Abstract
Background
Cardiac myxomas present a diagnostic challenge due to their ability to mimic various cardiovascular and systemic conditions. Timely identification is crucial for implementing surgical intervention and averting life-threatening complications.
Case presentation
We reported the case of a 49-year-old male patient who presented sudden legs weakness and slurred speech and was admitted 10 h later in emergency department. Physical examination was significant for paraparesis and paraphasia. Cardiac and carotid auscultation was normal. CT brain revealed multiple acute ischemic strokes and MRA was suggestive of cerebral vasculitis. As pre-therapy assessment, the EKG revealed no electrical abnormalities and the chest X-ray showed signs of left atrial enlargement. Transthoracic and transesophageal echocardiography showed a left atrial mass attached to the interatrial septum, measuring 9*5*4 cm and extending into the left ventricular cavity during diastole, which suggested the diagnosis of left atrial myxoma. The patient was referred for open-heart surgery and histopathological examination confirmed the diagnosis of myxoma. The patient weaned off from cardiopulmonary bypass and the postoperative period was uneventful.
Conclusion
We reported an interesting case with an unusual and misleading neurological presentation of a cardiac myxoma. The unpredictability of serious complications occurrence must awaken our medical flair, for an early diagnosis among a long list of differentials.
Similar content being viewed by others
Background
Cardiac myxomas present a considerable diagnostic challenge in medical practice. These tumors, primarily originating from the endocardium, can masquerade as various cardiovascular and systemic conditions, complicating their timely diagnosis and management [1]. Literature underscores the importance of a high index of suspicion for cardiac myxomas, particularly in patients with unexplained cardiac or systemic symptoms, in order to provide surgical treatment and prevent life-threatening complications [1].
Case presentation
We reported the case of a 49-year-old male patient with no cardiovascular risk factors or medical history, who experienced sudden onset of leg weakness and slurred speech. He presented to the emergency department 10 h later, with a heart rate of 100 bpm and blood pressure of 130/85 mmHg in both arms. Physical examination revealed paraparesis and paraphasia. Cardiac and carotid auscultation was normal.
CT brain showed multiple acute ischemic strokes. MRA revealed frontal, occipital and cerebellar white matter signal abnormalities suggestive of cerebral vasculitis (Fig. 1). The patient was subsequently transferred to the internal medicine department for further investigations and vasculitis treatment.
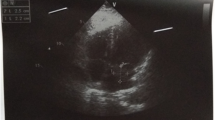
Pre-therapy assessments included an EKG which revealed a coronary sinus rhythm without electrical abnormalities (Fig. 2), a chest X-ray which showed cardiomegaly with a double density sign and splaying of the carina indicative of left atrial enlargement (Fig. 3), and a transthoracic echocardiography (TTE) which revealed a mobile and heterogeneous mass measuring 9*5*4 cm in the left atrium, extending into the left ventricular cavity during diastole without significant inflow obstruction across the mitral valve (Fig. 4). Transesophageal echocardiography (TOE) confirmed that the mass was attached to the interatrial septum at the site of the fossa ovalis, consistent with a diagnosis of left atrial myxoma (Fig. 5).
The patient underwent preoperative supra-aortic trunk echo-Doppler and coronary calcium scan which were unremarkable.
Laboratory examinations revealed microcytic and hypochromic anemia (hemoglobin: 10 g/dL) and elevated inflammatory biomarkers (C-reactive protein: 39 mg/L, ferritinemia: 520 ng/mL), with no hydro-electrolyte disorder and normal liver and kidney functions. Serological tests for syphilis, HIV, VHB and VHC and autoimmune screening were negative.
The patient underwent open-heart surgery. An aorto-bicaval cannulation and a cardiopulmonary bypass were established. After left atriotomy, a giant tumor arising from the interatrial septum was resected, with cauterization of its implantation base in order to prevent local recurrence. The left atrium was closed, and the aortic cross-clamp was removed. The surgical intervention lasted 50 min (Fig. 6).
Macroscopically, the excised mass measured 9*4*4 cm and was soft-mulberry-shaped and friable, with gelatinous consistency and punctate hemorrhage on the outer surface (Fig. 7). Histopathological examination showed marked loose myxoid stroma with scattered spindle cells, but no mitotic figures or malignant features, confirming the diagnosis of myxoma (Fig. 8).
Postoperative TOE did not reveal an interatrial shunt. The patient was monitored in the ICU for 24 h. The postoperative period was uneventful. He was discharged home with long-term echocardiographic follow-up recommended. The patient reported complete neurological recovery one month later.
Discussion
Primary cardiac tumors are rare entities in medicine, with studies indicating an incidence ranging from 0.001% to 0.3% in non-selected populations.
Three-quarters of these tumors are benign, and 30% of benign cardiac tumors are represented by myxomas [2].
Myxomas are typically diagnosed between the ages of 30 and 60 years. They mostly develop in the left atrium, specifically from the interatrial septum at the fossa ovalis, compared to right atrial and ventricular locations [2].
Clinical features of cardiac myxomas are determined by their size, location, consistency, and mobility. Constitutional symptoms attributed to myxomas, such as fever, weight loss, and arthralgia, suggest systemic inflammatory responses that could extend to the vascular endothelium [3].
The understanding of cardiac myxomas extends beyond their local impact within the heart, with emerging considerations for systemic manifestations, including their potential association with cerebral vasculitis [4].
Studies have highlighted that cardiac myxomas can lead to ischemic acute cerebral strokes through embolic phenomena, either from thrombi formation on the tumor’s surface or by detachment of tumor fragments into the systemic circulation. These emboli may result in cerebral infarctions, and there is growing interest in whether myxomas could also trigger inflammatory responses affecting cerebral blood vessels, resembling aspects of vasculitis [5].
Diagnosing cardiac myxomas can be challenging. TTE is the primary imaging modality due to its high sensitivity, allowing the assessment of the tumor’s characteristics (morphology, size, mobility, and attachment site). Doppler evaluation assesses the hemodynamic consequences of the mass. Complementary TOE provides a more detailed evaluation, and cardiac MRI can be useful in establishing a differential diagnosis with other cardiac masses [5].
Currently, there is no effective medical treatment to stop myxomas growth, and surgical removal is necessary to prevent life-threatening complications [6].
The timing of surgery is controversial in patients with recent neurological ischemic injuries, due to the risk of bleeding conversion during cardiopulmonary bypass [7].
Surgical resection is optimized by inducing cardioplegic arrest to provide a motionless operative field and realize a piecemeal removal. Aortic cross-clamping helps minimize perioperative tumor dislodgement and massive embolization, particularly with papillary and friable myxomas.
The usual approach is via left atrial incision, posterior to interatrial groove to explore the tumor. In cases of giant myxomas or restricted left atrial access, the approach can be via right atriotomy. There is controversy regarding radical resection with excision of a full-thickness portion of the interatrial septum versus conservative excision, especially with sporadic myxomas [8].
Surgical excision has an excellent long-term prognosis. The overall risk of recurrence is 22% for Carney complex myxomas, 12% for familial myxomas, and only 1% to 3% for sporadic cases [9].
Although surgical resection is curative with a low recurrence rate, patients should undergo regular follow-up with serial TTE [10].
Conclusion
We presented an interesting case featuring an uncommon and misleading neurological presentation of cardiac myxoma.
This case underscores the critical need for heightened clinical suspicion and thorough diagnostic evaluation to achieve early recognition among a broad range of differential diagnoses.
Recognizing and promptly treating cardiac myxomas is imperative to prevent life-threatening complications.
Availability of data and materials
No datasets were generated or analyzed during the current study.
Abbreviations
- CT:
-
Computed tomography
- MRA:
-
Magnetic resonance angiography
- EKG:
-
Electrocardiogram
- TTE:
-
Transthoracic echocardiography
- TOE:
-
Trans esophageal echocardiography
- ICU:
-
Intensive care unit
- IL-6:
-
Interleukin-6
References
Griborio-Guzman AG, Aseyev OI, Shah H, Sadreddini M (2022) Cardiac myxomas: clinical presentation, diagnosis and management. Heart 108(11):827–833
Yuan SM, Yan SL, Wu N (2017) Unusual aspects of cardiac myxoma. Anatol J Cardiol 17(3):241–247
Fabijanic D, Carević V, Bečić T, Pešutić-Pisac V (2022) Cardiac myxoma: benign, but deadly disease. Med Ultrason 24(2):248–249
Yuan SM, Humuruola G (2015) Stroke of a cardiac myxoma origin. Rev Bras Cir Cardiovasc 30(2):225–234
Grebenc ML, Rosado-de-Christenson ML, Green CE, Burke AP, Galvin JR (2002) Cardiac myxoma: imaging features in 83 patients. Radiographics 22(3):673–689
Samanidis G, Khoury M, Balanika M, Perrea DN (2020) Current challenges in the diagnosis and treatment of cardiac myxoma. Kardiol Pol 78(4):269–277
Schaff HV, Mullany CJ (2000) Surgery for cardiac myxomas. Semin Thorac Cardiovasc Surg 12(2):77–88
Gür AK, Aykaç MC (2018) Surgical treatment of cardiac myxomas: a 23-case experience. Heart Surg Forum 21(5):E370–E374
Zhang S, Zhang Q, Yu H, Liu L, Sun R, Song X et al (2020) Neuroimaging characteristics and long-term prognosis of myxoma-related intracranial diseases. Neuroradiology 62(3):307–317
Sheng WB, Luo BE, Liu Y, Zhang H, Zou LJ et al (2012) Risk factors for postoperative recurrence of cardiac myxoma and the clinical managements: a report of 5 cases in one center and review of literature. Chin Med J (Engl). 125(16):2914–2918
Acknowledgements
Not applicable.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
SE, BE, ME and AB conceived the idea to report the case report and were responsible for manuscript’s writing. SA, AE, FN, NM and AB supervised and reviewed this work. The final paper had been reviewed and approved by all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed written consent was obtained from the patient to publish his clinical data.
Competing interests
All authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Mhadi, S., El Hajjaj, B., Benatmane, A. et al. Cardiac myxoma and cerebral vasculitis: Is there a link?. Egypt Heart J 76, 61 (2024). https://doi.org/10.1186/s43044-024-00488-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-024-00488-0