatr 5:29
Pichler G, Binder C, Avian A, Beckenbach E, Schmölzer GM, Urlesberger B (2013) Reference ranges for regional cerebral tissue oxygen saturation and fractional oxygen extraction in neonates during immediate transition after birth. J Pediatr 163(6):1558–1563
Binder-Heschl C, Urlesberger B, Schwaberger B, Koestenberger M, Pichler G (2016) Borderline hypotension: how does it influence cerebral regional tissue oxygenation in preterm infants? J Matern Fetal Neonatal Med 29(14):2341–2346
Watzman HM, Kurth CD, Montenegro LM, Rome J, Steven JM, Nicolson SC (2000) Arterial and venous contributions to near-infrared cerebral oximetry. Anesthesiol 93(4):947–953
Clement P, Mutsaerts H-J, Václavů L, Ghariq E, Pizzini FB, Smits M et al (2018) Variability of physiological brain perfusion in healthy subjects–A systematic review of modifiers. Considerations for multi-center ASL studies. J Cereb Blood Flow Metab 38(9):1418–1437
Nastase L, Stoicescu SM, Banceanu G (2017) Cerebral regional oxygen saturation of the neonate during the transition to extrauterine life. Obstet Ginecol 6:235–240
Sood BG, McLaughlin K, Cortez J (2015) Near-infrared spectroscopy: applications in neonates. Semin Fetal Neonatal Med 20(3):164–172
Wolf M, Greisen G (2009) Advances in near-infrared spectroscopy to study the brain of the preterm and term neonate. Clin Perinatol 36(4):807–834
Fawzy A, Wu TD, Wang K, Robinson ML, Farha J, Bradke A et al (2022) Racial and ethnic discrepancy in pulse oximetry and delayed identification of treatment eligibility among patients with COVID-19. JAMA Intern Med 182(7):730–738
Couch L, Roskosky M, Freedman BA, Shuler MSJAJoAC (2015) Effect of skin pigmentation on near infrared spectroscopy. Am J Anal Chem 6(12):911
Bashambu MT, Whitehead HV, Hibbs AM, Martin RJ, Bhola M (2012) Evaluation of interobserver agreement of Apgar scoring in preterm infants. Pediatrics 130:e982-7
Kapadia VS, Lal CV, Kakkilaya V, Heyne R, Savani RC, Wyckoff MH (2017) Impact of the neonatal resuscitation program-recommended low oxygen strategy on outcomes of infants born preterm. J Pediatr 191:35–41
Farag MM, Khedr AAEAE, Attia MH, Ghazal HAE (2021) Role of near-infrared spectroscopy in monitoring the clinical course of asphyxiated neonates treated with hypothermia. Am J Perinatol https://doi.org/10.1055/s-0041-1740513
Baik N, Urlesberger B, Schwaberger B, Schmölzer GM, Mileder L, Avian A, Pichler G (2015) Reference ranges for cerebral tissue oxygen saturation index in term neonates during immediate neonatal transition after birth. Neonatol 108(4):283–286
Fuchs H, Lindner W, Buschko A et al (2012) Brain oxygenation monitoring during neonatal resuscitation of very low birth weight infants. J Perinatol 32:356–362
Murray DM, O’Connor CM, Ryan CA, Korotchikova I, Boylan GB (2016) Early EEG grade and outcome at 5 years after mild neonatal hypoxic ischemic encephalopathy. Pediatrics 138(4):e20160659
Walsh BH, Neil J, Morey J, Yang E, Silvera MV, Inder TE et al (2017) The frequency and severity of magnetic resonance imaging abnormalities in infants with mild neonatal encephalopathy. J Pediatr 187:26–33
Lodygensky GA, Battin MR, Gunn AJ (2018) Mild neonatal encephalopathy—how, when, and how much to treat? JAMA Pediatr 172(1):3–4
Conway J, Walsh B, Boylan G, Murray D (2018) Mild hypoxic ischaemic encephalopathy and long term neurodevelopmental outcome-a systematic review. Early Hum Dev 120:80–87
Robertson CM, Finer NN, Grace MG (1989) School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. J Pediatr 114(5):753–760
Chalak L, Latremouille S, Mir I, Sánchez PJ, Sant’Anna G (2018) A review of the conundrum of mild hypoxic-ischemic encephalopathy: Current challenges and moving forward. Early Human Dev 120:88–94
Tina LG, Frigiola A, Abella R, Artale B, Puleo G, D’Angelo S et al (2009) Near infrared spectroscopy in healthy preterm and term newborns: correlation with gestational age and standard monitoring parameters. Curr Neurovasc Res 6(3):148–154
Schwaberger B, Pichler G, Binder C, Avian A, Pocivalnik M, Urlesberger B (2014) Even mild respiratory distress alters tissue oxygenation significantly in preterm infants during neonatal transition. Physiol Meas 35(10):2085–2099
Urlesberger B, Kratky E, Rehak T, Pocivalnik M, Avian A, Czihak J et al (2011) Regional oxygen saturation of the brain during birth transition of term infants: comparison between elective cesarean and vaginal deliveries. J Pediatr 159(3):404–408
Kehrer M, Blumenstock G, Ehehalt S, Goelz R, Poets C, Schöning M (2005) Development of cerebral blood flow volume in preterm neonates during the first two weeks of life. Pediatr Res 58(5):927–930
Noori S, Wlodaver A, Gottipati V, McCoy M, Schultz D, Escobedo M (2012) Transitional changes in cardiac and cerebral hemodynamics in term neonates at birth. J Pediatr 160(6):943–948
Schwaberger B, Pichler G, Urlesberger B (2016) Does cerebral vasoconstriction following delivery protect against hyperoxia? J Pediatr 173(1):266–266
Acknowledgements
We are grateful and thankful all the patients, parents and staff of the NICU of Alexandria University that participated in the study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Hesham Abd EL-Rahim Ghazal conceived of the presented idea. Bahaa Salah-El Din Hammad, Alaa Ibrahim and Marwa M. Farag worked out all of the technical and medical details. Alaa Ibrahim collected the data. Marwa M. Farag and Alaa Ibrahim verified the analytical methods. Alaa Ibrahim, Marwa Farag, and Bahaa Salah-El Din Hammad contributed in interpretation of results. Marwa Farag and Alaa Ibrahim wrote the first draft of the manuscript. Hesham Abd EL-Rahim Ghazal supervised the findings of this work. All authors provided critical feedback and helped shape the research, analysis, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol has been approved by the Research Ethics Committee of Alexandria faculty of medicine. Approval no. is 0106540 in 17 September 2020 (date of approval). IRB no. is 00012098 and FWA no. is 00018699. Written informed consent was obtained from parents or authorized legal representatives of all newborns who participated in the study for publication of anonymous patients’ data.
Consent for publication
Informed written consent was obtained from parents or authorized legal representatives of all newborns who participated in the study for publication of anonymous patients’ data.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure 1:
flow chart of patients
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farag, M.M., Ghazal, H.A.E.LR., Ibrahim, A. et al. Near-infrared spectroscopy measured cerebral oxygenation in full-term infants during transition: an observational study. Egypt Pediatric Association Gaz 70, 53 (2022). https://doi.org/10.1186/s43054-022-00149-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43054-022-00149-4