Abstract
Background
Over recent years, spontaneous coronary artery dissection (SCAD) has emerged as a no longer rare cause of acute coronary syndrome (ACS). On the other hand, coronary artery spasm (CAS) is the main cause of ischemic heart disease with non-obstructive coronary lesions. Clinical manifestations of both vary from stable angina to ACS or, rarely, sudden cardiac death. These entities may be underdiagnosed on a coronary angiography.
Case presentation
We report the case of a young woman presenting with acute chest pain and no coronary risk factors. Angiography revealed a focal subcritical stenosis of the right coronary artery. Coronary wiring resulted in diffuse and critical spasm. However, optical coherence tomography (OCT) and intravascular ultrasound (IVUS) showed extensive SCAD. She was therefore treated conservatively. On the fourth day, cardiac computed tomography angiography (CCTA) excluded disease progression, and then she was discharged on medical therapy.
Conclusions
Combined IVI plays a vital role in providing accurate and detailed visualization of the coronary anatomy and thus allowing for more precise diagnosis, risk stratification, and treatment planning. CCTA can be considered a valuable tool in the noninvasive follow-up of SCAD.
Similar content being viewed by others
Background
SCAD is defined as a non-traumatic, non-atherosclerotic separation within the epicardial coronary artery wall leading to the development of a false lumen (FL) and eventually an intramural hematoma (IMH). Blood flow in the true lumen (TL) can be reduced by external compression, resulting in clinical manifestations [1]. CAS is a reversible vasoconstriction that narrows the lumen of normal or atherosclerotic coronary arteries, impairing myocardial blood flow.
Recent estimates suggest that SCAD accounts for between 2 and 4% of all ACS cases, predominantly affecting young women [1]. However, CAS was estimated at 50% in patients presenting with angina and 57% in those presenting with ACS [2].
To date, few reports have described an association between these two conditions [3, 4], and to the best of our knowledge, this is the first case with a comprehensive intravascular study (IVUS and OCT) demonstrating their simultaneous presence in the same vascular territory.
Case presentation
A 36-year-old woman presented to the emergency department with acute, retrosternal pain. She reported similar episodes over the past month and had experienced a syncopal episode following a bereavement event. She had no coronary risk factors. She had given birth more than two years previously.
Electrocardiography and echocardiography were within normal limits. Laboratory tests showed an elevated troponin level (1576 ng/ml, n.v. <11). The patient was referred to the catheter laboratory with a diagnosis of non-ST elevation acute myocardial infarction. Aspirin and loading dose of ticagrelor were administered.
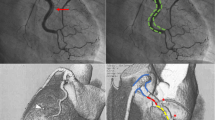
The left coronary angiogram showed unobstructed coronary arteries (Fig. 1a). The right coronary cannulation, however, drew more attention for a slight damping after ostium engagement and the subsequent angiogram showed a mild stenosis at the end of the first tract (Fig. 1b). Immediately after wiring with Runthrough TM NS Floppy, we observed extensive spasm unresponsive to intracoronary nitrates (Fig. 1c). IVUS showed a large amount of organized cloth in contact with the adventitia surrounding the probe and sparse echo-free spaces (Fig. 2a). OCT documented increased vessel diameters due to SCAD and IMH, which compressed the true lumen, spearing the ostium (Fig. 2b–f). We decided to adopt a supportive and watchful waiting strategy with prescription of acetylsalicylic acid, beta-blocker, and anxiolytics. Serial cardiac enzyme measurements showed gradually decreasing levels. On the fourth night of hospitalization, she had a sudden onset chest pain associated with ST segment elevation in the inferior leads. Intravenous nitrates were administered with prompt resolution of symptoms and ECG. The following day, CCTA excluded disease progression (Fig. 3). During the remaining stay, she was always asymptomatic and hemodynamically stable performing a two-week rehabilitation cycle. The patient was discharged with acetylsalicylic acid and beta-blocker on the twenty-third day of hospitalization, when angiography showed SCAD partial healing (Fig. 4). At six months of follow-up, she reported excellent quality of life and absence of chest pain.
IVI of SCAD and CAS: a IVUS shows a crescent-shaped cloth (white stars). b–e OCT highlights the dissection (b) separating the TL from the FL; probable fenestration (c, white arrow); the “shuriken effect” of the TL compressed by IMH and CAS with reduced light penetration (light blue arrows) (d); the volume reduction of IMH at RCA para-ostium (e). Longitudinal view (f) showing IMH distribution
Conclusions
This case highlights the limits of angiography and the crucial role of combined IVI in the early diagnosis of SCAD (which can sometimes be masked by other conditions, such as CAS), the management of which significantly diverges from that of atherosclerotic ACS or vasospastic angina. It also highlights the importance of close inpatient monitoring due to the risk of disease extension and complications for which there are no clear predictors. Finally, CCTA may be the ideal surveillance tool for follow-up, especially in conservatively managed patients, as it is a noninvasive, low-risk study.
In our case, angiography showed nonspecific images that could have been ignored or mistaken for subcritical atherosclerotic lesions. IVUS immediately ruled out CAD and showed a large amount of organized IMH in contact with the adventitia, extending over almost the entire probe run (Fig. 2a). At this point, some doubts remained: Could we definitively rule out the possibility of intraluminal thrombus? Was the course of the guidewire completely in the TL? Furthermore, could we exclude the presence of intimal tears, especially near the RCA ostium? Subsequent imaging with OCT confirmed the diagnosis of SCAD and superimposed CAS: The classic crescentic semi-lunar FL compressed the TL in a star-shaped image that we called “Shuriken effect” (Fig. 2d, the famous ninja weapon). The entire intima-lumen interface was unaffected by thrombotic formations, showing only in the second tract some continuity solutions (the so-called “fenestrations,” Fig. 2c) connecting the FL with the TL. Finally, the OCT pullback clearly depicted a progressive reduction of the IMH volume until its complete disappearance about 5 mm from the RCA ostium (Fig. 2e), reassuring us of the possibility of SCAD extension into the ascending aorta.
Considering both the clinical status (patient was asymptomatic and hemodynamically stable) and anatomical parameters (peri-ostium sparing, thrombolysis in myocardial infarction flow 3 and absence of intraluminal thrombus), we decided to adopt a conservative approach with close monitoring. Currently, based on expert opinion, overall conservative treatment is the preferred management strategy in SCAD patients, unless revascularization is mandated (i.e., in the case of ongoing ischemia, hemodynamic instability, or left main coronary dissection) [1]. In fact, percutaneous coronary intervention is associated with high failure rates and poor outcomes in the setting of compromised arterial wall integrity [5,6,7]; furthermore, in most cases (70% to 90%) the natural history of SCAD appears to be spontaneous healing within 4 to 6 weeks [5], with subsequent architectural changes that may lead to late stent malapposition or graft failure [8].
Although the prognosis for long-term survival is favorable [1, 7], these patients are at high risk of chronic angina, coronary spasm, and SCAD recurrence [9]. In this scenario, the risk-benefit ratio must be carefully assessed before repeating CAG, and CCTA has been proposed as a valid noninvasive alternative to CAG. Although there is a possibility of missing distal small-vessel disease, CCTA may conversely be of greater benefit in patients with large vessel proximal SCAD, as in our case [1, 10]. Therefore, we opted for noninvasive delayed imaging with CCTA during the patient’s unique in-hospital anginal episode. This imaging methodic clearly depicted the dissection (Fig. 3), allowing us to definitively exclude the possibility of local disease extension, right coronary sinus involvement, or disease progression to other territories, and to identify CAS as the precipitating event.
Among the precipitating factors of ACS, SCAD is a unique entity that still has several “gray areas” in clinical practice. Conversely, CAS is a transient condition, often associated with SCAD, that may mimic some features of the latter, leading to a misleading diagnosis. Our findings suggest that combined IVI may help to clarify these scenarios, by providing unique and specific insights into the most relevant morphologic changes of the vessel, improving diagnostic accuracy and guiding the management strategy. Finally, CCTA represents a noninvasive and effective way to reassess SCAD lesions and to make a differential diagnosis with CAS, moreover in medically managed patients.
Availability of data and materials
Raw data for images are not publicly available to preserve individuals’ privacy under the European General Data Protection Regulation.
Abbreviations
- ACS:
-
Acute coronary syndrome
- CAS:
-
Coronary artery spasm
- CCTA:
-
Cardiac computed tomography angiography
- FL:
-
False lumen
- IMH:
-
Intramural hematoma
- IVI:
-
Intravascular imaging
- IVUS:
-
Intravacular ultrasound
- OCT:
-
Optical coherence tomography
- SCAD:
-
Spontaneous coronary artery dissection
- TL:
-
True lumen
References
Adlam D, Alfonso F, Maas A, Vrints C, Writing Committee (2018) European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J 39(36):3353–3368
Hung M-J, Cherng W-J, Cheng C-W, Li L-F (2006) Comparison of serum levels of inflam-matory markers in patients with coronary vasospasm without significant fixed coronary artery disease versus patients with stable angina pectoris and acute coronary syndromes with sig-nificant fixed coronary artery disease. Am J Cardiol 97(10):1429–1434
Chung H, Lee SJ, Park JK, Choi IS, Won HY, Kim S, Cha JJ et al (2013) Spontaneous coronary artery dissection mimicking coronary spasm diagnosed by intravascular ultrasonography. Korean Circ J 43(7):491–496
Tsujita K, Miyazaki T, Kaikita K, Chitose T, Takaoka N, Soejima H et al (2012) Premenopausal woman with acute myocardial infarction caused by spontaneous coronary artery dissection and potential association with coronary vasospasm. Cardiovasc Interv Ther 27(2):121–126
Main A, Saw J (2019) Percutaneous coronary intervention for the treatment of spontaneous coronary artery dissection. Interv Cardiol Clin 8(2):199–208
Tweet MS, Kok SN, Hayes SN (2018) Spontaneous coronary artery dissection in women: what is known and what is yet to be understood. Clin Cardiol 41(2):203–210
Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ et al (2012) Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 126(5):579–588
Tweet MS, Eleid MF, Best PJ, Lennon RJ, Lerman A, Rihal CS et al (2014) Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 7:777–786
Rogowski S, Maeder MT, Weilenmann D, Haager PK, Ammann P, Rohner F et al (2017) Spontaneous coronary artery dissection: angiographic follow-up and long-term clinical outcome in a predominantly medically treated population. Catheter Cardiovasc Interv 89:59–68
Pristera N, Chaudhury P, Van Iterson EH, Cho LS (2021) Spontaneous coronary artery dissection: Principles of management. Cleve Clin J Med 88(11):623–630
Acknowledgements
I would like to express my deepest gratitude to all those who have contributed to this scientific work. Particularly, I am thankful to all the colleagues who provided insightful comments, feedback, and suggestions.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
PD and DR contributed to conceptualization, writing-original draft preparation, and writing—review and editing; VP contributed to resources-literature search; and PC supervised the study and contributed to project administration. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study did not require ethical approval due to its observational retrospective nature.
Consent for publication
Written informed consent was collected at the time of admission.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Desario, P., Rutigliano, D., Palumbo, V. et al. Uncovering a hidden danger: a case report of diffuse coronary spasm concealing spontaneous coronary artery dissection. Egypt Heart J 76, 83 (2024). https://doi.org/10.1186/s43044-024-00514-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-024-00514-1