Abstract
Background
The prognostic value of the level of programmed death ligand 1 (PD-L1) expression in non-Hodgkin lymphoma (NHL) is still debatable. This study examined the effect of the level of PD-L1 expression on the clinicopathological characteristics and prognosis of diffuse large B cell lymphoma (DLBCL).
Methods
A retrospective study was conducted on formalin-fixed paraffin-embedded tissue blocks of one hundred de novo DLBCL patients diagnosed from 2013 to 2016. PD-L1 expression was defined by a modified Combined-Positive Score (CPS) and their medical records were reviewed to collect their clinical, laboratory and radiological data, treatment, and outcome.
Results
The included patients were aged from 23 to 85 years and treated by rituximab- cyclophosphamide, doxorubicin, oncovin, prednisone (R-CHOP); 49% were males; 85% of the cases were presented at Ann Arbor stages III, IV; 33% of patients were seropositive for HCV and 87% of cases were presented with intermediate and high IPI. All included cases expressed PD-L1 using modified CPS. 27% of patients showed low PD-L1 expression (≥ 5% to < 50% of total tumor cellularity) while 73% of patients showed high PD-L1expression (≥ 50% of total tumor cellularity). High PD-L1 expression is statistically correlated with advanced stage (p 0.01), high IPI score (p 0.017), high incidence of stationary and progressive disease (p 0.002) and high incidence of relapse (p value 0.01). Five-year disease-free survival (DFS) was 29% for patients with high PD-L1 expression compared with 84.8% for patients with low PD-L1 expression (p 0.001).
Conclusions
This study suggests that high PD-L1 expression in DLBCL is associated with aggressive clinicopathological features and a decreased response to R-CHOP. The level of PD-L1 expression could be an independent predictor of DFS of DLBCL. More research is mandatory to standardize the cutoff value and scoring methods.
Similar content being viewed by others
Background
The incidence of non-Hodgkin lymphoma (NHL) has shown a dramatic increase over the past few decades. Diffuse large B cell lymphoma (DLBCL) represents the common type of NHL [1]. In Egypt, NHL was ranked the fifth most frequent malignancy in both genders [2]. The Ann Arbor staging system and the International Prognostic Index (IPI) are commonly used as prognostic factors [3]. However, the prognosis varies among the different histological types of NHL. The prognosis also differs among patients with DLBCL despite the same treatment strategy due to variable clinical features and molecular alternations. Therefore, the identification of new biomarkers could accurately predict the prognosis has great clinical and therapeutic implications [4].
Recently immunotherapy has enriched the landscape of cancer therapeutics. Immune checkpoint blockage with antibodies against programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1), and also cytotoxic T-lymphocyte antigen (CTLA-4) has produced hopeful results in various cancers [5].
PD-1, also referred to as CD279, is a receptor that is expressed on B lymphocytes (B cells), activated T lymphocytes (T cells), dendritic cells, macrophages, natural killer cells (NK cells), and monocytes [6]. PD-1 interacts with the PD-L1; also known as CD274 and PD-L2; also known as CD273, which lead to inhibition of the immune response. Both hematopoietic and non-hematopoietic cells exhibit high levels of PD-L1 expression. PD-L1 is constitutively expressed on T cells, B cells, antigen-presenting cells (APCs) and further up-regulated upon their activation. Comparatively fewer cell types express PD-L2 than PD-L1, mainly on APCs [7].
PD-L1 is expressed on malignant cells and tumor-infiltrating non-malignant stromal cells [8]. Recently, the impact of PD-L1 expression on the prognosis of various cancers has attracted the interest of many researchers because PD-1/PD-L1-mediates the tumor immune escapes [6].
The prognostic value of PD-L1 expression in DLBCL remains controversial. Some studies have proposed that the expression of PD-L1 is correlated with poor prognosis and could represent a valuable therapeutic target [8,9,10]. Kwon et al. found that positive PD-L1 expression carried no prognostic value, or correlated with favorable prognoses than those without [11].
In this study, we assessed the level of expression of PD-L1 in DLBCL using modified CPS and correlated these levels with other clinicopathological features and patients’ outcome.
Methods
This retrospective study was performed on formalin-fixed paraffin embedded tissue from archives from the pathology laboratory of our oncology center. One hundred patients were included in the period from 2013 to 2016. All patients were diagnosed with de novo DLBCL and underwent excisional or incisional biopsies. Fine needle aspiration cytology specimens and core biopsies were excluded from the study. The medical records were reviewed to collect their clinical, laboratory and radiological data, treatment, and outcome. The achieved response was classified according to the Lugano classification [12].
The Institutional Review Board of our faculty reviewed and approved this study (IRB code: R.20.10.1038). The authors declare that the guidelines of the World Medical Association Declaration of Helsinki were followed.
Histopathological features were reviewed from H&E-stained slides. Cases were assigned as centroblastic, immunoblastic, and anaplastic. Immunohistochemistry for CD20, CD3, CD10, BCL-6, MUM-1, BCL-2, P53, and C-MYC was done through DAKO Autostainer Link 48. The cases were assigned as germinal/non-germinal center cells of origin using the Hans algorithm according to the 2017 WHO classification of hematopoietic and lymphoid tumors [13]. Tissue microarray blocks were constructed. Manual immunohistochemical staining for PD-L1 was done using polyclonal anti-PD-L1 antibody–Biospes–Catalog # YPA1637. Immunohistochemical study was conducted according to manufacturer instructions with appropriate positive and negative controls.
Here, PD-L1 expression was defined by modified Combined-Positive Score (CPS). In contrast to solid tumors, it was difficult to distinguish lymphoma cells from immune cells in DLBCL. For easier interpretation, we used the modified CPS method to assess the level of PD‐L1 expression as follows: the percentage of positive lymphoma cells and immune cells/total tumor tissue cellularity [14].
The cut off used in this study was 5% of the cellularity expressing cytoplasmic and or membranous PD-L1. Positive cases were considered as low (≥ 5 to < 50% of cells) or high expression (≥ 50% of cells) [15, 16].
P53 over expression was defined as strong nuclear expression of more than 30% of tumor cells [17]. BCL-2 was considered positive if ≥ 50% of the tumor cells showed cytoplasmic staining. C-MYC was considered positive if ≥ 40% of the tumor cells showed nuclear staining [13]. Double expressor (DE) cases were positive for BCL-2 and C-MYC. Triple expressor (TE) cases are positive for BCL-6 in addition [18].
Statistical analysis
Data were analyzed using IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. Qualitative data were defined as number and percentage. Quantitative data were defined as mean ± standard deviation for parametric data after testing normality using Kolmogrov-Smirnov test.
Data analysis
-
Chi-square test or Fisher’s exact test for comparison of 2 or more groups was used for qualitative data.
-
Disease-free survival (DFS) was measured, in months, since date of complete response to the date of death, relapse, or the last follow-up visit. Overall survival (OS) was measured, in months, from the date of initial diagnosis to the date of death or the last follow-up visit. Survival data were estimated using the Kaplan–Meier curve method and the log-rank test was used for comparison.
-
Cox regression analysis of factors potentially related to survival was performed to identify which independent factors might jointly have a significant influence on survival
-
The p value is considered significant if < 0.05 at confidence interval 95%.
Results
Clinicopathological characteristics of the cases being studied
As shown in Table 1, the study included 100 cases of de novo DLBCL, the median age of studied cases was 59 years (ranged from 23 to 85 years), 49% were males, 33% were seropositive for HCV. Serum LDH was elevated in 79% of patients, 44% of cases had B symptoms. Bone Marrow was infiltrated in 20% of cases. Extra-nodal involvement was found in 26% of cases. 85% of cases were presented at an advanced stage (Ann Arbor stages III, IV). Eighty-seven percent of cases were presented with intermediate and high IPI.
Centroblastic variant was the commonest histological subtype, representing 72% of cases. According to Hans classifier, 78% of cases were of non-germinal center cell of origin. Over expression of P53 was noticed in 19 cases. Twenty-eight patients were double expresser and 8 patients were triple expresser.
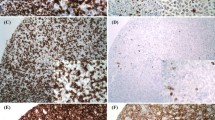
All included patients were treated by rituximab-cyclophosphamide, doxorubicin, oncovin, prednisone (R-CHOP protocol). Regarding treatment response; 42 patients achieved CR, 11 patients achieved PR, 3 patients had SD, and 44 patients showed disease progression. PD-L1 expression was detected in all included cases; 27 patients showed low expression; 73 patients showed high expression, as illustrated in Fig. 1.
PD-L1 expression and clinic-pathological characteristics
High PD-L1 expression is statistically correlated with advanced stage (p 0.01), high IPI score (p 0.017), high incidence of stationary and progressive disease (p 0.002) and a high incidence of relapse (p value 0.01). Twenty patients of the thirty-three HCV seropositive DLBCL patients had high PD-L1 expression (p value 0.05).
No statistical association was found between level of PD-L 1 expression and gender (p 0.73), age (p 0.18), serum LDH (p 0.9), B symptoms (p 0.39), involved sites (p 0.3), bone marrow infiltration (p 0.76), histological types (p 0.124), Hans classification (p 0.56), P53 over expression (p 0.72), double expresser cases (p 0.07), triple expresser cases (p 0.67) as illustrated in Table 2.
Factors affecting DFS and OS
The estimated one and 5-year DFS for the study cases were 92.2% and 58.5%, respectively. PD-L1 expression was a powerful prognostic factor with a 5-year DFS of 29% for patients with high PD-L1 expression compared with 84.8% for patients with low PD-L1 expression (p 0.001) (Table 3) (Fig. 2a). Cox regression analysis (Table 4) revealed that expression of PD-L1 is an independent predictor of DFS in DLBCL patients (p 0.028).
One and 5-year OS estimated for the studied cases were 78.4% and 65.1%, respectively. Statistically longer OS was observed in patients without bone marrow infiltration (p 0.02), low and low intermediate IPI risk (p 0.001) and low PD-L1 expression (p 0.009) (Table 5) (Fig. 2b). Cox regression analysis (Table 6) revealed that only IPI risk was an independent predictor for OS in DLBCL patients (p 0.028).
Discussion
DLBCL is a diverse disease as regards clinical, histopathological, immunohistochemical and genetic features. Patients with DLBCL do not show uniform response to the first line immunochemotherapy. This highlights the need for novel markers to accurately predict the response and prognosis and may be used as a tool for target therapy [19].
PD-1 is usually up-regulated in lymphoma cells. PD-L1and PD-L2 could be expressed by tumor cells and the surrounding tumor microenvironment. PD-1/PD-Ls interaction participates in immune escape and subsequent lymphomagenesis [9]. PD-L1 and PD-L2 were up regulated in malignant B-cell lymphomas through intracellular and extracellular mechanisms and, variations in the structure of the 3′-untranslated region of the PD-L1 gene affect PD-L1 expression [20, 21].
Regarding the hematological malignancies, pembrolizumab and nivolumab (monoclonal antibodies to PD-1 that block the interaction between PD-1 and its ligands, PDL1 and PDL-2) have been approved for treating relapsed and refractory classical Hodgkin lymphoma and primary mediastinal large B cell lymphoma to date [22,23,24].
Assessment of the level of PD-L1 expression in tumor tissue helps to define patients who are most likely to respond to treatment. In relapsed and refractory lymphoma, the over-expression of PD-L1 on the surface of tumor cells was associated with a better response to anti-PD-1 therapy [25].
Several difficulties are met during the assessment of PD-L1 expression. These encompassed the specificity of different clones of anti–PD-L1 antibodies for IHC and technical aspects including tissue fixation, processing and antigen retrieval [26]. Various commercially available PD-L1 IHC companion/complementary diagnostic assays are available. The companion diagnostic assay may define patient eligibility to anti-PD-L1 therapy. Validity, cut-off, and reporting show marked-variability among different platforms [27]. Regarding the cellular localization of PD-L1, difficult scoring systems are adopted. The tumor proportion score (TPS) measures the proportion of positive PD-L1 tumor cells among the total viable tumor cells. The combined proportion score (CPS) estimates the ratio of the overall positive cells (tumoral and non-tumoral) to the total number of viable tumor cells multiplied per 100. Modification of CPS relates the positive tumor cells and immune cells to the overall cellularity. While, the area filled by PD-L1-positive immune cells in relation to the whole tumor area is referred to as the immune cell score (IC). Furthermore, some studies included the intensity of staining in their scoring algorithms [28].
There is no consensus of what is the relevant cut-off that splits up positive from negative results. The cut-off for positive PD-L1 across different studies ranged from 1 to 50% [29].
Hawkes et al. published that PD-L1 is not commonly expressed in B cell NHL [30], Only 10–24% of DLBCL cases express PD-L1. Higher rates of PD-L1 expression have been detected in certain subtypes as EBV-associated DLBCL, T cell histiocyte-rich DLBCL, primary mediastinal LBCL, and activated B cell DLBCL [21, 31, 32].
Using modified CPS in this study, all included patients diagnosed with DLBCL expressed PD-L1; 27% patients showed low expression (≥ 1 to < 50% of cells) and 73% patients showed high expression (≥ 50% of cells).
Gravelle et al. published that approximately 20–30% of DLBCL expressed PD-L1 [9].
Unfortunately, EBV encoded RNA (EBER-ISH) or IHC for latent membrane protein (LMP) were not available in this retrospective study as EBV test was not routinely requested for NHL work up in our center. Chen et al. found that 100% of EBV-positive DLBCL expressed PD-L1 in tumor cells, as well as in the tumor microenvironment considering cut-off for PD-L1 positivity more than 5% of tumor cells with intensity level of 2 + or 3 + or PD-L1 positivity more than 20% of the tumor microenvironment (TME) with the level of staining intensity of 2 + or 3 + [31]. Kwon et al. found 61.1% of DLBCL expressed PD-L1 using 10% cut-off and included the intensity in scoring [11].
It is worth noting that 33% of our included patients were HCV sero-positive and HCV sero-positivity was associated with the level of PDL-1 expression (p 0.05). Abdellatif and Shiha found that PD-L1 expression in CD34 + hematopoietic stem cells was upregulated in chronic HCV infection [33]. Chen et al. found that PD-L1 was overexpressed on malignant cells and tumor infiltrating macrophages in virus-associated malignancies [31]. Mofrad et al. found that tumorigenic viruses can inhibit the anti-cancer immune system by several mechanisms; one of them is by overexpression of PD-1/PD-L1 [34].
We found no significant correlation between levels of PD-L1 expression and patients’ age, gender, serum LDH, B symptoms but the levels of PD-L1 expression were statistically associated with IPI score and poor prognosis. These results were consistent with that of Zhao et al. who meta-analyzed nine studies (five of them were DLBCL subtype) to assess the correlation between PD-L1 expression and clinicopathological characteristics and prognosis of NHL [35].
Dissimilar to our results, Zhao et al. found that PD-L1 expression in DLBCL was not associated with Ann Arbor stage (p 0.44) but they mentioned some limitations to the results of their meta-analysis including different sources PD-L1 antibodies, different cut-off values and a possible publication bias [35]. Another meta-analysis was carried out by Zeng et al. on 12 studies of NHL (6 of them were DLBCL). Their results revealed that the overexpression of PD-L1 was associated with B symptoms, higher IPI score ≥ 3, and Ann Arbor stages (III and IV) as well as poor prognosis in the patients diagnosed with DLBCL [36].
Regarding the PD-L1 correlation with prognosis, high PD-L1 expression was associated with a dismal outcome [35,36,37]. Qiu et al. found that positive PD-L1 expression is statistically associated with shorter PFS and OS in DLBCL and its prognostic significance increased significantly when the cutoff value was ≥ 30% [37]. The results of meta-analysis conducted by Geng et al. indicate that PD-L1 expression detected by immunohistochemistry was a promising marker for the identification of patients who may benefit from blocking PD-1/PD-L1 by immunotherapy [25]. Smith et al. found that the CR rate and 2-year PFS were improved when pembrolizumab was added to R-CHOP in previously untreated PD-L1 expressing DLBCL [38].
Conclusions
PDL-1 overexpression in DLBCL is associated with aggressive clinicopathological features and a lower response to standard RCHOP and worse prognosis. PD-L1 inhibitors may be promising in the initial treatment regimen for those patients.
However, more prospective multicentric research on larger study population is mandatory to validate and standardize the cut-off, scoring method, and site of expression.
Availability of data and materials
All the clinical, radiological, and pathological data used in this manuscript is available on Mansoura University medical system (Ibn Sina Hospital management system). http://srv137.mans.edu.eg/mus/newSystem/.
IHC results for PDL1 are available from Assistant Professors of Pathology Dr. Sherine Refaat and Afaf Taha Ibrahiem and Lecturer of pathology Dr. Eman Mohamad Ibrahim on reasonable request.
Abbreviations
- APC:
-
Antigen-presenting cells
- B cells:
-
B lymphocytes
- BCL2:
-
B cell lymphoma 2
- CD:
-
Cluster differentiation
- CTLA:
-
Cytotoxic T-lymphocyte antigen
- DLBCL:
-
Diffuse large B cell lymphoma
- DFS:
-
Disease-free survival
- DE:
-
Double expressor
- EBER-ISH:
-
EBV encoded RNA in situ hybridization
- EBV:
-
Epstein-Barr virus
- HCV:
-
Hepatitis C virus
- IC:
-
Immune cells
- IPI:
-
International Prognostic Index
- LDH:
-
Lactate dehydrogenase
- LMP:
-
Latent membrane protein
- mCPS:
-
Modified Combined-Positive Score
- MUM-1:
-
Multiple myeloma 1
- NK cells:
-
Natural killer cells
- NHL:
-
Non-Hodgkin lymphoma
- OS:
-
Overall survival
- PD-1:
-
Programmed death protein 1
- PD-L1:
-
Programmed death ligand 1
- R-CHOP:
-
Rituximab-cyclophosphamide, doxorubicin, oncovin, prednisone
- T cells:
-
T lymphocytes
- TE:
-
Triple expressor
- TME:
-
Tumor microenvironment
References
Ekberg S, K Smedby E, Glimelius I, Nilsson-Ehle H, Goldkuhl C, Lewerin C, et al. Trends in the prevalence, incidence and survival of non-Hodgkin lymphoma subtypes during the 21st century–a Swedish lymphoma register study. Br J Haematol. 2020;189(6):1083–92.
Ibrahim AS, Khaled HM, Mikhail NNH, Baraka H, Kamel H. Cancer incidence in Egypt: results of the national population-based cancer registry program. J Cancer Epidemiol. 2014;2014:437971.
Abdelhamid T, Samra M, Ramadan H, Mehessin M, Mokhtar N. Clinical prognostic factors of diffuse large B cell non-Hodgkin lymphoma: a retrospective study. J Egypt Natl Canc Inst. 2011;23(1):17–24.
Beham-Schmid C. Aggressive lymphoma 2016: revision of the WHO classification. Memo. 2017;10(4):248–54.
Janakiram M, Pareek V, Cheng H, Narasimhulu DM, Zang X. Immune checkpoint blockade in human cancer therapy: lung cancer and hematologic malignancies. Immunotherapy. 2016;8(7):809–19.
Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):727.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Kiyasu J, Miyoshi H, Hirata A, Arakawa F, Ichikawa A, Niino D, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126(19):2193–201.
Gravelle P, Burroni B, Péricart S, Rossi C, Bezombes C, Tosolini M, et al. Mechanisms of PD-1/PD-L1 expression and prognostic relevance in non-Hodgkin lymphoma: a summary of immunohistochemical studies. Oncotarget. 2017;8(27):44960.
Dong L, Lv H, Li W, Song Z, Li L, Zhou S, et al. Co-expression of PD-L1 and p-AKT is associated with poor prognosis in diffuse large B-cell lymphoma via PD-1/PD-L1 axis activating intracellular AKT/mTOR pathway in tumor cells. Oncotarget. 2016;7(22):33350.
Kwon D, Kim S, Kim P-J, Go H, Nam SJ, Paik JH, et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology. 2016;68(7):1079–89.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumors of hematopoietic and lymphoid tissues (Revised 4th edition). Lyon: IARC; 2017.
Huang S, Nong L, Liang L, Zheng Y, Wang W, Liu J, et al. Comparison of PD-L1 detection assays and corresponding significance in evaluation of diffuse large B-cell lymphoma. Cancer Med. 2019;8(8):3831–45.
Gatalica Z, Bilalovic N, Vranic S, Arguello D, Reddy S, Ghosh N. PD-L1 and PD1 expression in lymphomas. Washington, DC: American Society of Hematology; 2015.
Miyoshi H, Kiyasu J, Kato T, Yoshida N, Shimono J, Yokoyama S, et al. PD-L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T-cell leukemia/lymphoma. Blood J Am Soc Hematol. 2016;128(10):1374–81.
Xie Y, Bulbul MA, Ji L, Inouye CM, Groshen SG, Tulpule A, et al. p53 expression is a strong marker of inferior survival in de novo diffuse large B-cell lymphoma and may have enhanced negative effect with MYC coexpression: a single institutional clinicopathologic study. Am J Clin Pathol. 2014;141(4):593–604.
Peña C, Villegas P, Cabrera ME. Double or triple-expressor lymphomas: prognostic impact of immunohistochemistry in patients with diffuse large B-cell lymphoma. Hematol Transfus cell Ther. 2020;42(2):192–3.
Menon MP, Pittaluga S, Jaffe ES. The histological and biological spectrum of diffuse large B-cell lymphoma in the WHO classification. Cancer J. 2012;18(5):411.
Kataoka K, Shiraishi Y, Takeda Y, Sakata S, Matsumoto M, Nagano S, et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature. 2016;534(7607):402–6.
Marletta S, Fusco N, Munari E, Luchini C, Cimadamore A, Brunelli M, et al. Atlas of PD-L1 for pathologists: indications, scores, diagnostic platforms and reporting systems. J Pers Med. 2022;12(7):1073.
Armand P, Shipp MA, Ribrag V, Michot J-M, Zinzani PL, Kuruvilla J, et al. Pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure: long-term efficacy from the phase 1b keynote-013 study. Blood. 2016;128(22):1108.
Armand P, Rodig S, Melnichenko V, Thieblemont C, Bouabdallah K, Tumyan G, et al. Pembrolizumab in relapsed or refractory primary mediastinal large B-cell lymphoma. J Clin Oncol. 2019;37(34):3291.
Armand P, Engert A, Younes A, Lee HJ, Santoro A, Zinzani PL, et al. Nivolumab for relapsed or refractory classical Hodgkin lymphoma (cHL) after autologous hematopoietic cell transplantation (auto-HCT): extended follow-up of the phase 2 single-arm CheckMate 205 study. Blood. 2018;132:2897.
Geng Z, Xiao Y, Zhu X-J, Ye C, Zhou J-F. Anti-PD-1 therapy for clinical treatment of lymphoma: a single-arm meta-analysis. Oncotarget. 2018;9(82):35343.
Cheung CC, Barnes P, Bigras G, Boerner S, Butany J, Calabrese F, et al. Fit-for-purpose PD-L1 biomarker testing for patient selection in immuno-oncology: guidelines for clinical laboratories from the Canadian Association of Pathologists-Association Canadienne Des Pathologistes (CAP-ACP). Appl Immunohistochem Mol Morphol. 2019;27(10):699.
Udall M, Rizzo M, Kenny J, Doherty J, Dahm S, Robbins P, et al. PD-L1 diagnostic tests: a systematic literature review of scoring algorithms and test-validation metrics. Diagn Pathol. 2018;13(1):1–11.
Gavrielatou N, Shafi S, Gaule P, Rimm DL. PD-L1 expression scoring: noninterchangeable, noninterpretable, neither, or both. J Natl Cancer Inst. 2021;113:1613–4 Oxford University Press.
Ribas A, Hu-Lieskovan S. What does PD-L1 positive or negative mean? J Exp Med. 2016;213(13):2835–40.
Hawkes EA, Grigg A, Chong G. Programmed cell death-1 inhibition in lymphoma. Lancet Oncol. 2015;16(5):e234–45.
Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MGM, Xu ML, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin cancer Res. 2013;19(13):3462–73.
Andorsky DJ, Yamada RE, Said J, Pinkus GS, Betting DJ, Timmerman JM. Programmed death ligand 1 is expressed by non–Hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin cancer Res. 2011;17(13):4232–44.
Abdellatif H, Shiha G. PD-L1 expression on circulating CD34+ hematopoietic stem cells closely correlated with T-cell apoptosis in chronic hepatitis c infected patients. Int J stem cells. 2018;11(1):78.
Mofrad MG, Maleki DT, Faghihloo E. The roles of programmed death ligand 1 in virus-associated cancers. Infect Genet Evol. 2020;84:104368.
Zhao S, Zhang M, Zhang Y, Meng H, Wang Y, Liu Y, et al. The prognostic value of programmed cell death ligand 1 expression in non-Hodgkin lymphoma: a meta-analysis. Cancer Biol Med. 2018;15(3):290.
Zeng Q, Liu Z, Liu T. Prognostic value and clinicopathological characteristics of PD-L1 overexpression in non-Hodgkin lymphoma: a meta-analysis. BMC Cancer. 2020;20(1):1–10.
Qiu L, Zheng H, Zhao X. The prognostic and clinicopathological significance of PD-L1 expression in patients with diffuse large B-cell lymphoma: a meta-analysis. BMC Cancer. 2019;19(1):1–12.
Smith SD, Till BG, Shadman MS, Lynch RC, Cowan AJ, Wu QV, Voutsinas J, Rasmussen HA, Blue K, Ujjani CS, et al. Pembrolizumab with R-CHOP in previously untreated diffuse large B-cell lymphoma: potential for biomarker driven therapy. Br J Haematol. 2020;189(6):1119–26.
Acknowledgements
Not applicable.
Funding
Not applicable as this work was not funded or supported by any organization.
Author information
Authors and Affiliations
Contributions
EMI, SR, and ATI interpreted histopathologic and immunohistochemical stained slides, captured figures provided in this study; also, they contributed in the study design. MWF and SE equally participated in the design of the study, collecting clinical, radiological, histopathological data, and statistical analysis. Also, all authors participated in writing and editing of final version of manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board of Faculty of Medicine, Mansoura University reviewed and approved this study (IRB code: R.20.10.1038). The authors declare that the guidelines of the World Medical Association Declaration of Helsinki were followed.
This is a retrospective study so we did not take informed consents.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, E.M., Refat, S., El-Ashwah, S. et al. Programmed death ligand 1 expression in diffuse large B cell lymphoma: correlation with clinicopathological prognostic factors. J Egypt Natl Canc Inst 35, 12 (2023). https://doi.org/10.1186/s43046-023-00171-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43046-023-00171-6