Abstract
Background
The conventional standard treatment for ovarian cancer is not very effective, and the disease is fatal for women. Cancer Stem Cells (CSCs) that express CD44+/CD24- can contribute to chemoresistance and a poor prognosis. We seek to investigate the expression of CSCs (CD44+/CD24-) in ovarian cancer and their predictive significance.
Methods
The ambispective cohort was performed on 64 patients (32 patients in each group) at four hospitals (Cipto Mangunkusumo, Tarakan, Fatmawati, and Dharmais Hospital). Debulking surgery was performed on the patients, followed by histopathological analysis. The patients had six rounds of chemotherapy and were under monitoring for six months. The therapeutic responses were evaluated using the RECIST criteria (Response Criteria in Solid Tumors) and categorized as chemoresistant or chemosensitive. Using immunohistochemistry, we directly assess the CSCs from ovarian cancer tissue and using flow cytometry to assess the CSCs from the blood.
Results
High CSCs expression and ovarian cancer chemoresistance were significantly related in both trials (p 0.05). A better outcome was obtained using CD44+/CD24- immunohistochemistry.
Conclusions
We conclude that there is a substantial association between high CSCs expression and chemoresistance in ovarian cancer and that CSCs immunohistochemistry has a higher predictive value.
Similar content being viewed by others
Introduction
Ovarian cancer is a deadly women cancer [1]. Only 20% of women survive more than five years after being diagnosed with ovarian cancer, which has a risk of 1.3% [2]. Patients with ovarian cancer experience different symptoms that cannot be showcased to generate a definitive diagnosis. Therefore, further examination is necessary to identify ovarian cancer if symptoms such as abdominal distress or distension are detected [3]. Initially, 65–80% of patients respond to treatment, but 50% continue to develop platinum chemoresistance, which has a bad prognosis [4]. With standard therapies, twelve months of progression-free survival (PFS) and thirty months of overall survival (OS) would be achieved, which include debulking surgery followed by chemotherapy [5]. Chemotherapy resistance and a poor prognosis were caused by the cancer stem cells (CSCs) proteins. CSCs may be a target for cancer genetic therapy, according to recent studies [6].
The growth, metastasis, recurrence, and medication resistance of tumours are significantly influenced by cancer stem cells. Tumour recurrence was associated with high CD44 expression in chemoresistant ovarian cancer cell lines [6]. According to recent research by Klemba et al., ovarian cancer with high CD44+/CD24- has a stronger capacity for invasion and differentiation [2]. High CD44 expression was linked to chemoresistance, poor differentiation, high resistance, and recurrence rate, according to Zhang et al. [7]. Therefore, miR-199a's downregulation of CD44 in ovarian cancer may inhibit the cell cycle, diminish the ability for proliferation, and decrease invasiveness in vitro and tumour development in vivo [8].
The G1, S, G2, and M cell cycle stages are typical. Because the cells are not a part of the cell cycle but are still metabolically active, G0 is an early G1 phase and is referred to as the resting phase. The G1, S, and G2 phases are the interphases of cells, whereas the M phase is a mitotic phase (prophase, prometaphase, metaphase, anaphase, telophase). The G1 phase lasts the longest in normal cells, but CSCs have a shorter G1 phase that promotes rapid cell division to produce more stem cells [9]. The majority of chemotherapeutic targets are cells that are actively proliferating in the S or M phase [6]. CSCs, however, will stay in the G0 phase. CSCs' impact on the cell cycle results in chemoresistance of the cancer cells.
Identifying the association between CSCs and ovarian cancer chemotherapy response as well as the prediction capacity of CSCs is the goal of this project. We hypothesize that the CD44+/CD24- has a connection to ovarian cancer and is a reliable indicator of chemoresistance.
Methods
Research Design
This is a two-year ambispective cohort study conducted at the Cipto Mangunkusumo Hospital, Tarakan Hospital, Fatmawati Hospital, and Dharmais Hospital (February 2018 - February 2022).
Subjects
Patients with epithelial ovarian cancer in stages II-IV who agreed to participate in the study were the study's subjects. Pregnancy and other extra cancers met the exclusion criteria. Sixty-four patients made up the sample (32 in each group) using the consecutive sampling method. Thirty-two (50%) of the patients responded to the therapy with chemoresistance and 32 (50%) with chemosensitivity. For the flow cytometry and immunohistochemistry studies, samples were taken from every patient. After six months of surveillance, we observed no lost follow-up patients. Table 1 shows the distribution of patient profiles and clinical features.
Data collection
The patients received histopathological examination and surgery. Patients who had malignant outcomes got six chemotherapy sessions followed by six months of monitoring. The effectiveness of the medication was assessed using the RECIST Criteria, after which it was divided into chemoresistant and chemosensitive responses. We performed retrospective immunohistochemical analysis of the ovarian cancer tissue and prospective flow cytometry to analyze CD44+/CD24- blood expression. Serum Ca-125 levels, operation type, cancer histology type, cancer stage, tumour cell differentiation, cancer residue, cancer size, ascites, and lymph node metastases are among the other factors that were taken into account. The FIGO criteria were used to establish the disease stage. The Research Ethics Committee of Universitas Indonesia granted us ethical permission.
Slide preparations
Eight 3 μm paraffin-block preparations were employed in this study to analyze the tissue. The blocks were then heated to 600°C and dried (30 min). We rehydrated it with serial alcohol, deparaffinized it using graduated xylol, and then washed it with water (5 minutes). Using 1.5 per cent hydrogen peroxide in methanol for 10 minutes, we isolated the area before washing it with water (5 min). To stop endogenous peroxidase activity, we applied a blocking technique. In the pre-treatment, a 960°C Decloaking Chamber contained Tris EDTA acid (10 min). We cleaned it in phosphate-buffered saline after cooling the blocks for 45 min (PBS). With the help of the Universal Background Sniper formula, we repeated the blocking procedure (15 min).
Using antibodies directed specifically against CD44 and CD24, we were able to identify their expression. We used a 1:500 dilution of the antibodies to incubate the preparations. After an hour, we washed it with PBS (5 min). Cocktail staining (double staining), was employed. After that, it was washed with PBS and incubated for 20 minutes with a Trekkie Universal Link solution (5 min). After that, we washed it in PBS and incubated it for 15 min with track-Avidin-HRP labelled (5 min). Diaminobenzene (DAB) was added to the 1 mL of a substrate, and it was vortexed (15 s). We drizzled it with the preparation, incubated it for two minutes, and then washed it (10 min).
After counterstaining with Counterstain Kit (CAT) hematoxylin for five seconds and washing with water, the preparations and substrate were examined (5 s). The preparations were submerged in distilled water at 5 % for 5 s before being rinsed with water (5 min). For dehydration procedures, we employed graded alcohol, and for cleaning procedures, graded xylol. A mounting solution and cover glass were used to finish the preparation. We decided to include stromal tissue internal positive controls and negative controls devoid of primary antibodies in each smear. To evaluate the preparations, we utilized a Leica ICC 50 HD microscope.
CSCs immunohistochemistry cell counts
The CSCs' (CD44+/CD24-) expression was found by using a 400× light microscope magnification and three different fields of view. While the CD24 marker was red in both the cytoplasm and cell membrane, the CD44 marker was mostly brown in the cell membrane [10]. There is no red staining and a predominance of brown staining in the high CD44+/CD24- cells. Low CD44+/CD24- expression was defined as being less than 10% of the total coloured cells, whereas high CD44+/CD24- expression was defined as being greater than 10% of the total colored cells.
Flow cytometry methods
Peripheral blood samples as large as 5 mL were centrifuged, and the supernatant was then removed, leaving 50 μL of the sample to be resuspended. Fluorescent CD44 and CD24 antibodies were used to react with the samples (labelled PerCP and APC respectively). We eliminated the leukocytes from the pacific blue-labelled CD45 reagents. Then, we added 2.5 µL of CD44 and 2.5 µL of CD24 markers. The samples were first incubated for 15 min, followed by the addition of 300 μL of lysing solution and incubation. We repeated the centrifugation after adding 1 mL of FACS flow solution. After adding 500 μL of perm wash buffer and centrifuging the samples, we discarded the supernatant. We centrifuged the mixture after adding 1mL of perm wash buffer once more. Then, we added 200 μL paraformaldehyde (1% solution) in phosphate-buffered saline (PBS). We analyzed the samples with a flow cytometer.
Statistical analysis
Data that were bivariate and univariate were examined. Chi-square or an alternate Fisher test were used to analyze the categorical variables. Both studies then investigated the AUC (area under curves) values and ROC (receiver operating characteristic) curves as a predictor of therapeutic response. In all research, we compared the power of CSCs using logistic regression.
Results
Ovarian cancer blood flow cytometry test
By using a flow cytometer to measure the number of cells, CSCs were identified as those with positive CD44+/CD24- marker expression. The percentage of the total cell count was used to compute the CSCs proportion. Figures 1 and 2 describe the flow cytometry analysis. The findings show that CSCs were substantially expressed in chemoresistance-positive ovarian cancer patients.
CD44+/CD24- Staining at ovarian cancer tissue
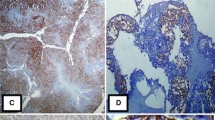
When compared to CD24+ cells, which have a red cytoplasm and membrane (blue arrows in Fig. 3(A)), CD44+ cells have a brown cell membrane (400x magnification). In chemoresistant ovarian cancer tissue, there is significant CD44+/CD24- cell expression, with brown staining predominating over red. Low CD44+/CD24- cell expression is seen in chemosensitive ovarian cancer tissue in Fig. 3(B). It appears like most of the cells are red.
CD44+/CD24− expression in ovarian cancer tissue (400× magnification). CD44+ cells have brown cell membrane (orange arrow). CD24+ cells have red cytoplasm and cell membrane (blue arrow). CD44+/CD24− cells have a more dominant brown color than red color. A Chemoresistant ovarian cancer tissue, brown staining predominating over red. B Chemosensitive ovarian cancer tissue, almost all cells are colored red
Bivariate analysis
Some variables had results in Table 2 that were significantly different (p 0.05). Compared to the flow cytometry CSCs, the immunohistochemistry CSCs have an OR of 10.7 and 3.19. Immunohistochemistry CSCs hence have a higher OR and RR value. But CSCs immunohistochemistry and flow cytometry were reliable indicators of ovarian cancer chemoresistance.
AUC (area under the curve) and ROC (receiver operating characteristic) curve
The immunohistochemistry of CD44+/CD24− has a greater value, as evidenced by the AUC values (Table 3) and ROC curve (Fig. 4). The immunohistochemistry CSCs have a 96% specificity, 81% sensitivity, and a substantially excellent accuracy (AUC value 0.891, p 0.05). The sensitivity is 78%, the specificity is 75%, and the flow cytometry CSCs have a substantial fair accuracy (AUC value 0.766, p 0.05). Therefore, ovarian cancer chemoresistance may be accurately predicted using immunohistochemistry and flow cytometry CSCs.
Multivariate analysis
Table 4 displays the results of the regression. A better outcome suggests that the immunohistochemistry CSCs are a better predictor.
Discussion
The expression of CD44+/CD24- in ovarian cancer tissue and blood flow in ovarian cancer patients was compared for the first time in our study. Only cultured cell lines were employed in the experiments conducted in the past. Both the blood and ovarian cancer tissue have significant CSC expression. Better outcomes come from ovarian cancer tissues that exhibit CD44+/CD24−.
In both investigations, we discovered that CSCs were highly expressed in chemoresistant ovarian cancer patients. The development of ovarian cancer is linked to high CSCs expression. A strong ability for differentiation, migration, tumorigenesis, and aggressiveness was also seen in cancer stem cell tumors. The stemness characteristics of cancer cells have been described using the combination of CD44 and CD24 [11]. Ovarian cancer cells with high CD44+/CD24- are more treatment-resistant, more capable of differentiating, and more invasive. The median PFS and cancer recurrence were both shorter in patients with greater CD44+/CD24- ovarian cancer cells [2].
Additionally, different malignancies benefit from the prognostic value of CSCs expression. Low patient survival is associated with high CSCs expression in patients with gastric cancer [12]. High CSCs expression was also associated with breast cancer tumour development, metastasis, chemoresistance, and a poor prognosis [11, 13]. Higher levels of CD44 and CD24 expression were also seen in the pancreatic ductal adenocarcinoma and were related to its development [14].
Most cell types, including leukocytes, epithelial cells, and malignant cells have Cluster of Differentiation 44 (CD44) as cell surface adhesion receptor protein [15]. CD44 has been reported in the colorectal cancer [16], breast cancer [17], urothelial bladder cancer [18], gastric cancer [19], and pancreatic cancer [20]. According to a meta-analysis, CD44 is substantially associated with patients' survival rates being low [21]. A cell adhesion molecule in ovarian cancer is the Cluster of Differentiation 24 (CD24) [22]. Ovarian, lung, and breast cancer have all been linked to high CD24 expression [23]. Therapeutic CD24 genetic ablation may slow the growth of tumours in vivo and lengthen patient life [23].
A transmembrane protein found on cancer stem cells is called CD44. Cell proliferation, adhesion, migration, and invasion are facilitated by signalling pathways that are triggered by the binding and activation of CD44 by its ligands. These pathways also modify cytoskeletal alterations and boost cellular motility. Hyaluronic acid is the primary ligand for CD44 in cancer cells. When undergoing epithelial to mesenchymal transition (EMT) processes, epithelial ovarian cancer cells require certain stem cell characteristics. As a result, these cancer cells will express CD44 more often, be more invasive, and be more chemotherapy-resistant [24]. Inducing self-renewal processes, promoting metastasis and cell invasion are possible effects of CD44's interactions with fibroblast growth factor (FGF), osteopontin, and epidermal growth factor (EGF) [12].
Human tumours and developing B and T lymphocyte surface both express the CD24 protein. Numerous cancers, including ovarian and breast cancer, have been observed to express CD24 highly. Tumour growth, migration, and invasion are all impacted by CD24. By expelling the tumour cells from the circulation, the interaction between CD24 and P-selectin on platelets might cause metastasis. The CD24 may cause Src kinase activation and promote tumour growth. The sialic acid-binding immunoglobulin (Ig)-like lectins (Siglecs) recognize CD24 as a ligand [25]. On granulocytes, lymphocytes, and monocytes, Siglec-10 is a crucial receptor [26]. Siglec-10 and CD24's association inhibits phagocytes and encourages immunological escape mechanisms. The fundamental trait of tumour formation and recurrence is its capacity to evade the immune system [25].
No recent study has been done to specifically look at CSCs in ovarian cancer tissue and blood circulation. Further research is still required since the CD44+/CD24- phenotype could not be the sole factor contributing to the chemoresistance, despite our significant data indicating a link between CD44+/CD24- and ovarian cancer chemoresistance. To validate these findings, external validation research is necessary.
Conclusion
The study found a substantial correlation between ovarian cancer chemoresistance and high CSC expression. A stronger predictor of CSCs than flow cytometry is immunohistochemistry CSCs in ovarian cancer tissue.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RECIST:
-
Response Criteria in Solid Tumors
- OS:
-
Overall Survival
- PFS:
-
Progression-free Survival
- CD44:
-
Cluster of differentiation 44
- CSCs:
-
Cancer stem cells
- CD24:
-
Cluster of differentiation 24
- PBS:
-
Phosphate-buffered Saline
- EDTA:
-
Ethylenediaminetetraacetic acid
- FIGO:
-
International Federation of Gynecology and Obstetrics
- DAB:
-
Diaminobenzene
- CAT:
-
Counterstain Kit
- AUC:
-
Area Under Curve
- ROC:
-
Receiver operating characteristic
- OR:
-
Odds Ratio
- RR:
-
Risk Ratio/relative risk
- FGF:
-
Fibroblast growth factor
- EGF:
-
Epidermal growth factor
- Siglec:
-
Sialic-acid-binding Ig-like Lectin
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Klemba A, Purzycka-Olewiecka JK, Wcislo G, et al. Surface markers of cancer stem-like cells of ovarian cancer and their clinical relevance. Contemp Oncol (Pozn). 2018;22(1A):48–55.
Funston G, Van Melle M, Baun M-LL, et al. Variation in the initial assessment and investigation for ovarian cancer in symptomatic women: a systematic review of international guidelines. BMC Cancer. 2019;19(1):1–13.
Li H, Li J, Gao W, et al. Systematic analysis of ovarian cancer platinum-resistance mechanisms via text mining. J Ovar Res. 2020;13(1):1–6.
du Bois A, Baert T, Vergote I. Role of Neoadjuvant Chemotherapy in Advanced Epithelial Ovarian Cancer. J Clin Oncol. 2019;37(27):2398–405.
Suster NK, Virant-Klun I. Presence and role of stem cells in ovarian cancer. World J Cells. 2019;11(7):383.
Zhang J, Yuan B, Zhang H, et al. Human epithelial ovarian cancer cells expressing CD105, CD44 and CD106 surface markers exhibit increased invasive capacity and drug resistance. Oncol Lett. 2019;17(6):5351–60.
Alshamrani AA. Roles of microRNAs in ovarian cancer tumorigenesis: two decades later, what have we learned? Front Oncol. 2020;10:1084.
Basu S, Dong Y, Kumar R, et al. Slow-cycling (dormant) cancer cells in therapy resistance, cancer relapse and metastasis. Semin Cancer Biol. 2022;78:90–103.
Moura JMBDO, Gonzaga AKG, Queiroz SIML, et al. Immunohistochemical expression of OCT4 and CD44 in major and minor salivary gland neoplasms. Braz Oral Res. 2021;35.
Li W, Ma H, Zhang J, et al. Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Sci Rep. 2017;7(1):1–15.
Hui D, Chen J, Jiang Y, et al. CD44+ CD24-/low sphere-forming cells of EBV-associated gastric carcinomas show immunosuppressive effects and induce Tregs partially through production of PGE2. Exp Cell Res. 2020;390(2):111968.
Shadbad MA, Hosseinkhani N, Asadzadeh Z, et al. A systematic review to clarify the prognostic values of CD44 and CD44+ CD24-phenotype in triple-negative breast cancer patients: lessons learned and the road ahead. Front Oncol. 2021;11.
Durko L, Wlodarski W, Stasikowska-Kanicka O, et al. Expression and clinical significance of cancer stem cell markers CD24, CD44, and CD133 in pancreatic ductal adenocarcinoma and chronic pancreatitis. Dis Markers. 2017;2017.
Chen L, Fu C, Zhang Q, et al. The role of CD44 in pathological angiogenesis. FASEB J. 2020;34(10):13125–39.
Wang Z, Tang Y, Xie L, et al. The prognostic and clinical value of CD44 in colorectal cancer: a meta-analysis. Front Oncol. 2019;9:309.
Hu J, Li G, Zhang P, et al. A CD44v+ subpopulation of breast cancer stem-like cells with enhanced lung metastasis capacity. Cell Death Dis. 2017;8(3):e2679.
Hu Y, Zhang Y, Gao J, et al. The clinicopathological and prognostic value of CD44 expression in bladder cancer: a study based on meta-analysis and TCGA data. Bioengineered. 2020;11(1):572–81.
Yang J. Identification of novel biomarkers, MUC5AC, MUC1, KRT7, GAPDH, CD44 for gastric cancer. Med Oncol. 2020;37(5):1–10.
Liu Y, Wu T, Lu D, et al. CD44 overexpression related to lymph node metastasis and poor prognosis of pancreatic cancer. Int J Biol Markers. 2018;33(3):308–13.
Han S, Huang T, Li W, et al. Prognostic value of CD44 and its isoforms in advanced cancer: a systematic meta-analysis with trial sequential analysis. Front Oncol. 2019;9:39.
Li S-S, Ma J, Wong AS. Chemoresistance in ovarian cancer: exploiting cancer stem cell metabolism. J Gynecol Oncol. 2018;29(2):e32.
Barkal AA, Brewer RE, Markovic M, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392–6.
Chen C, Zhao S, Karnad A, et al. The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol 2018;11(1):1-23.
Yin S-S, Gao F-H. Molecular mechanism of tumor cell immune escape mediated by CD24/Siglec-10. Front Immunol. 2020;11:1324.
Altevogt P, Sammar M, Hüser L, et al. Novel insights into the function of CD24: A driving force in cancer. Int J Cancer. 2021;148(3):546–59.
Acknowledgements
We would thank Cipto Mangunkusumo, Tarakan Hospital, Dharmais hospital, and Fatmawati Hospital for their support in this research. We would like to acknowledge Christine Pamphila, M.D., Rasendah, M.D., Kristabella Gianina, M.D., for their contribution to data analysis and data collection.
Funding
The funding of the research was from all the authors. We have no support in the form of grants, equipment, drugs, etc.
Author information
Authors and Affiliations
Contributions
UHMS done the concept of the study, organized the data set, performed the surgery, performed the immunohistochemistry and flow cytometry data retrieval, analyzed the data, validate the data, and major contributor in writing the manuscript. AA done the concept of the study, organized the data set, performed the surgery, performed the immunohistochemistry and flow cytometry data retrieval, analyzed the data, validate the data, and contributor in writing the manuscript. GP done the concept of the study, organized the data set, performed the surgery, performed the immunohistochemistry and flow cytometry data retrieval, analyzed the data, validate the data, and contributor in writing the manuscript. SG done the concept of the study the study, organized the data set, performed the surgery, performed the immunohistochemistry and flow cytometry data retrieval, analyzed the data, validate the data, and contributor in writing the manuscript. ARH done the concept of the study, organized the data set, performed the immunohistochemistry and flow cytometry data retrieval, analyzed the data, validate the data, and contributor in writing the manuscript. PR done the concept of the study, organized the data set, performed the immunohistochemistry and flow cytometry data retrieval, analyzed the data, validate the data, and contributor in writing the manuscript. AK done the concept of the study, organized the data set, performed the immunohistochemistry and flow cytometry data retrieval, analyzed the data, validate the data, and contributor in writing the manuscript. RW done the concept of the study, organized the data set, performed the immunohistochemistry and flow cytometry data retrieval, analyzed the data, validate the data, and contributor in writing the manuscript. DRF done the concept of the study, organized the data set, performed the immunohistochemistry and flow cytometry data retrieval, analyzed the data, validate the data, and major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was granted by the Health Research Ethics Committee of the Universitas Indonesia, Cipto Mangunkusumo Hospital No. KET-230/UN2.F1/ETIK/PPM.00.02/2021, March 15th, 2021. Informed consent was obtained from all participants included in the study.
Consent for publications
Not applicable. We have no individual person’s data in the manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sihombing, U.H.M., Andrijono, A., Purwoto, G. et al. CD44+/CD24- Expression as predictors of ovarian cancer chemoresistance: immunohistochemistry and flow cytometry study. J Egypt Natl Canc Inst 34, 44 (2022). https://doi.org/10.1186/s43046-022-00143-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43046-022-00143-2