Abstract
Background
Atrial fibrillation (AF) is one of the leading causes of hospitalization and even death worldwide. Complex bidirectional associations have been suggested between psychiatric disorders and AF disease. This study was conducted to investigate the prevalence of psychiatric symptoms in a cohort of Egyptian population presented with symptomatic non-valvular AF (NVAF) and to identify the high-risk subjects in need for professional psychiatric consultation. A total of 100 eligible symptomatic NVAF patients were recruited in this cross-sectional study. Each patient was subjected to: (1) cardiac evaluation included electrocardiogram, trans-esophageal echocardiography, and the European Heart Rhythm Association (EHRA). (2) Psychiatric evaluation consisted of clinical psychiatric interviewing, Hospital Anxiety and Depression Scales (HADS), Mini–Mental State Examination (MMSE), type-D personality screening, and the short form-36 (SF-36) health survey for the assessment of health-related quality of life (HRQoL).
Results
Forty-four percent of our enrolled AF patients had anxiety symptoms, 32% had depressive symptoms, 24% had mild cognitive impairment, and 32% had type-D personality. Linear regression analysis demonstrated that the left atrial dimension (LAD) and the age were the main significant predictors of MMSE, while the main predictors of HADS were SF-36 (physical functioning and general health) and the age. Neither psychiatric symptoms, nor type-D personality was a significant predictor for the evaluated cardiac parameters.
Conclusions
Mild cognitive impairment as well as depressive and anxiety symptoms is not uncommon associates with NVAF patients. Assessment of cognitive function and HRQoL is strongly advised for AF patients presented with enlarged LAD particularly among old adults.
Similar content being viewed by others
Background
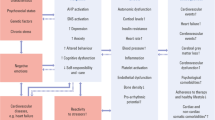
Mental disorders and cardiovascular diseases often coexist with a complex bi-directional relationship [1]. The underlying mechanism of this coupling is multifaceted and has not been fully clarified. Psychosocial factors are deemed to play a substantial role in the expression, course, and magnitude of cardiac illness [2]. Their adverse consequences may be mediated by the lower chance of receiving preventive measures and by the poor adherence to lifestyle interventions and medications [3, 4].
Globally, AF is the commonest sustained cardiac arrhythmia in adults, which is associated with a remarkable morbidity and mortality, thus posing a noteworthy burden to patients, clinicians, and healthcare systems [5]. It affects approximately 1 to 4% of the overall population and the prevalence of NNAF increases with age and reach up to 9% among elderly [6].
In patients with established AF, psychiatric manifestations (including anxiety, depression, and symptom preoccupation) are not uncommon, with a reported prevalence of 25 to 50% across various studies. On the one hand, the deleterious effects caused by arrhythmias are usually not restricted to the AF episode only, as anxiety resulting from panic of developing new events in patients with paroxysmal AF may sometimes become more annoying and restraining than the arrhythmia itself [7]. On the other hand, patients complaining from anxious or depressive symptoms are more prone to an amplified risk of developing various arrhythmias, including AF [8].
As well, growing evidence from recent meta-analytical researches established that AF may be a major predictor of cognitive impairment and increased risk of dementia with or without a history of stroke. Additionally, the relationship to dementia was found to be evident when AF starts in mid-adulthood period and when its duration is longer [9].
The negative impact of AF on the HRQoL has been extensively documented in several previous studies, which also signified the potential detrimental effect of associated mental disorders on increasing the burden of AF managing. This may be additionally intensified by the existence of co-morbidities such as diabetes mellitus (DM), hypertension (HTN), and obesity [10, 11]. However, there is still debate regarding the impacts of different socio-demo-graphical (age, sex, occupation, habits), clinical (ejection fraction (EF), LAD, EHERA score, co-morbidities, treatment approaches), and psychiatric confounders (anxiety, depression, personality type) on HRQoL in patients suffering AF [12].
Recent systematic reviews have focused mainly on AF symptoms severity and changes in HRQoL after rate- and rhythm-control interventions. Nevertheless, the recognition of individuals at higher risk of developing co-morbid psychiatric symptoms with AF seems crucial as it could direct the preventive strategies and the screening programs for early discovery of psychiatric symptoms among vulnerable AF populations, in order to avoid the potential dire consequences resulting from the reluctance in initiating the appropriate psychiatric treatment services.
The primary objective of this study was to investigate the prevalence of co-morbid psychiatric symptoms and cognitive decline as well as quality of life that may associate NVAF patients. The correlations of different variables that could affect the severity of psychiatric symptoms were our secondary goal. The third objective was to identify the high-risk patients who are in need for a qualified psychiatric consultation and intervention.
Methods
Study setting and design
A total of 100 consecutive eligible NVAF inpatients were enrolled in this cross-sectional study by convenience sampling. The study was carried out by the collaboration of Cardiology and Neuropsychiatry Departments at Tanta University Hospitals within 16 months starting from November 2020 after the approval of the responsible institutional ethical committee under the code 34283/11/20.
Patient selection criteria
Inclusion criteria incorporated adults aged 18–65 years of both sex with > 12 years of basic education (completed at least their secondary school education to ensure their ability to perform the psychometric tests properly), who were presented with de novo symptomatic NVAF. Our study was planned to selectively include a group of NVAF patients to avoid heterogeneity of our sample, as the other variant (i.e., the valvular type) has different etiology which is often seen in people suffering from a heart valve disorder or a prosthetic heart valve, who have poorer prognosis with an increasing risk for cardiovascular death or disabling stroke and hence requires different assessment methods and treatment strategies compared to their NVAF counterparts.
Exclusion criteria included patients not fitting the inclusion criteria as well as those presented by other types of AF, acute coronary syndromes, heart failure with reduced EF, thyroid disorders, advanced hepatic/renal diseases, or malignancies, as well as patients with a history of head trauma, substance abuse, psychotropic medications, dementia, or any psychiatric/neurological diseases affecting the cognitive abilities. The information of all eligible participants was recorded for results analysis without any missing data. All patients in this study were subjected to the following:
-
1)
Cardiac evaluation was focused mainly on the following:
-
a
Resting 12 lead electrocardiogram for diagnosis of AF.
-
b
Transthoracic echocardiographic examination to assess left ventricular (LV) functions and LAD.
-
c
The European Heart Rhythm Association (EHRA) symptoms severity score was applied to measure the AF-related symptoms. Arrhythmia‐related symptoms (including palpitations, fatigue, dizziness, dyspnea, and chest pain) are usually evaluated, and the patients attain scores from 1 to 4 with regard to how these symptoms influence their daily activity (ranging from no symptoms to severe symptoms that lead to discontinuation of everyday activities, respectively) [13]. EHERA score ≥ 2 was found to be related to adverse outcomes and more frequent hospitalizations [14].
-
a
-
2)
General examination and routine laboratory workup to exclude patients who had serious metabolic or neurological disorders that might affect cognitive abilities.
-
3)
Psychiatric evaluation included clinical psychiatric interviewing as well as assessment of psychometric tests as follows:
-
a- The Arabic form of the Mini–Mental State Examination (MMSE), which is a widely used brief screening tool, was applied for quantitative evaluation of cognitive impairment [15]. It consists of 11 simple questions including tests of orientation, registration, attention, recall, and language. The MMSE score is the number of correct items with a maximum total score of 30. A cutoff score of 27 for cognitive impairment was recommended by many researchers [16,17,18,19] as it yields the best sensitivity and specificity in detecting even minor decline in cognitive functions. Accordingly, our participants were classified, based on their cognitive scores, into: no impairment (≥ 27), mild impairment (21–26), moderate impairment (11–20), or severe impairment (≤ 10).
-
b- The Hospital Anxiety and Depression Scale (HADS) in its Arabic version, which offers an applicable and replicable screening tool for the evaluation of anxiety and depressive symptoms in non-psychiatric inpatients units. It is a self-report rating scale of 14 items on a 4-point Likert scale scored 0–3 based on recent symptoms. It contains two subtests: HADS-anxiety subscale (HADS-A) and HADS-depression subscale (HADS-D), each of them includes 7 items. Each of the anxiety and depression subscales are obtained by summing up the scores of the seven items, yielding values between 0 and 21. The HADS have three ranges for each subscale: 0–7 (normal), 8–10 (borderline), and 11–21 (cases) [20, 21].
-
c- Type-D (distressed) personality scale (DS14) was used to assess negative affectivity (NA) and social inhibition (SI). It includes 14 items which were rated using a 5-point scale ranging from 0 = false to 4 = true [22]. The 7 NA items from the DS14 cover the tendency to experience feelings of dysphoria, anxiety, and irritability with possible total score from 0 to 28 based on 7 questions. The 7 SI items cover: social discomfort, reticence, and lack of social poise, with a possible total score from 0 to 28 based on 7 questions. Type D scores can be calculated either as the sum of the NA and SI scores (i.e., additive method) or as the result of NA and SI scores (i.e., synergistic method). The simple interpretation is that a person who scores equal or higher than 10 points on both scales can be classified as having a type-D personality [22].
-
d- The short form-36 (SF-36) health survey questionnaire in its Arabic version was applied to evaluate HRQoL. It measures 8 key health concepts of physical and mental components over a 4-week recall period. The eight measured domains are vitality, physical functioning, bodily pain, general health, physical role functioning, emotional role functioning, social role functioning, and mental health. Item scores for each health domain were coded, summed, and transformed into a scale from 0 (worst possible health state) to 100 (best possible health state) [23, 24].
-
Statistical analysis
Data were analyzed using Statistical Program for Social Science (SPSS) version 22.0. Quantitative data were expressed as mean ± standard deviation (± SD). Qualitative data were expressed as frequency and percentage. Pearson’s correlation coefficient (r) test was used for correlating data. Regression analysis was used to predict the value of a variable based on the value of another variable by linear regression analysis.
The sample size and power analysis was calculated using Epi-Info statistics program for public health professionals (Center for Disease Control and Prevention [CDC], Atlanta, GA, USA, 2011). The calculation was based on determining the frequency of psychiatric disorders in a population (patients with NVAF). The criteria used were as follows: (a) an anticipated frequency of 30% of the psychiatric disorders, based on previous works citing similar frequencies; (b) absolute precision of 10%; (c) confidence interval of 95%; (d) design effect of 1; and (e) the population was left as large (1,000,000). The software calculation was based on the following equation:
where the values entered are between brackets, N is the population size (1,000,000), P is the hypothesized % frequency of outcome in the population (30%), d is the confidence limits (precision) as % of 100 (10%), DEFF is the design effect (1), and Z is the Z value for 95% confidence limits (1.96). The equation yielded a minimal sample size of 81 at confidence interval of 95%, but we intentionally increased the number to 100 to compensate for incomplete data of patients.
Results
A total of 100 consecutive adult NVAF patients (mean age 55.63 ± 9.68 years) were enrolled in this cross-sectional study. Of them, 40% were smokers, 71% were males, and 36% were unemployed. As regards the co-morbidities, 29% patients had HTN, and 23% had DM, while co-morbid DM and HTN represented 8% of all participants (Table 1).
The study revealed that 44% of patients had anxiety symptoms, 32% had depressive symptoms, 32% had type-D personality, and 24% of patients had mild cognitive impairment. The mean values of depressive and anxiety symptoms according to HADS were 9.41 ± 1.87 and 9.980 ± 2.01, respectively. The mean value of MMSE was 27.1 ± 1.06. The mean values of both components of type-D personality scale were 9.97 ± 2.06 for NA and 9.29 ± 2.08 for SI. In addition, the mean values of all components of SF-36 Scale were presented in Table 2.
The mean values of the evaluated cardiac parameters were 2.60 ± 0.64, 59.41 ± 4.92, and 4.519 ± 0.50 for EHRA score, EF and LAD, respectively (Table 2).
As regards gender differences in the present sample, there were no statistically significant differences in all the studied parameters (HADS, MMSE, EHRA, EF, LAD, type-D personality, and SF-36) between both sexes (p > 0.05).
Apart from EHERA score which was significantly higher (p = 0.009) in smokers compared to non-smokers, all other studied cardiac and psychiatric parameters had no significant correlation with smoking (p > 0.05).
Regarding the effect of clinical co-morbidities on the outcomes, EHRA score was statistically significantly higher in patients with DM (p = 0.007) and with combined DM and HTN (p = 0.031) compared to patients without co-morbidities. Also, NA of type-D personality was statistically significantly higher (p = 0.035) among patients with co-morbid HTN and DM compared to others without such co-morbidities. However, all other studied parameters were not affected significantly by the associated co-morbidities.
Apart from the LAD and the pain-component of SF-36 which had a statistically significant association to patients with intellectual work (p = 0.033) and patients with manual work (p = 0.009), respectively, compared to unemployed patients, the relation between the occupation and all other studied parameters didn't reach level of significance.
The correlation between the studied psychiatric-, HRQoL-, and cardiac-variables highlighted the followings: the LAD has a significant inverse correlation with both MMSE and SF-36 (physical function) and a significant positive correlation with SF-36 (role of limitation due to physical health). The EF has a significant positive correlation with both SF-36 components (physical function and vitality), while EHRA has no significant correlation with all SF36 items. No significant correlations were detected between cardiac variables with any of type-D personality, HADS-A, or HADS-D (Table 3 and Fig. 1).
Linear regression analysis of predictors affecting the studied parameters underlined the followings: there were no predictor factors affecting cardiac EF significantly. Clinical co-morbidities and special habits were the main predictors affecting EHERA score. LAD and age were the main predictor factors of MMSE, while age and both the physical functioning and general health components of SF-36 were the main predictors affecting HADS as shown in Table 4.
Discussion
AF is the most widespread arrhythmia in medical practice and one of the leading causes of hospitalization. Notably, clinical risk factors among AF populations are currently receiving more attention_among them HTN, diabetes, and alcohol abuse [25]. Nerveless, the co-morbid psychiatric symptoms are also crucial and should receive the same attention in view of the fact that the Current European Society of Cardiology guidelines have recently delineated psychiatric distress and depressed mood as known consequences of AF [26].
This study investigated 100 eligible NVAF adult patients (mean age 55.63 ± 9.68 years, 71% male, 40% smokers, 36% unemployed, 29% hypertensive, 23% diabetic, and 8% were both diabetic and hypertensive) for the prevalence of co-morbid psychiatric symptoms and cognitive decline and for assessing the HRQOOL. Our demographic results were in line with several previous researches [27, 28]. Nevertheless, a higher age was recorded in studies of Freeman et al. [14] and Balcı et al. [29] that could be explained by the exclusion of elderly > 65 years in our series (to reduce the possible age-related cognitive decline) on the one hand, and by rising-up of human longevity in high-income countries compared to our developing countries on the other hand [30]. Good and early screening for HTN and DM as well as higher age group (which is considered risk factor for both DM and HTN) in other studies could explain the higher prevalence of both co-morbidities in previous series [31]. Regarding smoking, the disparity between various researches could be clarified by the substantial regional differences in smoking rate. In addition, the slight dissimilarity in the percentage of unemployment between studies may reflect the higher job opportunities available in developed countries [32].
In the current study, 44% and 32% of the enrolled patients had anxiety and depressive symptoms, respectively. These findings were in line with earlier studies [33,34,35] that reported equivalent rates of anxiety (34.9–38%) and depression (16.9–39.4%) among their AF series.
Accumulating evidence pointed to the potential role of AF in triggering psychiatric diseases caused by patient awareness of their prospective illness, and high worry and nervousness [36]. On the other direction, patients complaining from anxious and depressive symptoms are more prone to increased risks of initiation and/or recurrence of various arrhythmias, including AF [8]. Several mechanisms have been proposed to explain this possible bidirectional coupling, including elevated inflammatory markers, triggered autonomic nervous system with consequent catecholamine overload, dys-regulated hypothalamic–pituitary–adrenal axis, and activated renin–angiotensin–aldosterone system [8]. These alterations could trigger recurrent episodes of AF, either directly by disturbing the electro-physiologic characteristics of the atria, or indirectly by promoting atrial fibrosis and stress-cardiomyopathy through microvascular endothelial damage and catecholamine cardiotoxic effects [37].
Regarding cognitive function analysis in this work, we found that 24% of our patients had mild cognitive impairment, which coincided with the findings of Alonso et al. [38] who detected up to 28% rate of cognitive impairment among their series. It was suggested that AF may speed cognitive impairment and increase the danger of dementia with time through several pathways. Firstly, AF is associated with an increased cerebro-vascular events and stroke repetition leading to associated gradual atrophy of brain volume [39]. Even in individuals with normal baseline cognitive functions, the incident AF may be associated with concomitant mild cognitive decline, even in the absence of clinically manifested stroke, via silent (subclinical) cerebral infarcts through an AF-related embolic lesion [40]. Additionally, the beat-to-beat variations with diminished cardiac output present in AF may cause transient or chronic cerebral hypo-perfusion [41], all contributing to cognitive decline.
Type-D personality is distinguished by either negative affectivity or social inhibition. In this work, approximately one third (32%) of the enrolled patients had type-D personality. Earlier studies [42, 43] recorded comparable prevalence (30.1–32%) of type-D personality among AF population. It was hypothesized that the chronic stress, which characterized individuals with type-D personality, could stimulate the sympathetic activation and promote more arousal symptoms (tachycardia, sweating) [44]. Type-D personality has been listed by the European Cardiovascular Prevention guideline as a fundamental psychosocial risk factor to screen for [45].
The current research revealed no significant correlations between different cardiac variables (EHERA, EF, LAD) and both HADS scores. In the same line, Pavlicek et al. [46] noted that EHRA did not link significantly to the mean values of anxiety and depression scores. On the contrary, Watkins et al. [47] concluded that HADS-A subscale was associated with left ventricular EF. Also, Wang et al. [48] found that anxiety had significant association with LAD and suggested that AF-caused anxiety is arbitrated by poor quality of life as AF patients are usually marked by discomfortable symptoms, limited daily activities, complicated treatment, and probable upsetting complications. The discrepancy between various studies may be due to different sample size, selection criteria, and/or age categories.
Regression analysis of our results showed that the age and both physical functioning and general health components of SF-36 were the main predictors affecting HADS. Similar to these findings, Polikandrioti et al. [35] noted that HADS-D were statistically significantly correlated with age among AF patients. Also, Younes et al. [49] reported a significant correlation between both anxiety and depressive symptoms and HRQoL. As well, Özabacı et al. [50] revealed that overall HRQoL scores significantly predict depression scores. It is assumed that unhealthy life style, reduced physical activity, deprived incomes, sense of loneliness, despair, and not being a part of society increasing risk of depression. Hence, quality of life assessment is strongly recommended during the assessment of depression and anxiety especially for the elderly as well.
In the present study, LAD was negatively correlated with cognitive functions (MMSE), which was consistent with both earlier and recent studies [51, 52], as higher LAD measurements were significantly correlated with lower MMSE scores. This was additionally confirmed by the linear regression analysis in our study, which identified LAD and age as the two prominent predictors impacting cognitive functions. Consequently, early cognitive assessment is strongly recommended for all AF patients with moderate-severe enlarged left atrium (with LAD > 4.6 cm) chiefly among old-aged adults as they are most likely to have worsen MMSE scores. In partial agreement to the current findings, Alonso et al. [38] revealed a significant correlation between age and cognitive functions in AF patients.
The literatures revealed conflicting results regarding the association between personality-type and cardiovascular disease. Some studies [42, 53], in line with the current one, did not underscore any significant correlation between type-D personality and cardiovascular risk. Conversely, others [54] concluded that type-D personality was significantly associated with arousal symptoms. Biological factors and health-related behaviors are deemed to take part in the association between type-D personality and the progression of cardiovascular diseases. The adherence to medication, lifestyle modification, and post-event cardiac rehabilitation are essential prerequisites to prevent disease progression during the long-term follow-up of cardiac patients and all these issues may be held up by the distressed personality. Enrolling older patients in the latter study [54] and selecting cases with lone AF (common in older > 60 years, associated with cardiomyopathy), not NVAF like ours, may explain this contradiction between results of the two studies.
There is a great controversy regarding the effect of various factors on HRQoL in AF patients. Previous studies [55,56,57], in agreement to our results, reported that EF has a major effect on SF-36 (physical function and vitality). Additionally, our work showed that LAD has significant correlations with SF-36 (physical function and role of limitation due to physical health), whilst EHRA has no such correlation with any of the SF36 items. On the contrary, other studies [58,59,60] established that AF patients with higher EHRA scores were most likely to have lower HRQoL as fear of complications associated with treatment, such as bleeding, can isolate patients from social activities and lead to a decreased HRQoL and increase cardiac symptoms [61]. This discrepancy in the results could be related to different age categories, and dissimilar methodology using various questionnaire in assessing HRQoL in the previous series.
Despite the study failed to catch any psychiatric predictor that could affect cardiac symptoms considerably, clinical co-morbidities were identified as the significant predictors affecting AF severity (EHRA score) in our patients, while special habits were the second ones. This may shed light on the possibility that the com-morbid psychiatric symptoms in our sample were the consequence rather than the cause of AF, but this should be regarded with caution for further future confirmation. In the same line, recent researches [62] emphasized on the importance of optimization and controlling of all coexisting medical conditions that can worsen AF, such as hyperthyroidism, DM, HTN, congestive heart failure, stimulant use, and excessive alcohol consumption. Likewise, other genetic, environmental, ethnic, and racial factors may also play a crucial role in the pathogenesis of AF and should be considered in future evaluations.
It is worth mentioning that recent studies highlighted that substantial proportion of AF patients may experience early unrecognized or non-specific intermittent symptoms (fatigue, dyspnea, tightness) for long period (up to 1 year) before seeking cardiac consultation and attributes their symptoms to overwork, inadequate sleep, and/or deconditioning [63, 64]. This may shed light on the possible delay in confirming AF diagnosis particularly with the lack of regular routine check-up in our developing countries and that most of our patients regard their initial chaotic non-specific symptoms as not very serious and amenable to self-management.
Limitations
This study should be considered in the background of some limitations such as its cross-sectional design. The study could be more valuable if we could do follow-up of the cases to evaluate the long-term effect of NVAF on psychiatric symptoms and vice versa. This could be regarded in future longitudinal studies on a larger sample size to confirm our findings. In addition, further trials are essential to elucidate the benefits of using cognitive enhancement therapy and antidepressants in improving cognitive abilities and psychiatric symptoms as well as quality of life among NVAF patients.
We may be criticized for the absence of a control group including healthy participants to differentiate the age-related psychiatric and cognitive troubles from those potentially induced by AF. As the study excluded patients who had previous (pre-AF) history of dementia, psychiatric troubles or any organic illness affecting cognition as well as the more advanced age groups (> 65 years; who are usually more prone to the normal age-related cognitive changes [65, 66]), we assume that the psychiatric troubles detected in our sample were related to AF. Salthouse [65] conducted a comprehensive analysis to delineate the patterns (trends) of normal age-related cognitive changes among apparently healthy adults to introduce a reference guide for distinguishing the abnormal (pathological) cognitive decline from those of normal aging. He revealed that the main age-related cognitive decline in reasoning and memory most often occurs after the age of 65 years.
Another shortcoming across several studies (including the present one) is related to the use of MMSE as a standalone single test for cognitive assessment, as the use of inconsistent cut-off points (to differentiate between the normal and cognitively-impaired participants) is likely to compromise comparability and generalizability of the findings across different studies. So, adding another screening tool for cognitive assessment could be more helpful in this regard and will be one of our future plan.
Conclusions
Considerable number of our NVAF patients had psychiatric symptoms and mild cognitive impairment that should not be ignored to avoid the possible detrimental complications. So, the “consultation-liaison psychiatry” for assessing anxiety, depression, cognitive ability, and HRQOL is fundamental for designing an appropriate intervention plan to address mental burdens of patients living with NVAF. Old-adult AF patients with enlarged LAD are of greater risk to develop cognitive impairment, hence they requires early and precise psychiatric attention. Psychiatric staff members are supposed to be a part of a multidisciplinary team-work together with the cardiologists for early prevention and treatment of any cognitive or psychiatric complications that might associate NVAF.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Change history
08 February 2023
A Correction to this paper has been published: https://doi.org/10.1186/s43045-023-00292-7
Abbreviations
- AF:
-
Atrial fibrillation
- DM:
-
Diabetes mellitus
- EF:
-
Ejection fraction
- EHRA:
-
The Modified European Heart Rhythm Association
- HADS:
-
Hospital Anxiety and Depression Scales
- HADS-A:
-
HADS-anxiety subscale
- HADS-D:
-
HADS-depression subscale
- HRQoL:
-
Health-related quality of life assessment
- HTN:
-
Hypertension
- LAD:
-
Left atrial dimension
- MMSE:
-
Mini–Mental State Examination
- NVAF:
-
Non-valvular AF
- SF-36:
-
Short form-36 health survey
References
De Hert M, Detraux J, Vancampfort D (2018) The intriguing relationship between coronary heart disease and mental disorders. Dialogues Clin Neurosci 20(1):31–40
Khayyam-Nekouei Z, Neshatdoost H, Yousefy A, Sadeghi M, Manshaee G (2013) Psychological factors and coronary heart disease. ARYA Atherosclerosis 9(1):102
Jones EA, Mitra AK, Bhuiyan AR (2021) Impact of COVID-19 on mental health in adolescents: a systematic review. Int J Environ Res Public Health 18(5):2470
Fenger-Grøn M, Vestergaard CH, Ribe AR, Johnsen SP, Frost L, Sandbæk A et al (2021) Association between bipolar disorder or schizophrenia and oral anticoagulation use in Danish adults with incident or prevalent atrial fibrillation. JAMA Netw Open 4(5):e2110096–e2110096
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP et al (2019) American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 139(10):e56–e528. https://doi.org/10.1161/CIR.0000000000000659. (Erratum in: Circulation. 2020 Jan 14;141(2):e33.)
Wu J, Zhang Y, Liao X, Lei Y (2020) Anticoagulation therapy for non-valvular atrial fibrillation: a mini-review. Front Med 7:350
PenelaMaceda D, Berruezo A (2019) Atrial fibrillation: not just an electric and single organ disease. Eur J Prev Cardiol 26(2):185–186
Nef H, Möllmann H, Akashi Y, Hamm C (2010) Mechanisms of stress (Takotsubo) cardiomyopathy. Nat Rev Cardiol 7(4):187–193. https://doi.org/10.1038/nrcardio.2010.16
Islam MM, Poly TN, Walther BA, Yang HC, Wu CC, Lin MC et al (2019) Association between atrial fibrillation and dementia: a meta-analysis. Frontiers in Aging Neuroscience 11:305
Lane DA, Langman CM, Lip GY, Nouwen A (2009) Illness perceptions, affective response, and health-related quality of life in patients with atrial fibrillation. J Psychosom Res 66(3):203–210
Leon BM, Maddox TM (2015) Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes 6(13):1246
Gleason KT, Nazarian S, Himmelfarb CRD (2018) Atrial fibrillation symptoms and sex, race, and psychological distress: a literature review. J Cardiovasc Nurs 33(2):137
Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener HC et al (2007) Outcome parameters for trials in atrial fibrillation: recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NET work and the European Heart Rhythm Association. Europace 9:1006–1023
Freeman JV, Simon DN, Go AS, Spertus J, Fonarow GC, Gersh BJ et al (2015) Association between atrial fibrillation symptoms, quality of life, and patient outcomes: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circ Cardiovasc Qual Outcomes 8(4):393–402
Albanna M, Yehya A, Khairi A, Dafeeah E, Elhadi A, Rezgui L et al (2017) Validation and cultural adaptation of the Arabic versions of the Mini-Mental Status Examination - 2 and Mini-Cog test. Neuropsychiatr Dis Treat 13:793–801
Folstein MF, Folstein SE, McHugh PR, Fanjiang G (2001) Mini-Mental State Examination User’s Guide. Psychological Assessment Resources, Odessa
O’Bryant SE, Humphreys JD, Smith GE et al (2008) Detecting dementia with the Mini-Mental State Examination in highly educated individuals. Arch Neurol 65(7):963–967
Tischer TS, Nitschke D, Krause I, Kundt G, Öner A, D’Ancona G et al (2019) Prevalence and progression of cognitive impairment in atrial fibrillation patients after treatment with catheter ablation or drug therapy. Cardiol Res Pract 2019:7216598. https://doi.org/10.1155/2019/7216598
Ciesielska N, Sokołowski R, Mazur E, Podhorecka M, Polak-Szabela A, Ke˛dziora-Kornatowska K, (2016) Is the Cardiology Research and Practice 7 montreal cognitive assessment (MoCA) test better suited than the mini-mental state examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis Psychiatria Polska 50(5):1039–1052
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67(6):361–370
El-Rufaie OE, Absood G (1987) Validity study of the Hospital Anxiety and Depression Scale among a group of Saudi patients. Br J Psychiatry 151(5):687–688
Denollet J (2005) DS14: standard assessment of negative affectivity, social inhibition, and type D personality. Psychosom Med 67:89–97
Garratt AM, Ruta DA, Abdalla MI, Russell IT (1994) SF 36 health survey questionnaire: II. Responsiveness to changes in health status in four common clinical conditions. Qual Health Care 3(4):186–192
Al-Ghamdi MS, Taha AZ, Ahmad B, Khalil MS (2002) Quality of life in a sample of hypertensive patients attending primary health care facilities in Al-khobar. Saudi Arabia J Family Community Med 9(1):25–32
Verdecchia P, Angeli F, Reboldi G (2018) Hypertension and atrial fibrillation: doubts and certainties from basic and clinical studies. Circ Res 122(2):352–368
Herrmann-Lingen C, Wachter R (2021) Psychological stress and incidence of atrial fibrillation. Eur J Prev Cardiol 28(6):631–632
Szymanski FM, Filipiak KJ, Karpinski G, Platek AE, Opolski G (2014) Occurrence of poor sleep quality in atrial fibrillation patients according to the EHRA score. Acta Cardiol 69:291–296
Gallo WT, Bradley EH, Falba TA, Dubin JA, Cramer LD, Bogardus ST et al (2004) Involuntary job loss as a risk factor for subsequent myocardial infarction and stroke: findings from the Health and Retirement Survey. Am J Ind Med 45(5):408–416
Balcı KG, Balcı MM, Canpolat U, Şen F, Akboğa MK, Süleymanoğlu M et al (2016) Comparison of health-related quality of life among patients using novel oral anticoagulants or warfarin for non-valvular atrial fibrillation. Anatol J Cardiol 16(7):474–481
Ho JY, Hendi AS (2018) Recent trends in life expectancy across high income countries: retrospective observational study. BMJ 362:k2562
Kapil U, Khandelwal R, Ramakrishnan L, Khenduja P, Gupta A, Pandey RM et al (2018) Prevalence of hypertension, diabetes, and associated risk factors among geriatric population living in a high-altitude region of rural Uttarakhand, India. J Family Med Prim Care 7:1527–1536
Craig L, Mullan K (2010) Parenthood, gender and work-family time in the United States, Australia, Italy, France, and Denmark. J Marriage Fam 72:1344–1361
Thrall G, Lip GY, Carroll D, Lane D (2007) Depression, anxiety, and quality of life in patients with atrial fibrillation. Chest 132(4):1259–1264
Thompson TS, Barksdale DJ, Sears SF, Mounsey JP, Pursell I, Gehi AK (2014) The effect of anxiety and depression on symptoms attributed to atrial fibrillation. Pacing Clin Electrophysiol 37(4):439–446
Polikandrioti M, Koutelekos I, Vasilopoulos G, Gerogianni G, Gourni M, Zyga S et al (2018) Anxiety and depression in patients with permanent atrial fibrillation: prevalence and associated factors. Cardiol Res Pract 2018:7408129
Gehi AK, Sears S, Goli N, Walker TJ, Chung E, Schwartz J et al (2012) Psychopathology and symptoms of atrial fibrillation: implications for therapy. J Cardiovasc Electrophysiol 23(5):473–478
Garg PK, O’Neal WT, Diez-Roux AV, Alonso A, Soliman EZ, Heckbert S (2019) Negative affect and risk of atrial fibrillation: MESA. J Am Heart Assoc 8(1):e010603
Alonso A, Arenas de Larriva AP (2016) Atrial fibrillation, cognitive decline and dementia. Eur Cardiol 11(1):49–53
Hu YF, Chen YJ, Lin YJ, Chen SA (2015) Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol 12(4):230–243
Chen LY, Lopez FL, Gottesman RF, Huxley RR, Agarwal SK, Loehr L et al (2014) Atrial fibrillation and cognitive decline-the role of subclinical cerebral infarcts: the atherosclerosis risk in communities study. Stroke 45(9):2568–2574
Gardarsdottir M, Sigurdsson S, Aspelund T, Rokita H, Launer LJ, Gudnason V et al (2018) Atrial fibrillation is associated with decreased total cerebral blood flow and brain perfusion. Ep Europace 20(8):1252–1258
Jeon SW, Lim HE, Yoon S, Na KS, Ko YH, Joe SH et al (2017) Does type D personality impact on the prognosis of patients who underwent catheter ablation for atrial fibrillation? A 1-year follow-up study. Psychiatry Investig 14(3):281–288
Son YJ, Song EK (2012) The impact of type D personality and high-sensitivity C-reactive protein on health-related quality of life in patients with atrial fibrillation. Eur J Cardiovasc Nurs 11:304–312
Hering D, Lachowska K, Schlaich M (2015) Role of the sympathetic nervous system in stress-mediated cardiovascular disease. Curr Hypertens Rep 17(10):80
Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG et al (2017) Decline in cardiovascular mortality: possible causes and implications. Circ Res 120(2):366–380
Pavlicek V, Wedegärtner SM, Millenaar D, Wintrich J, Böhm M, Kindermann I et al (2022) Heart-focused anxiety, general anxiety, depression and health-related quality of life in patients with atrial fibrillation undergoing pulmonary vein isolation. J Clin Med 11(7):1751
Watkins LL, Koch GG, Sherwood A, Blumenthal JA, Davidson JR, O’Connor C et al (2013) Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. J Am Heart Assoc 2(2):e000068
Wang Z, Qin H, Chen G, Dai Y, Cai Y, Cheng X et al (2020) Anxiety is associated with increased risk for atrial cardiopathy. Acta Neurol Belg 120(6):1383–1388
Younes O, Amer R, Fawzy H, Shama G (2019) Psychiatric disturbances in patients undergoing open-heart surgery. Middle East Current Psychiatry 26(1):1–7
Özabacı N (2010) Quality of life as a predictor of depression. Procedia Soc Behav Sci 2(2):2458–2463
Alosco ML, Gunstad J, Jerskey BA, Clark US, Hassenstab JJ, Xu X et al (2013) Left atrial size is independently associated with cognitive function. Int J Neurosci 123(8):544–552
Tang J, Cheng X (2020) Analysis of risk factors associated with cognitive dysfunction in patients with atrial fibrillation. Yangtze Medicine 4:319–330
Grande G, Romppel M, Vesper JM, Schubmann R, Glaesmer H, Herrmann-Lingen C (2011) Type D personality and all-cause mortality in cardiac patients–data from a German cohort study. Psychosom Med 73(7):548–556
Kupper N, van den Broek K, Haagh E, van der Voort P, Widdershoven J, Denollet J (2018) Type D personality affects health-related quality of life in patients with lone atrial fibrillation by increasing symptoms related to sympathetic activation. J Psychosom Res 115:44–52
Reddy YNV, Rikhi A, Obokata M, Shah SJ, Lewis GD, AbouEzzedine OF et al (2020) Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail 22(6):1009–1018
van den Berg MP, Hassink RJ, Tuinenburg AE, van Sonderen EF, Lefrandt JD, de Kam PJ et al (2001) Quality of life in patients with paroxysmal atrial fibrillation and its predictors: importance of the autonomic nervous system. Eur Heart J 22(3):247–253
Dorian P, Paquette M, Newman D, Green M, Connolly SJ, Talajic M et al (2002) Quality of life improves with treatment in the Canadian Trial of Atrial Fibrillation. Am Heart J 143(6):984–990
Witassek F, Springer A, Adam L, Aeschbacher S, Beer JH, Blum S et al (2019) Swiss-AF study investigators. Health-related quality of life in patients with atrial fibrillation: the role of symptoms, comorbidities, and the type of atrial fibrillation. PLoS One 14(12):e0226730
Son YJ, Baek KH, Lee SJ, Seo EJ (2019) Health-related quality of life and associated factors in patients with atrial fibrillation: an integrative literature review. Int J Environ Res Public Health 16(17):3042
Theunissen LJHJ, Cremers HP, van Veghel D, van der Voort PH, Polak PE, de Jong SFAMS et al (2022) Age-dependency of EHRA improvement based on quality of life at diagnosis of atrial fibrillation. J Arrhythm 38(1):50–57
Charitakis E, Barmano N, Walfridsson U, Walfridsson H (2017) Factors predicting arrhythmia-related symptoms and health-related quality of life in patients referred for radiofrequency ablation of atrial fibrillation: an observational study (the SMURF Study). JACC Clin Electrophysiol 3(5):494–502
Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S et al (2020) Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med 382(1):20–28
Wilson RE, Rush KL, Hatt L, Reid RC, Laberge CG (2020) The symptom experience of patients with atrial fibrillation before their initial diagnosis. J Cardiovasc Nurs 35(4):347–357
Ladwig KH, Goette A, Atasoy S, Johar H (2020) Psychological aspects of atrial fibrillation: a systematic narrative review: impact on incidence, cognition, prognosis, and symptom perception. Curr Cardiol Rep 22(11):137
Salthouse TA (2019) Trajectories of normal cognitive aging. Psychol Aging 34(1):17–24
Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D et al (2018) Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 90(3):126–135
Acknowledgements
We would like to express our deep gratitude to the entire medical team in Cardiology and Neuropsychiatry Departments at Tanta University Hospital who helped in managing the patients. We also express our appreciation to our participated subjects and their families.
Funding
This study was self-funded. No funds were received.
Author information
Authors and Affiliations
Contributions
MY: Literature search, psychiatric assessment of all participants, data acquisition, data analysis, statistical analysis, manuscript preparation and first draft. R. A: Conception and design of the work, literature search, reviewing data analysis, reviewing the statistical analysis, manuscript writing and reviewing. HE: Conception and design of the work, literature search, cardiology assessment of the participants, reviewing data analysis and statistical analysis. GS: Conception and design of the work, reviewing data analysis, reviewing the statistical analysis, and critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethical Research Committee of the Faculty of Medicine, Tanta University, under the code 34283/11/20. An informed written consent was obtained from all participants to be included in this study.
Consent for publication
Each of the participants was given a code number, and his anonymity was preserved (his individual person’s data was omitted and not included in the submitted manuscript).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: “Authors' contribution statement has been updated”.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yossef, M., Amer, R., Elsokkary, H. et al. Psychiatric symptoms in patients with non-valvular atrial fibrillation. Middle East Curr Psychiatry 29, 105 (2022). https://doi.org/10.1186/s43045-022-00268-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43045-022-00268-z