Abstract
Background
Esophageal cancer has a poor survival outcome with 5-year OS at 16.7% despite treatment. Some inflammation-based prognostic indicators like the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) have been previously studied as potential biomarker for predicting outcome in esophageal cancer. Recently, platelet-to-albumin ratio (PAR) has been reported as a promising prognostic factor in gastrointestinal malignancies.
Methods
We performed a retrospective analysis of prospectively treated patients of carcinoma esophagus to evaluate the prognostic significance of inflammation-based prognostic indicators—neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and a composite inflammation-nutrition index: platelet-to-albumin ratio (PAR) in esophageal cancer. Based on previous studies, the optimal cut-off value of PAR was kept at 5.7 × 10^9, and 2.62 for NLR.
Results
A total of 71 patients of locally advanced esophageal cancer treated between 2019 and 2022, with either neoadjuvant or definitive chemoradiotherapy, were included. Median follow-up time was 19 months [range: 7–44 months]. Median OS and PFS in our study cohort were 11.3 months [range: 7–23 months] and 7.8 months [range: 3–17 months], respectively. In univariate analysis, lower PAR was found to be significantly correlated with shorter survival time (HR = 2.41; 1.3–4.76; p = 0.047). There was no association found between the OS and the NLR [HR = 1.09; 0.95–1.26; p = 0.222]. Univariate and multivariate linear and logistic regressions found no association between V15, V10, V5, or V2 of spleen and nadir lymphocyte count or between Dmax or Dmean and nadir lymphocyte counts.
Conclusion
Present analysis found a trend toward an inverse association between PAR and OS. PAR, in the not-so-distant future, may evolve as a novel, convenient, and inexpensive prognostic indicator in esophageal cancer.
Similar content being viewed by others
Introduction
Burden of esophageal cancer worldwide makes it the ninth most common cancer and the sixth leading cause of cancer-related mortality worldwide [1, 2]. Treatment of esophageal cancer depends on the stage, nodal involvement, and tumor location. In resectable esophageal cancer, standard of care is pre-operative concurrent chemoradiotherapy followed by resection [3]. In others, concurrent chemoradiotherapy followed by consolidation chemotherapy forms the backbone of the treatment [4]. Despite the advances in all available modalities of treatment, oncologic outcome of esophageal cancer remains dismal with 5-year survival rates ranging from 20 to 30% only [5, 6]. In light of these precarious statistical figures, it becomes all the more necessary to identify various prognostic markers that may help us in further treatment escalation.
Studies have shown that baseline nutritional status, cancer-related inflammation, the immune system, and thrombosis affect oncological outcomes across various solid tumors including stomach, lung, and prostate cancer, in terms of carcinogenesis, proliferation, progression, and metastasis [7,8,9,10,11,12]. Thus, assessing a patient’s pre-treatment nutritional and inflammation status becomes imperative in order to attempt to bring about a positive survival impact. However, screening tools for evaluating the perioperative nutrition and inflammation status in esophageal cancer patients are currently limited. Some inflammation-based prognostic indicators like the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) have been previously studied in esophageal cancer [13]. Recently, platelet-to-albumin ratio (PAR) has been reported as a promising prognostic factor in gastrointestinal malignancies [14,15,16]. Previous reports have shown that platelets are a marker of systemic inflammation status, and albumin is one of the most important markers of nutritional status which might make PAR a composite practical low-cost surrogate marker of both the nutritional status and systemic inflammation status. Some studies also suggest that unintended splenic irradiation in lower thoracic esophageal cancer correlates to the severity of lymphopenia and, ultimately, a lower survival outcome [17,18,19].
We wanted to evaluate the prognostic significance of inflammation-based prognostic indicators—neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and a composite inflammation-nutrition index: platelet-to-albumin ratio (PAR) in esophageal cancer. We also wanted to identify if unintended spleen irradiation correlated to the nadir lymphocyte count. The protocol was approved by institute ethics committee.
Materials and methods
Patients
Data of patients with esophageal cancer who underwent chemoradiotherapy on curative lines between November 2019 and October 2022 were retrospectively reviewed. Eligible patients had histologically confirmed SCC of the esophagus; ECOG PS 0–2; and treated with definitive or neoadjuvant chemoradiotherapy. Patients with a history of previous malignancy or chronic/acute inflammatory diseases were not considered for analysis.
Treatment protocol
All patients were staged according to the 8th AJCC TNM staging for esophageal cancer, with the aid of imaging techniques for assessment of the locoregional extent of the disease and to rule out distant metastases. A multidisciplinary board comprising of a radiation oncologist, a medical oncologist, a radiologist, a nuclear medicine physician, and a surgical oncologist finalized the treatment plan. Patients deemed resectable underwent neoadjuvant chemoradiotherapy to a dose of 41.4 Gy in 23 fractions treated five times a week over 4.5 weeks with concurrent weekly carboplatin-paclitaxel. If not eligible for surgery, patients underwent definitive chemoradiotherapy till 50.4 Gy in 25 fractions over 5 weeks with weekly 5FU-cisplatin followed by two cycles of consolidation chemotherapy. The patients deemed resectable by the MDT underwent Ivor Lewis esophagectomy or VATS assisted TTE.
Radiation planning
Gross tumor volume (GTV) delineation for radiotherapy was done after co-registration of the PET-CT images with the planning CT images and correlation with the upper GI endoscopy findings. A 3-cm craniocaudal expansion along the esophageal mucosa and a 1-cm circumferential margin (anatomically constrained) were applied to form the CTV. Lymph nodes if seen were contoured separately; a 1-cm margin was given to form the nodal CTV. A 1-cm margin was applied to the CTV to form the planning target volume (PTV) (Fig. 1a). Radiotherapy was delivered by the IMRT (intensity-modulated radiation therapy) or the VMAT (volumetric modulated arc therapy) using the Eclipse version 15.5 treatment planning system (Varian Medical, Palo Alto, CA) (Fig. 1b). VMAT/IMRT was delivered with 6-MV Acuros-XB algorithm version 15.6.05 photon beams generated from the Varian TrueBeam SVC linear accelerator equipped with a 120-leaf Millenium multi-leaf collimator. Spleen was retrospectively contoured for all the patients and the spleen dosimetry was evaluated in terms of the mean spleen dose (Dmean), the maximum spleen dose (Dmax), V2, V5, V10, V15, and V20 (Fig. 2).
Follow-up
Patients were reviewed weekly during the course of treatment for acute toxicities. After completion of treatment, follow-up examinations were conducted 1 month after finishing radiotherapy, and then every 3 months in the first year, every 6 months over the next 2 years, and once a year thereafter with physical examination, thoracic CT scanning, or 18FDG whole body PET-CT scan. Overall survival (OS) was defined as the period from treatment initiation to the date of last follow-up or death from any cause. Progression-free survival (PFS) was defined as the period from treatment initiation to the date of disease progression or death from any cause. Disease progression was evaluated by the standard Response Evaluation Criteria in Solid Tumors (RECIST criteria).
Definition of the indices
The baseline hematological parameters, namely, the hemoglobin, neutrophil, lymphocyte, platelet counts, and serum albumin levels, were collected from the central database system of the hospital and the corresponding neutrophil–lymphocyte ratio (NLR) and platelet-albumin ratio (PAR) were calculated. The same was repeated for weekly laboratory values during the course of concurrent chemoradiotherapy and for values obtained 1 month after completion of the designated treatment. The nadir lymphocyte count during the course of radiotherapy was considered in every patient and it was graded according to the Common Terminology Criteria for Adverse Events version 4.0.
NLR and PAR were defined as NLR = absolute neutrophil count/absolute lymphocyte count and PAR = platelet counts/serum albumin level (g/L). Based on previous studies, the optimal cut-off value of PAR was kept at 5.7 × 10^9, and 2.62 for NLR [20]. The cohort of the patients selected was stratified into a low PAR (PAR < 5.7 × 10^9) and a high PAR group (PAR ≥ 5.7 × 10^9).
Statistical analysis
All statistical analysis was performed with SPSS 26.0 (SPSS, Chicago, IL). Association between the PAR groups, the NLR, and clinicopathological characteristics was analyzed by the χ2 test. Survival curves for OS were plotted via the Kaplan–Meier method and compared by the log-rank test to assess the prognostic influence of the NLR and the PAR. Univariate and multivariate Cox analyses were conducted to identify the independent risk or prognostic factors. A p value < 0.05 was considered statistically significant. Spearman correlation coefficients were used to evaluate the associations between the spleen dose-volume parameters and the nadir lymphocyte count.
Results
Patient demography
Baseline clinicopathological characteristics of the patients eligible for the analysis have been presented in Table 1.
Prognostic significance of PAR and NLR
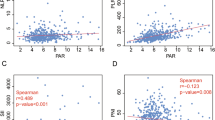
Median follow-up time was 19 months [range: 7–44 months]. Median OS in the study cohort was 11.3 months [range: 7–23 months] while median PFS was 7.8 months [range: 3–17 months]. In Kaplan–Meier survival analysis, lower PAR significantly correlated with shorter survival time (HR = 2.41; 1.3–4.76; p = 0.047) (Fig. 3). One- and 2-year OS rates were 76.5% and 58.9%, respectively, in the high PAR group and 87.1% and 82.4%, respectively, in the low PAR group. Kaplan–Meier analysis showed no association found between survival and NLR [HR = 1.09; 0.95–1.26; p = 0.222]; nadir lymphocyte count [HR = 1.21; 0.87–1.36; p = 0.34]; platelet-lymphocyte ratio (PLR) [HR = 1.1; 0.98–1.21; p = 0.56]; or the serum albumin [HR = 0.99; 0.87–1.29; p = 0.78]. Univariate analyses showed that lymph node metastasis, T stage, TNM stage group, and PAR were predictive of overall survival (OS) (Table 2). Multivariate analyses were performed using the Cox proportional hazards model for these variables, which identified the TNM stage group (p = 0.005) and lymph node metastasis (p = 0.031) as predictors of OS. PAR lost its significant impact on OS on multivariate analysis (Table 3). Spearman correlation analysis revealed that V2, V5, V10, V15, V20, Dmax, and mean splenic dose did not significantly correlate with nadir lymphocyte count (p = 0.41, 0.61, 0.21, 0.09, 0.87, 0.58, 0.06, respectively) (Fig. 4). Subset analysis did not show any relation between these even for middle and lower esophagus subsites. Spleen Dmean was significantly more with VMAT than IMRT (p = 0.02). Out of the 71 patients audited, 45 patients (63.4%) were planned for definitive CTRT while the remaining underwent neoadjuvant chemoradiotherapy and were subsequently planned for surgery. However, only 15 (60%) of those planned for surgery did actually got operated. In survival analysis, lower PAR significantly correlated with shorter survival time in both groups individually (HR = 2.62; 1.7–4.7; p = 0.037) (HR = 2.42; 1.3–4.3; p = 0.043). However, other hematological markers analyzed did not show significant survival impact in either of the groups, nor did the survival among the two groups vary significantly.
Discussion
Esophageal carcinoma is a highly aggressive malignancy which makes it imperative to identify various prognostic factors besides the TNM staging system, to screen high-risk patients who are more pronounced to experience or distant metastasis, and to implement aggressive early intervention in an attempt to improve the outcomes on a personalized basis. Recent studies suggest that cancer-related inflammation (CRI) is the seventh hallmark of cancer and presence of a smoldering inflammation in the tumor microenvironment contributes to proliferation and sustenance of malignant cells, metastasis, angiogenesis, and aversion of the host tumor-related immune response which ultimately lead to a resistance pattern toward systemic anticancer therapies [21].
Platelets, a critical coin in the phenomenon of hemostasis, are also now being linked to systemic inflammation and are gaining recognition as an immune modulatory cell [22, 23]. Moreover, platelet could shield peripheral circulating tumor cells and interfere with natural killer cells for recognition of tumor cells, which enhanced their metastatic potential [24]. Nutritional status is also an important aspect of the holistic management of cancer. Malnutrition and cachexia have been independently evaluated as poor clinical prognostic factors in patients with advanced cancer [25,26,27]. Earlier, albumin was reported to be a biomarker of malnutrition which significantly correlated with poor clinical outcomes in patients of esophageal cancer [28, 29]. Moreover, albumin synthesis is a negative inflammatory marker. Therefore, risk stratification based on inflammation-nutritional indicators is of great significance and will help the clinical physician to provide timely and effective nutritional intervention. That is how arose the novel practical surrogate marker known as the platelet-albumin ratio (PAR) which takes into account both the inflammatory and nutritional status of the patient. This marker can be easily calculated from a simple hemogram and a liver function test, which makes it very practical. Huang et al. showed that PAR could be an independent indicator of PFS and OS. Patients with a low pre-treatment PAR (< 5.7 × 109) had a significantly better prognosis in both PFS and OS than those with a high pre-treatment PAR (≥ 5.7 × 109) [20]. Another study found a marginally significant difference in the post-operative surgical complications and long-term oncological outcomes between the PAR-high and PAR-low groups [30]. Neutrophils, the major inflammatory cells, can promote tumor cell proliferation, angiogenesis, and metastasis by inhibiting T cells. Lymphocytes, on the other hand, can prevent tumor progression by enhancing immune surveillance. However, the associated inflammation during tumor development inhibits the lymphocytes, leading to immune escape [31]. Xu et al. indicated that the neutrophil–lymphocyte ratio (NLR) could be a sensitive parameter for evaluating the prognosis in esophageal cancer [32]. Similarly, high pre-treatment NLR was reported to be associated with worse DFS and OS in patients with resectable esophageal cancer [33]. A meta-analysis by Yang et al. also suggested that high NLR is associated with poor prognosis in patients with esophageal cancer [34].
In our study, we found an inverse relationship between the PAR value and overall survival, whereby low PAR values correlated to better survival. Our results did not show any apparent impact of NLR, PLR, nadir lymphocyte count, or serum albumin, on survival. Spleen is the largest lymphoid organ in the body where white pulp activates the immune response when antigens and antibodies are present in blood [35]. An unintended increase in spleen dose can have a significant impact on absolute lymphocyte count and, thereby, tumor immunity. Previous studies had shown a significant correlation between high spleen irradiation doses and low lymphocyte counts after RT in patients with hepatocellular carcinoma and pancreatic cancer [36, 37]. Sakaguchi et al. demonstrated that spleen dose to be associated with decreased lymphocyte count and an increased ratio of NLR after treatment in patients with esophageal cancer [38]. Alexandru et al. suggested that spleen unintentional V15 and maximum dose irradiation were associated with lymphopenia during chemoradiotherapy [39]. However, the present analysis failed to show any statistically significant association between spleen dosimetry (in terms of the V2Gy, V5Gy, V10Gy, V15Gy, V20Gy, the mean, and maximum doses) and lymphopenia.
Our analysis suggested a prognostic implication of the PAR value in patients of esophageal cancer with an inverse association between PAR and OS. Certain limitations of our analysis must be considered. This was a retrospective single-center analysis which may have led to selection bias. Secondly, platelet counts and serum albumin levels could be influenced by other factors such as coagulation disorder and liver dysfunction, which confound the results. Thirdly, the optimal cut-off for PAR and NLR might be different for the Indian population than for the Western population, whose studies have been used to define the cut-off points in this analysis. However, PAR, in the not-so-distant future, may evolve as a novel, convenient, and inexpensive prognostic indicator in esophageal cancer. Future validation from prospective larger-scale studies is warranted.
Conclusion
This analysis showed that PAR could be a novel and independent predictive variable with survival connotations. Measurement of PAR is a relatively inexpensive, convenient, and reliable endeavor in routine clinical practice. With future studies and robust evidence to show the prognostic importance of PAR, it may become one of the simplest indices that will help in clinical decision-making regarding the intensification of treatment.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Shapiro J, Van Lanschot JJ, Hulshof MC, van Hagen P, van Berge Henegouwen MI, Wijnhoven BP, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–8.
Herskovic A, Martz K, Al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326(24):1593–8.
Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–12. https://doi.org/10.1016/S0140-6736(12)60643-6.
He H, Chen N, Hou Y, Wang Z, Zhang Y, Zhang G, Fu J. Trends in the incidence and survival of patients with esophageal cancer: a SEER database analysis. Thoracic cancer. 2020;11(5):1121–8.
Wu G, Yao Y, Bai C, Zeng J, Shi D, Gu X, et al. Combination of platelet to lymphocyte ratio and neutrophil to lymphocyte ratio is a useful prognostic factor in advanced non-small cell lung cancer patients. Thorac Cancer. 2015;6(3):275–87.
Kim MR, Kim AS, Choi HI, Jung JH, Park JY, Ko HJ. Inflammatory markers for predicting overall survival in gastric cancer patients: a systematic review and meta-analysis. PLoS ONE. 2020;15(7):e0236445.
Akgül Ö, Çetinkaya E, Yalaza M, Özden S, Tez M. Prognostic efficacy of inflammation-based markers in patients with curative colorectal cancer resection. World J Gastrointest Oncol. 2017;9(7):300–7.
Peng H, Luo X. Prognostic significance of elevated pretreatment systemic inflammatory markers for patients with prostate cancer: a meta-analysis. Cancer Cell Int. 2019;19:70.
Sakurai K, Ohira M, Tamura T, Toyokawa T, Amano R, Kubo N, et al. Predictive potential of preoperative nutritional status in long-term outcome projections for patients with gastric cancer. Ann Surg Oncol. 2016;23(2):525–33.
Toyokawa T, Kubo N, Tamura T, Sakurai K, Amano R, Tanaka H, et al. The pretreatment controlling nutritional status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer. 2016;16:722.
Xu GW, Wu HR, Xiong R, Li CW, Liu CQ, Xu MQ, Xie MR. Value of the preoperative neutrophil-to-lymphocyte ratio as a prognostic factor for long-term survival in postoperative esophageal squamous cell carcinoma patients. Thoracic Cancer. 2018;9(12):1707–15.
Haksoyler V, Topkan E. High pretreatment platelet-toalbumin ratio predicts poor survival results in locally advanced nasopharyngeal cancers treated with chemoradiotherapy. Ther Clin Risk Manag. 2021;17:691–700.
Huang C, Xia YQ, Xiao L, Huang J, Zhu ZM. Combining the platelet-to-albumin ratio with serum and pathologic variables to establish a risk assessment model for lymph node metastasis of gastric cancer. J Biol Regul Homeost Agents. 2021;35(2):811–7.
Huang Z, Zheng Q, Yu Y, Zheng H, Wu Y, Wang Z, et al. Prognostic significance of platelet-to albumin ratio in patients with esophageal squamous cell carcinoma receiving definitive radiotherapy. Sci Rep. 2022;12(1):3535.
Saito T, Toya R, Yoshida N, Shono T, Matsuyama T, Ninomura S, et al. Spleen dose-volume parameters as a predictor of treatment-related lymphopenia during definitive chemoradiotherapy for esophageal cancer. In Vivo. 2018;32:1519–25.
Van Rossum PSN, Deng W, Routman DM, Liu AY, Xu C, Shiraishi Y, et al. Prediction of severe lymphopenia during chemoradiation therapy for esophageal cancer: development and validation of a pretreatment nomogram. Pract Radiat Oncol. 2019;10:e16-26.
Davuluri R, Jiang W, Fang P, Xu C, Komaki R, Gomez DR, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017;99:128–35.
Huang Z, Zheng Q, Yu Y, Zheng H, Wu Y, Wang Z, et al. Prognostic significance of platelet-to-albumin ratio in patients with esophageal squamous cell carcinoma receiving definitive radiotherapy. Sci Rep. 2022;12(1):3535.
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–81.
Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost. 2015;114(09):449–58.
Kral JB, Schrottmaier WC, Salzmann M, Assinger A. Platelet interaction with innate immune cells. Transfusion Medicine and Hemotherapy. 2016;43(2):78–88.
Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. Platelets and fibrin (ogen) increase metastatic potential by impeding natural killer cell–mediated elimination of tumor cells. Blood. 2005;105(1):178–85.
Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NE, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36(5):1187–96.
Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10(2):90–9.
Arends J. Struggling with nutrition in patients with advanced cancer: nutrition and nourishment—focusing on metabolism and supportive care. Ann Oncol. 2018;29:ii27-34.
Lindenmann J, Fink-Neuboeck N, Koesslbacher M, Pichler M, Stojakovic T, Roller RE, et al. The influence of elevated levels of C-reactive protein and hypoalbuminemia on survival in patients with advanced inoperable esophageal cancer undergoing palliative treatment. J Surg Oncol. 2014;110(6):645–50.
Sun SY, Chen PP, Meng LX, Li L, Mo ZX, Sun CH, et al. High preoperative plasma fibrinogen and serum albumin score is associated with poor survival in operable esophageal squamous cell carcinoma. Dis Esophagus. 2019;32(1):doy057.
Aoyama T, Ju M, Komori K, Tamagawa H, Tamagawa A, Morita J, et al. Clinical impact of platelet-to-albumin ratio on esophageal cancer patients who receive curative treatment. In Vivo. 2022;36(4):1896–902.
Wang J, Jia Y, Wang N, Zhang X, Tan B, Zhang G, et al. The clinical significance of tumor-infiltrating neutrophils and neutrophil-to-CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma. J Transl Med. 2014;12:1.
Xu GW, Wu HR, Xiong R, Li CW, Liu CQ, Xu MQ, et al. Value of the preoperative neutrophil-to-lymphocyte ratio as a prognostic factor for long-term survival in postoperative esophageal squamous cell carcinoma patients. Thoracic Cancer. 2018;9(12):1707–15.
Sakin A, Alay M, Sahin S, Aydemir O, Aldemir MN, Sakin A, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in esophageal squamous cell carcinoma. North Clin Istanb. 2021;8(5):435–42.
Yang X, Huang Y, Feng JF, Liu JS. Prognostic significance of neutrophil-to-lymphocyte ratio in esophageal cancer: a meta-analysis. Onco Targets Ther. 2015;10:789–94.
Varga I, Babala J, Kachlik D. Anatomic variations of the spleen: current state of terminology, classification, and embryological background. Surg Radiol Anat. 2018;40:21–9.
Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2015;38(3):259.
Zhao Q, Xu X, Yue J, Zhu K, Feng R, Jiang S, et al. Minimum absolute lymphocyte counts during radiation are associated with a worse prognosis in patients with unresectable hepatocellular carcinoma. Ther Adv Gastroenterol. 2017;10(2):231–41.
Sakaguchi M, Maebayashi T, Aizawa T, Ishibashi N, Okada M. Association between unintentional splenic radiation and lymphopenia and high neutrophil/lymphocyte ratio after radiotherapy in patients with esophageal cancer. Translational Cancer Research. 2021;10(12):5076.
Alexandru M, Rodica A, Dragos-Eugen G, Mihai-Teodor G. Assessing the spleen as an organ at risk in radiation therapy and its relationship with radiation-induced lymphopenia: a retrospective study and literature review. Adv Radiat Oncol. 2021;6(6):100761.
Acknowledgements
Nil.
Funding
Nil.
Author information
Authors and Affiliations
Contributions
Adrija Ghosh: Design, Data collection, manuscript writing, Approval. Abhilash Dagar: Design, Data collection, manuscript writing, Approval. Ram Pukar Bharat: Data collection, manuscript writing, Approval. Jaswin Raj: Planning, Data collection, Approval. Dyuti Shah: Stats, Data collection, Approval. Jyoti Sharma: Design, Data collection, manuscript writing, Approval. Akash Kumar: Design, Data collection, manuscript writing, Approval. Pritee A. Patil: Design, Data collection, manuscript writing, Approval. Aman Sharma: Design, Approval. Dayanand Sharma: Design, Approval. Supriya Mallick: Concept, Design, Data collection, manuscript writing, Approval.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval: AIIMS ethics committee - IEC-917/13.12.2022, RP-10/2022. Informed written consent to participate in the study was provided by all participants.
Consent for publication
Written informed consent for the publication was obtained from the participants.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghosh, A., Dagar, A., Bharat, R.P. et al. Platelet-to-albumin ratio and radiation-induced lymphopenia—prognostic biomarker for carcinoma esophagus. J Egypt Natl Canc Inst 36, 4 (2024). https://doi.org/10.1186/s43046-024-00208-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43046-024-00208-4