Abstract
Background
The association of homocysteine with coronary artery disease (CAD) has been explored previously with mixed findings. The present Systematic Review and Meta-Analysis (SRMA) has assessed the pooled estimate of association between homocysteine (Hcy) and CAD, and its variation over the period and geography.
Methods
Systematic literature search was done in PubMed, Scopus and Cochrane to identify the observational studies that have reported mean Hcy among cases (CAD) and control. The SRMA was registered in PROSPERO (ID-CRD42023387675).
Results
Pooled standardized mean difference (SMD) of Hcy levels between the cases and controls was 0.73 (95% CI 0.55–0.91) from 59 studies. Heterogeneity was high (I2 94%). The highest SMD was found among the Asian studies (0.85 [95% CI 0.60–1.10]), while the European studies reported the lowest SMD between the cases and controls (0.32 [95% CI 0.18–0.46]). Meta-regression revealed that the strength of association was increasing over the years (Beta = 0.0227, p = 0.048).
Conclusions
Higher homocysteine levels might have a significant association with coronary artery diseases, but the certainty of evidence was rated low, owing to the observational nature of the studies, high heterogeneity, and publication bias. Within the population groups, Asian and African populations showed a greater strength of association than their European and American counterparts, and it also increased over the years.
Similar content being viewed by others
Background
Cardiovascular disease (CVD) is a major public health and clinical problem across the world. Though mortality due to CVD is decreasing in developed countries, the proportion of deaths due to coronary heart disease (CHD) is increasing (of all the deaths among people over 35 years of age, around one-third are due to CHD) [1]. Over ninety percent of CAD events occur in individuals with at least one risk factor. Among the risk factors, few are non-modifiable, like increasing age, male gender, history of premature CAD among first-degree family members, and some genetic factors, while many are modifiable or preventable risk factors like hypertension, high fasting plasma blood glucose, obesity or overweight, physical inactivity, dyslipidemia (high low-density lipoprotein and low high-density lipoprotein) cigarette smoking, stress, suboptimal diet, etc. [2] But in recent years, studies have reported that known classical risk factors can explain only half to one-third of atherosclerotic vascular events [3]. Certain novel potential risk factors that are being studied and hypothesized are high-sensitivity C-reactive protein, lipoprotein (a), plasma fibrinogen, plasma homocysteine, plasminogen inhibitor type I, endogenous tissue plasminogen activator (tPA), estrogen deficiency, microalbuminuria, increased levels of the leukocyte enzyme myeloperoxidase, etc. [2, 3]
Homocysteine (Hcy) has been studied as an independent risk factor for vascular disease. Researchers across the world are interested in finding out its association with CAD, cerebrovascular disease, and peripheral vascular disease. Fasting plasma Hcy concentrations between 5 and 15 µmol/L are considered normal [3]. The correlation between hyperhomocysteinemia and atherosclerotic disease was first detected more than 50 years ago by McCully in 1969 [4]. One of the earlier meta-analysis done by Boushey et al. concluded that a 5 µmol/L increment in total homocysteine level and 0.5 µmol/L (20 mg/dL) increase in serum cholesterol elevate the similar risk of CAD [5]. Arnesen et al. observed a positive relationship between homocysteine and the risk of myocardial infarction in their large prospective study [6]. Schnyder G. et al. suggested that total homocysteine, along with age and gender, strongly predicts the severity of coronary artery disease and should be assessed for the CVD risk profile of patients as an independent cardiovascular marker [7]. On the contrary, a few studies did not find a significant association between homocysteine and coronary artery disease [8,9,10]. Thus, the studies have reported mixed findings in terms of the relationship between plasma homocysteine and cardiovascular disease. Preliminary systematic search showed that previously published SRMAs in 2008 [11] and 2022 [12] reported a significant association between Hcy and CHD. However, these analyses had the following limitations: The SRMA published in 2008 included studies representing population from North America and European regions only, undertook Medline and Cochrane database search only [11], while three primary database search is recommended for SRMAs [13]. The outcomes of the included studies were also diverse like any CHD event including CHD death, MI, revascularization procedures, CVD death or stroke [11]. In the 2022 SRMA, the search strategies (database specific), inclusion–exclusion criteria and the outcomes were not comprehensively defined and/or published. Publication bias was not assessed quantitatively, and outlier determination was not done [12]. Certainty of the evidence was not determined [11, 12]. Since studies considered different cut-off values of Hcy based on median, tertiles, quartiles or quintiles, risk ratio (RR) was estimated for each study based on assumed log-linear association between CHD risk and Hcy. So, actual RR of the studies was not used in meta-analysis. Based on the above critical gaps identified, we undertook the index meta-analysis to determine the association between Hcy and the CAD by adopting the following comprehensive and transparent methodology.
Methods
Systematic search
The index SRMA adhered to the PRISMA guidelines (Additional file 1: Table S1). The research question for the index SRMA was: What is the association between the plasma homocysteine level and the occurrence of coronary artery disease? which is elaborated in Additional file 1: Table S2. An extensive literature search was done in various databases: PubMed, Scopus and Cochrane. The authors also searched Google Scholar to find other related articles. The base search strategy was formulated for PubMed. The same search strategy was used for the rest of the database as per the required format (Additional file 1: Table S3). We also reviewed the references of the eligible articles and found more studies that could be included in this systematic review and meta-analysis.
Selection of study
After the removal of duplicates, screening of the studies was done based on the criteria elaborated in Additional file 1: Table S2. Titles and abstracts were screened for the outcome of interest, i.e., Acute Coronary Syndrome or Coronary Artery Disease, and the exposure of interest, i.e., total plasma homocysteine concentration. Through title and abstract screening, observational studies like cross-sectional, case–control and nested case–control studies were identified where the case group represents acute coronary syndrome (ACS) or CAD patients and the control group comprises healthy individuals or patients free from coronary artery disease and exposure, i.e., plasma homocysteine level, has been measured. This initial title-abstract screening of the studies was done independently by two reviewers (SVU and AG). Full-text articles of these short-listed studies were retrieved and again screened by the same two authors (SVU and AG) against predefined study eligibility criteria. At any level, discrepancies about the eligibility of the study were discussed and resolved by the reviewers, and adjudication was sought from the third reviewer (BKP), if consensus was not achieved between the two reviewers.
Data extraction
All the relevant data were extracted independently by the two authors (SVU and AG). For that, a common data extraction format was prepared using Microsoft Excel. Information like the name of the author, publication year, study type, number of cases and controls, and characteristics of cases and controls were retrieved. Information about exposure, i.e., plasma homocysteine, was collected, like mean and SD among case and control groups, fasting or non-fasting blood samples and methods of homocysteine estimation. If the data were reported as a mean and standard error in the study, then SD was calculated. Other relevant information was also recorded, like the mean age, age group and gender of the study participants.
Assessment of the quality of the studies
To assess the study quality, the National Heart, Lung, and Blood Institute (NHLBI) tool was used, which was developed for observational studies [14]. Two reviewers assessed the quality of the studies and third reviewer resolved if any discrepancy or disagreement raised. Studies fulfilling at least 75% criteria were labeled good-quality studies, while those with 50% to 75% were fair, and less than 50% were considered poor-quality studies.
Data analysis
The pooled estimates of the outcomes, along with a 95% confidence interval (95% CI), were measured in terms of standardized mean difference (SMD). I2 statistics were applied to assess the heterogeneity of studies, and I2 > 50% was considered substantial to high heterogeneity [15]. If I2 > 50% was found, a random-effects model (the Der Simonian and Laird method) [16] was applied. The prediction interval of the pooled estimate was determined based on the Tau2 statistics [17]. Heterogeneity was explored by undertaking subgroup analyses based on the following variables: case group (ACS/CAD), geography (continent of study), gender (males and females/males only), type of blood sample (fasting or non-fasting), and study period. Assessment of publication bias was planned by means of the Funnel plot, Doi plot, and LFK index if more than ten studies were found eligible for meta-analysis. Baujat plot and influential plots were done to identify the influential studies. A leave-one-out analysis was planned to estimate the impact of each study on the pooled outcome estimate and the heterogeneity. A sensitivity analysis was planned after eliminating the poor-quality studies and influential studies. A p-value of < 0.05 was deemed significant.
The meta-analysis was performed using R statistical software (version 4.2.2 (2022–10–31 urct) following the standard codes [18]. Other R packages used were ‘meta’ (version 6.2–0) and ‘metasens’ (version 1.5–2).
Certainty in the evidence
The study evaluated and summarized the pooled estimate's certainty for each outcome using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) methodology [19].
The systematic review and meta-analysis was registered with PROSPERO (ID-CRD42023387675).
Results
Eligible studies
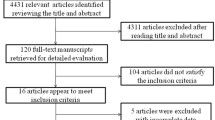
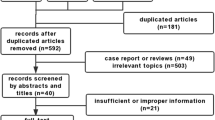
The databases were thoroughly searched, resulting in a collection of 2504 distinct records. These records were then subjected to title-abstract screening, leading to the identification of 379 studies that were deemed suitable for a comprehensive review. After conducting a thorough examination of the full texts of these studies, 59 of them were determined to be eligible for data extraction and were thus included in the systematic review and meta-analysis (Fig. 1).
Characteristics of the included studies
The included studies were conducted from 1990 to 2022. The sample size of the studies ranged from 28 to 875 patients in the CAD group and 15 to 2914 controls. Case–control study design was reported in 46 of the 59 studies, while 12 of the 59 were cross-sectional studies and one was a nested case–control study. Ten of the studies included only male participants. Most of the studies were conducted among individuals of any age group (41/59). The majority of the studies were conducted in India-12 [20,21,22,23,24,25,26,27,28,29,30,31], followed by Turkey-8 [32,33,34,35,36,37,38,39], Tunisia-7 [40,41,42,43,44,45,46], Pakistan-5 [47,48,49,50,51], United States of America (USA)-4 [52,53,54,55], Taiwan-4 [56,57,58,59], China-3 [60,61,62], Iran-2 [63, 64], and United Kingdom (UK)-2 [65]. Palestine [66], Norway [67], Indonesia [68], South Korea [69], Switzerland [70], Poland [71], France [72], Japan [73], Canada [74], Germany [75], Cyprus [76], and Greece [77] that reported one study each. According to the diagnosis of the cases, 30/59 studies included ACS patients, 16/59 studies included CAD patients with ≥ 50% occlusion, and 13/59 studies included CAD patients without mentioning the exact diagnostic criteria. Homocysteine levels were measured in the fasting blood samples in 49 studies and in non-fasting samples in three studies. Seven studies did not specify the fasting status of the sample (Table 1).
Risk of bias assessment
Upon assessing the risk of bias, it was determined that out of the 59 studies included in the meta-analysis, 55 were classified as being of good or fair quality, while four studies were of poor quality (Additional file 1: Table S4).
Outcomes
The pooled SMD of the homocysteine levels between the cases and controls was 0.73 (95% CI 0.55–0.91), which implies a significantly higher homocysteine levels among the cases than the controls. There was high heterogeneity between the studies (I2 = 94%, p < 0.01). The prediction interval was between − 0.60 and 2.06 (Fig. 2). Sensitivity analysis following the removal of poor-quality studies resulted in a similar pooled SMD of 0.73 (95% CI 0.54–0.91) (Additional file 2: Figure S1).
Two of the 59 studies were identified as potential outliers (Additional file 2: Figure S2), and the sensitivity analysis revealed a slightly lower SMD of 0.64 (95% CI 0.50–0.78). Although there was a minor decrease in heterogeneity, it still remained at a high level with an I2 value of 92% (p < 0.01). Additionally, the prediction interval became narrower, ranging from − 0.35 to 1.63 (Additional file 2: Figure S3).
The leave-one-out analysis revealed that there was not much variation observed in the heterogeneity, with I2 values ranging from 93 to 94%. Similarly, the pooled standardized mean difference (SMD) estimate remained relatively stable, varying from 0.68 to 0.75 throughout the analysis (Additional file 2: Figure S4).
Table 2 presents the pooled estimate resulting from the subgroup analysis. Except for the Europe subgroup (I2 51%), heterogeneity remained substantial and even among all the subgroup analysis. Diagnosis (ACS/CAD) and the fasting status of the blood sample did not significantly affect the SMD between the cases and controls. There was a significant difference in the homocysteine levels association with the CAD/ACS between different regions of the world, with the highest SMD among the Asian studies (0.85 [95% CI 0.60–1.10]), while the European studies reported the lowest SMD between the cases and controls (0.32 [95% CI 0.18–0.46]). Similarly, the time period of the study had a significant impact on the pooled SMD of the Hcy levels, with the post-2000 SMDs being significantly higher than the pre-2001 levels. Meta-regression also revealed that the strength of association was increasing over the period in the index meta-analysis (Beta = 0.0227, p = 0.048).(Fig. 3). Relatively, studies that included only males had a significantly lower SMD in the homocysteine levels (0.40 [95% CI 0.28;0.53]) than the studies where both genders were included (0.81 [95% CI 0.59–1.03]) (Table 2).
Funnel plot and Doi plot asymmetry analysis indicated the presence of potential publication bias. This bias was statistically represented by the LFK index, which yielded a value of 2.69 (Fig. 4a, b).
The certainty of the evidence was rated low, owing to the observational nature of the studies, high heterogeneity, and publication bias (Table 3).
Discussion
Of the novel biomarkers of CAD, plasma homocysteine is being studied to determine whether it is an independent modifiable risk factor or not. The mechanism by which high concentrations of plasma homocysteine lead to atherothrombosis is endothelial cell damage impairing its function, which has been shown in many in vitro studies as well. High Hcy levels increase oxidative stress, inducing inflammatory processes, increasing endoplasmic reticulum stress and apoptosis, increasing autoimmune reactions, and ultimately increasing the coagulation cascade [68, 78]. The index meta-analysis, which is the first to present the pooled estimate of this association, included a total of 9381 cases of CAD and 12,188 controls from the 59 studies and revealed a significantly higher plasma homocysteine concentration among the cases (SMD of 0.73, 95% CI: 0.55–0.91). This suggests a significant association of homocysteine with the occurrence of CAD, although the overall certainty of the findings is low, mostly due to high heterogeneity. The high heterogeneity in the association reported in the index meta-analysis could be due to the difference in inclusion and exclusion criteria of cases and controls, inadequate sample size, different methods of homocysteine measurement and fasting status of blood sample collection, the presence of other traditional risk factors, and the wide range of time periods of the included studies. Plasma homocysteine concentration is affected by many factors like age, gender, ethnicity, nutritional deficiencies like folate and Vitamin B12, and renal and liver function [79]. In addition to these factors, the enzyme methylene tetrahydrofolate reductase, encoded by the MTHFR gene, also regulates the homocysteine level. This gene metabolizes and removes homocysteine by using folate. A polymorphism of the gene MTHFR C677T reduces the efficiency of the enzyme and leads to an increase in plasma Hcy concentration [80], which might have also contributed to the heterogeneity. Twenty-one of the 59 studies have investigated the different genetic and environmental interactions involved in the occurrence of CAD. Girelli D. et al. concluded in their study that the MTHFR C677T mutation was not associated with CAD, but genetic–environmental interaction might contribute to the vascular risk by raising Hcy, which is why the folate level is low [81]. Similar findings were produced by Huh HJ et al. who found that gene–nutrient interactions can increase the risk for CAD based on specific threshold folate levels [69].
Studies adopted different case groups, like patients with acute conditions or those who had coronary events in the past, where the diagnosis was based on the extent of stenosis on angiography. The pooled estimate of SMD was high among the CAD group, where patients with any degree of stenosis were included. Heterogeneity was little reduced (from 95% in the CAD group with ≥50% stenosis, 94% in the ACS group and 92% in the CAD-criteria unspecified group). Various studies reported that patients with severe CAD in terms of the number of vessels affected or higher Gensini scores had significantly elevated mean total homocysteine levels [23, 82]. But Bozkurt A et al. reported that homocysteine concentration was unrelated to the extent (in terms of the number of vessels affected) and severity of the disease [34]. Pooled SMD is high for the studies done in Asian countries (pooled SMD 0.85, 95% CI: 0.60–1.10), followed by African countries (pooled SMD 0.75, 95% CI: 0.18–1.32). Heterogeneity is also high among studies done in countries on these two continents, while studies in European or American regions showed less heterogeneity as well as a small pooled SMD. Since homocysteine is believed to be determined by nutritional deficiencies like folate and Vitamin B12 as well as ethnicity, the finding of the index subgroup analysis can be explained by studies exploring the folate levels in various geographical regions. A meta-analysis of MTHFR polymorphism with CHD risk done by Clarke R et al. reported folate levels as low in Asian and European un-supplemented populations, intermediate folate levels in the supplemented European population and un-supplemented US and Australian populations, while high folate levels in the supplemented US and Australian populations [80]. Chambers JC et al., in their two parallel case–control studies done among two ethnic groups, European and Indian Asian men residing in Europe for an average of 27 years, reported that fasting Hcy concentrations were high in Indian Asian men compared to European men, and the age-adjusted difference was 6% [83]. Another study conducted to understand the impact of migration on the risk of coronary heart disease revealed that serum homocysteine levels were significantly higher among the Indian participants residing in India compared to Indian-origin participants who migrated to Sandwell, UK. This was consistent with low serum folate levels among Indian participants compared to migrated participants [84]. CAD is one of the major public health concerns across the world and is known for its multifactorial causation. Thus, genetic as well as environmental factors (folate levels) have an interactive effect on the Hcy levels.
Subgroup analysis based on gender showed that pooled SMD was lower in studies that included only male participants compared to studies with both genders. Studies have shown that males had significantly higher Hcy values than females at each age range, which could be a contributing factor to gender differences in developing CAD [34, 85, 86]. The majority of the studies have documented fasting homocysteine estimation through high-performance liquid chromatography, fluorescence polarization immunoassay, or ELISA methods. Subgroup analysis shows a lower but insignificant pooled SMD when homocysteine is estimated from a non-fasting blood sample. The estimated pooled SMD was lower in the studies published before 2001 (0.37, 95% CI: 0.23–0.52), with substantial heterogeneity. Almost all the studies were done in Europe or the American region except one, which was from Japan. After 2010, all the studies were published in Asian or African countries. So, this difference in pooled estimates over the period of time might be due to the region of study. It is also possible that over the span of three decades, lifestyle has changed considerably and all the known risk factors have become more prevalent, thus increasing the vulnerability for coronary artery disease. Food habits and probably the quality of food have also changed over time, so nutritional deficiencies particularly folate and Vitamin B12 deficiencies could have increased, which in turn is associated with a rise in homocysteine levels, particularly in low- and middle-income countries in Asia and Africa.
Strengths and limitations
The index meta-analysis is the first study to present the global, regional, and temporal pooled association estimates of the Hcy with CAD. The quality of the studies was evaluated by standard tools, and sensitivity analysis was undertaken to improve the robustness of the findings. Heterogeneity was explored by means of appropriate and feasible subgroup analyses. The GRADE profile was applied to present the certainty of the evidence from the meta-analysis. However, the index meta-analysis had the following limitations: High heterogeneity between the studies persisted even after subgroup analysis might be due to the presence of publication bias, language bias or genetic reasons. Although we searched three of the major databases (PubMed, Scopus and Cochrane) along with Google Scholar, more databases could not be included, because of limited resource availability.
Conclusions
Overall, even though higher homocysteine levels might have a significant association with coronary artery diseases, the certainty of evidence is low. Within the population groups, Asian and African populations showed a greater strength of association than their European and American counterparts. High heterogeneity, which is a major factor impacting the certainty of the evidence, needs to be explored in future studies by reporting and conducting subgroup analysis based on determinants such as genetics, sex and folate levels. Primary studies can be designed to alleviate all probable confounding factors to assess the predictive role of homocysteine in CAD and whether it is a modifiable risk factor.
Availability of data and material
The datasets used and/or analyzed during the current study are included in the manuscript and supplementary materials.
Abbreviations
- ACS:
-
Acute coronary syndrome
- CAD:
-
Coronary artery disease
- CHD:
-
Coronary heart disease
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular disease
- Hcy:
-
Homocysteine
- NHLBI:
-
National Heart, Lung, and Blood Institute
- RR:
-
Risk ratio
- SD:
-
Standard deviation
- SMD:
-
Standardized mean difference
- SRMA:
-
Systematic review and meta-analysis
- tPA:
-
Tissue plasminogen activator
References
Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A (2016) Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med 4(13):1–12
Mack M, Gopal A (2016) Epidemiology, traditional and novel risk factors in coronary artery disease. Heart Fail Clin 12(1):1–10. https://doi.org/10.1016/j.hfc.2015.08.002
Yuan MZ, Fang Q, Liu GW, Zhou M, Wu JM, Pu CY (2019) Risk factors for post-acute coronary syndrome depression: a meta-analysis of observational studies. J Cardiovasc Nurs 34(1):60–70
Ganguly P, Alam SF (2015) Role of homocysteine in the development of cardiovascular disease. Nutr J 14(1):1–10
Boushey CJ, Beresford SAA, Omenn GS, Motulsky AG (1995) A quantitative assessment of plasma homocysteine as a risk factor for vascular disease: probable benefits of increasing folic acid intakes. JAMA J Am Med Assoc 274(13):1049–57
Arnesen E, Refsum H, Bønaa KH, Ueland PM, Førde OH, Nordrehaug JE (1995) Serum total homocysteine and coronary heart disease. Int J Epidemiol 24(4):704–9
Schnyder G, Pin R, Roffi M, Flammer Y, Hess OM (2001) Association of plasma homocysteine with the number of major coronary arteries severely narrowed. Am J Cardiol 88(9):1027–30
Deepa R, Velmurugan K, Saravanan G, Karkuzhali K, Dwarakanath V, Mohan V (2001) Absence of association between serum homocysteine levels and coronary artery disease in south Indian males. Indian Heart J 53(1):44–7
Chacko KA (1998) Plasma homocysteine levels in patients with coronary heart disease. Indian Heart J 50(3):295–299
Sastry BK, Indira N, Anand B, Prabha BS, Raju BS (2001) A case-control study of plasma homocysteine levels in South Indians with and without coronary artery disease. Indian Heart J. 53(6):749–53
Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M (2008) Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clin Proc 83(11):1203–1212
Wang B, Mo X, Wu Z, Guan X (2022) Systematic review and meta-analysis of the correlation between plasma homocysteine levels and coronary heart disease. J Thorac Dis 14(3):646–653
Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, Metzendorf M-I, Noel-Storr A, Paynter R, Rader T, Thomas J WL. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane, 2022. [Internet]. [cited 2023 May 4]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools,
Study Quality Assessment Tools | NHLBI, NIH [Internet]. [cited 2023 May 4]. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 22(4):719–48
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials [Internet]. 7(3):177–88
Gandhi AP, Shamim MA, Padhi BK (2023) Steps in undertaking meta-analysis and addressing heterogeneity in meta-analysis. Evid. 1(1):78–92
Shamim MA, Gandhi AP, Dwivedi P, Padhi BK (2023) How to perform meta-analysis in R: a simple yet comprehensive guide. Evid. 1(1):93–113
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 336(7650):924–6
Abraham R, John MJ, Calton R, Dhanoa J (2006) Raised serum homocysteine levels in patients of coronary artery disease and the effect of vitamin B12 and folate on its concentration. Indian J Clin Biochem 21(1):95–100
Angeline T, Jeyaraj N, Tsongalis GJ (2007) MTHFR Gene polymorphisms, B-vitamins and hyperhomocystinemia in young and middle-aged acute myocardial infarction patients. Exp Mol Pathol. 82(3):227–33
Puri A, Gupta OK, Dwivedi RN, Bharadwaj RPS, Narain VS, Singh S (2003) Homocysteine and lipid levels in young patients with coronary artery disease. J Assoc Physicians India. 51:681–5
Shenoy V, Mehendale V, Prabhu K, Shetty R, Rao P (2014) Correlation of serum homocysteine levels with the severity of coronary artery disease. Indian J Clin Biochem 29(3):339–344
Bahulikar A, Tickoo V, Phalgune D (2018) Association of Non-HDL cholesterol, homocysteine and Vitamin D in acute coronary syndrome. J Assoc Physicians India. 66(8):22–5
Bhagwat VR, Yadav AS, Rathod IM (2009) Homocysteine, lipid indices and antioxidants in patients with ischaemic heart disease from Maharashtra. India Singapore Med J 50(4):418–424
Dogra RK, Das R, Ahluwalia J, Kumar RM, Talwar KK (2012) Prothrombotic gene polymorphisms and plasma factors in young north Indian survivors of acute myocardial infarction. J Thromb Thrombolysis. 34(2):276–82
Gupta M, Sharma P, Garg G, Kaur K, Bedi GK, Vij A (2005) Plasma homocysteine: an independent or an interactive risk factor for coronary artery disease. Clin Chim Acta. 352(1–2):121–5
Gupta MD, Girish MP, Sarkar PG, Gupta A, Kategari A, Bansal A et al (2018) Role of ApoE gene polymorphism and nonconventional biochemical risk factors among very young individuals (aged less than 35 years) presenting with acute myocardial infarction. Indian Heart J. 70:S146-56
Gupta SK, Kotwal J, Kotwal A, Dhall A, Garg S (2012) Role of homocysteine & MTHFR C677T gene polymorphism as risk factors for coronary artery disease in young Indians. Indian J Med Res 135(4):506–512
Karumarakkal J (2017) Homocysteine and lipid profile in patients with coronary artery disease. J Med Sci Clin Res 5(9):28105–28109
Palazhy S, Kamath P, Vasudevan DM (2015) Elevated oxidative stress among coronary artery disease patients on statin therapy: a cross sectional study. Indian Heart J 67(3):227–232
Akyürek O, Akbal E, Güneş F (2014) Increase in the risk of ST elevation myocardial infarction is associated with homocysteine level. Arch Med Res. 45(6):501–6
Aydin M, Gokkusu C, Ozkok E, Tulubas F, Unlucerci Y, Pamukcu B et al (2009) Association of genetic variants in Methylenetetrahydrofolate Reductase and Paraoxonase-1 genes with homocysteine, folate and vitamin B12 in coronary artery disease. Mol Cell Biochem 325(1–2):199–208
Bozkurt A, Toyaksi H, Acartürk E, Tuli A, Çayli M (2003) The effects of hyperhomocysteinemia on the presence, extent, and severity of coronary artery disease. Jpn Heart J. 44(3):357–68
Bozkurt E, Keles S, Acikel M, Islek M, Ateşal S (2004) Plasma homocysteine level and the angiographic extent of coronary artery disease. Angiology. 55(3):265–70
Gokkusu C, Tulubas F, Unlucerci Y, Ozkok E, Umman B, Aydin M (2010) Homocysteine and pro-inflammatory cytokine concentrations in acute heart disease. Cytokine. 50(1):15–8
Ozkan Y, Yardim-Akaydin S, Imren E, Torun M, Simşek B (2006) Increased plasma homocysteine and allantoin levels in coronary artery disease: possible link between homocysteine and uric acid oxidation. Acta Cardiol 61(4):432–439
Yildirir A, Tokgozoglu SL, Kabakci G, Ovunc K, Aksoyek S, Oto A et al (2001) Extent of coronary atherosclerosis and homocysteine affect endothelial markers. Angiology. 52(9):589–96
Yilmaz H, Isbir S, Agachan B, Ergen A, Farsak B, Isbir T (2006) C677T mutation of methylenetetrahydrofolate reductase gene and serum homocysteine levels in Turkish patients with coronary artery disease. Cell Biochem Funct 24(1):87–90
Bahri R, Esteban E, Moral P, Hassine M, Hamda KB, Chaabani H. Apolipoprotein gene polymorphisms and plasma levels in healthy Tunisians and patients with coronary artery disease. Lipids Health Dis [Internet]. 2008;7. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-58149389414&doi=10.1186%2F1476-511X-7-46&partnerID=40&md5=3bbb73e1356d0aa89507b0b2ae377065
Chalghoum A, Noichri Y, Karkouch I, Dandana A, Baudin B, Jeridi G et al (2015) Metabolic interactions between hyperhomocysteinemia and endothelin-1 among Tunisian patients with acute coronary diseases. Biol Res 48(1):32
Ghazouani L, Abboud N, Mtiraoui N, Zammiti W, Addad F, Amin H et al (2009) Homocysteine and methylenetetrahydrofolate reductase C677T and A1298C polymorphisms in Tunisian patients with severe coronary artery disease. J Thromb Thrombolysis. 27(2):191–7
Jemaa R, Achouri A, Kallel A, Ali SB, Mourali S, Feki M et al (2008) Association between the 2756A> G variant in the gene encoding methionine synthase and myocardial infarction in Tunisian patients. Clin Chem Lab Med. 46(10):1364–8
Kerkeni M, Addad F, Chauffert M, Myara A, Gerhardt M, Chevenne D et al (2006) Hyperhomocysteinaemia, methylenetetrahydrofolate reductase polymorphism and risk of coronary artery disease. Ann Clin Biochem 43(3):200–206
Noichri Y, Chalghoum A, Chkioua L, Baudin B, Ernez S, Ferchichi S, et al. Low erythrocyte catalase enzyme activity is correlated with high serum total homocysteine levels in tunisian patients with acute myocardial infarction. Diagn Pathol 2013;8(1). Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84876805889&doi=10.1186%2F1746-1596-8-68&partnerID=40&md5=41943f59f1b6c4eaa11c80c1ae49d159
El OM, Aouni Z, Mazigh C, Khochkar R, Gazoueni E, Haouela H et al (2010) Homocysteine and markers of inflammation in acute coronary syndrome. Exp Clin Cardiol 15(2):e25–e28
Ijaz A, Zamir S, Sattar A, Jan R, Ali S, Wazir F (2015) homocysteine levels in younger patients with coronary artery disease in Pakistan. Gomal J Med Sci. 13(4):202–6
Iqbal MP, Ishaq M, Kazmi KA, Yousuf FA, Mehboobali N, Ali SA et al (2005) Role of vitamins B6, B12 and folic acid on hyperhomocysteinemia in a Pakistani population of patients with acute myocardial infarction. Nutr Metab Cardiovasc Dis. 15(2):100–8
Iqbal MP, Mehboobali N, Tareen AK, Yakub M, Iqbal SP, Iqbal K et al (2013) Association of body iron status with the risk of premature acute myocardial infarction in a Pakistani population. PLoS ONE 8(6):e67981
Muzaffar R, Khan MA, Mushtaq MH, Nasir M, Khan A, Haq IU et al (2023) Hyperhomocysteinemia as an independent risk factor for coronary heart disease. Comparison with conventional risk factors. Brazil J Biol. 83:1–8
Shah H, Jan MU, Altaf A, Salahudin M (2018) Correlation of hyper-homocysteinemia with coronary artery disease in absence of conventional risk factors among young adults. J Saudi Hear Assoc. 30(4):305–10
Genest JJJ, McNamara JR, Salem DN, Wilson PW, Schaefer EJ, Malinow MR (1990) Plasma homocyst(e)ine levels in men with premature coronary artery disease. J Am Coll Cardiol 16(5):1114–1119
Giles WH, Croft JB, Greenlund KJ, Ford ES, Kittner SJ (2000) Association between total homocyst(e)ine and the likelihood for a history of acute myocardial infarction by race and ethnicity: Results from the third National Health and Nutrition Examination Survey. Am Heart J 139(3):446–53
Martin NJ, Collier AC, Bowen LD, Pritsos KL, Goodrich GG, Arger K et al (2009) Polymorphisms in the NQO1, GSTT and GSTM genes are associated with coronary heart disease and biomarkers of oxidative stress. Mutat Res - Genet Toxicol Environ Mutagen 674(1–2):93–100
Stampfer MJ, Malinow MR, Willett WC, Newcomer LM, Upson B, Ullmann D et al (1992) A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA J Am Med Assoc 268(7):877–881
Chen CYJ, Yang TC, Chang C, Lu SC, Chang PY. Homocysteine is a bystander for ST-segment elevation myocardial infarction: a case-control study. BMC Cardiovasc Disord. 2018;18(1)
Cheng ML, Ho HY, Lin JF, Chen YC, Chan EC, Chiu DTY (2008) Clinical relevance of plasma homocysteine levels in Taiwanese patients with coronary artery disease. Biofactors. 34(2):125–34
Chua S, Wu CJ, Chang HW, Hang CL, Chen CJ, Yang CH et al (2005) Impact of elevated plasma total homocysteine concentration on coronary atherosclerosis in Chinese patients with acute myocardial infarction undergoing primary coronary intervention. Int Heart J. 46(2):181–93
Lin PT, Huang MC, Lee BJ, Cheng CH, Tsai TP, Huang YC (2008) High plasma homocysteine is associated with the risk of coronary artery disease independent of methylenetetrahydrofolate reductase 677C–>T genotypes. Asia Pac J Clin Nutr. 17(2):330–8
Li S, Pan G, Chen H, Niu X (2019) Determination of serum homocysteine and hypersensitive c-reactive protein and their correlation with premature coronary heart disease. Heart Surg Forum. 22(3):E215-7
Wu DF, Liao QC, Lu F, Wang Z, Yu K, Deng JL (2022) Differential effects of hyperhomocysteinemia on the lipid profiles and lipid ratios between patients with and without coronary artery disease A retrospective observational study. Med (United States) 101(52):E32464
Zhang SY, Xuan C, Zhang XC, Zhu J, Yue K, Zhao P et al (2020) Association between MTHFR gene common variants, serum homocysteine, and risk of early-onset coronary artery disease: a case-control study. Biochem Genet 58(2):245–256
Golbahar J, Rezaian GR (2004) Association of hyperhomocysteinemia with coronary artery disease in Southern Iran. Iran J Med Sci. 29(3):116–9
Kazemi MBS, Eshraghian K, Omrani GR, Lankarani KB, Hosseini E (2006) Homocysteine level and coronary artery disease. Angiology 57(1):9–14
Chambers JC, Ireland H, Thompson E, Reilly P, Obeid OA, Refsum H et al (2000) Methylenetetrahydrofolate reductase 677 C–>T mutation and coronary heart disease risk in UK Indian Asians. Arterioscler Thromb Vasc Biol 20(11):2448–2452
Alawneh I, Saymeh A, Daraghmeh M, Jabri D, Yaseen L. Role of plasma homocysteine levels and other associated factors with coronary artery disease among palestinian patients in North Palestine: a case control study. Pan Afr Med J. 2022;42. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85137978211&doi=10.11604%2Fpamj.2022.42.180.34264&partnerID=40&md5=a552a444d543d70541a52328479bd51d
Christensen B, Landaas S, Stensvold I, Djurovic S, Retterstøl L, Ringstad J et al (1999) Whole blood folate, homocysteine in serum, and risk of first acute myocardial infarction. Atherosclerosis 147(2):317–326
Sugijo H, Sargowo D, Widjajanto E, Romdoni R. The role of methylenetetrahydrofolate reductase C677T gene polymorphism as a risk factor for coronary artery disease: a cross-sectional study in the Sidoarjo Regional General Hospital. Pan Afr Med J [Internet]. 2022;41. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85131291374&doi=10.11604%2Fpamj.2022.41.212.24916&partnerID=40&md5=2900d11806f445807f19dff8d835c256
Huh HJ, Chi HS, Shim EH, Jang S, Park CJ (2006) Gene–nutrition interactions in coronary artery disease: correlation between the MTHFR C677T polymorphism and folate and homocysteine status in a Korean population. Thromb Res 117(5):501–506
Loehrer FM, Angst CP, Haefeli WE, Jordan PP, Ritz R, Fowler B (1996) Low whole-blood S-adenosylmethionine and correlation between 5-methyltetrahydrofolate and homocysteine in coronary artery disease. Arterioscler Thromb Vasc Biol 16(6):727–733
Szczeklik A, Sanak M, Jankowski M, Dropiński J, Czachór R, Musiał J et al (2001) Mutation A1298C of methylenetetrahydrofolate reductase: Risk for early coronary disease not associated with hyperhomocysteinemia. Am J Med Genet. 101(1):36–9
Montalescot G, Ankri A, Chadefaux-Vekemans B, Blacher J, Philippe F, Drobinski G et al (1997) Plasma homocysteine and the extent of atherosclerosis in patients with coronary artery disease. Int J Cardiol 60(3):295–300
Kawashiri M, Kajinami K, Nohara A, Yagi K, Inazu A, Koizumi J et al (1999) Plasma homocysteine level and development of coronary artery disease. Coron Artery Dis. 10(7):443–7
Dalery K, Lussier-Cacan S, Selhub J, Davignon J, Latour Y, Genest J Jr (1995) Homocysteine and coronary artery disease in French Canadian subjects: Relation with vitamins B12, B6, pyridoxal phosphate, and folate. Am J Cardiol. 75(16):1107–11
Rothenbacher D, Fischer HG, Hoffmeister A, Hoffmann MM, März W, Bode G et al (2002) Homocysteine and methylenetetrahydrofolate reductase genotype: association with risk of coronary heart disease and relation to inflammatory, hemostatic, and lipid parameters. Atherosclerosis 162(1):193–200
Eftychiou C, Antoniades L, Makri L, Koumas L, Costeas PA, Kyriakou E et al (2012) Homocysteine levels and MTHFR polymorphisms in young patients with acute myocardial infarction: a case control study. Hell J Cardiol 53(3):189–194
Rallidis LS, Gialeraki A, Komporozos C, Vavoulis P, Pavlakis G, Travlou A et al (2008) Role of methylenetetrahydrofolate reductase 677C->T polymorphism in the development of premature myocardial infarction. Atherosclerosis. 200(1):115–20
Calim A, Turkoz FP, Ozturkmen YA, Mazi EE, Cetin EG, Demir N et al (2020) The relation between homocysteine levels in patients with acute coronary syndrome and grace score. Sisli Etfal Hastan tip Bul 54(3):346–350
Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J et al (2004) Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem 50(1):3–32
Clarke R, Bennett DA, Parish S, Verhoef P, Dötsch-Klerk M, Lathrop M, et al. Homocysteine and coronary heart disease: Meta-analysis of MTHFR case-control studies, avoiding publication bias. PLoS Med 2012;9(2). Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84857711683&doi=10.1371%2Fjournal.pmed.1001177&partnerID=40&md5=c2f79264057a18a9ec5ce581690a33c7
Girelli D, Friso S, Trabetti E, Olivieri O, Russo C, Pessotto R et al (1998) Methylenetetrahydrofolate reductase C677T mutation, plasma homocysteine, and folate in subjects from northern Italy with or without angiographically documented severe coronary atherosclerotic disease: Evidence for an important genetic-environmental intera. Blood 91(11):4158–4163
El Oudi M, Bouguerra C, Aouni Z, Mazigh C, Bellaaj R, Machghoul S (2011) Homocysteine and inflammatory biomarkers plasma levels, and severity of acute coronary syndrome. Ann Biol Clin (Paris) 69(2):175–180
Chambers JC, Obeid OA, Refsum H, Ueland P, Hackett D, Hooper J et al (2000) Plasma homocysteine concentrations and risk of coronary heart disease in UK Indian Asian and European men. Lancet [Internet]. 355(9203):523–7
Patel JV, Vyas A, Cruickshank JK, Prabhakaran D, Hughes E, Reddy KS et al (2006) Impact of migration on coronary heart disease risk factors: Comparison of Gujaratis in Britain and their contemporaries in villages of origin in India. Atherosclerosis 185(2):297–306
Xu R, Huang F, Wang Y, Liu Q, Lv Y, Zhang Q (2020) Gender- and age-related differences in homocysteine concentration: a cross-sectional study of the general population of China. Sci Rep. 10(1):1–11
Cohen E, Margalit I, Shochat T, Goldberg E, Krause I (2019) Gender differences in homocysteine concentrations, a population-based cross-sectional study. Nutr Metab Cardiovasc Dis. 29(1):9–14
Acknowledgements
The authors would also like to acknowledge Global Centre for Evidence Synthesis for providing a platform to learn, teach, collaborate, and perform systematic reviews and meta-analyses.
Funding
This research did not receive any grant from any funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SVU did conceptualization, formal analysis, data curation, writing—original draft. BKP done methodology, validation, formal analysis, software, supervision, writing—review & editing. AVB performed data curation, formal analysis, software, supervision, writing—review & editing. APG contributed to data curation, methodology, writing—review and editing. MAS performed formal analysis, software, supervision, writing—review & editing. ND was involved in methodology, formal analysis, supervision, writing—review & editing. PS and SR did methodology, formal analysis, software, supervision, writing—review & editing. MNK performed formal analysis, supervision, writing—review & editing. AG contributed to methodology, formal analysis, supervision, writing—review & editing. QSZ done methodology, formal analysis, supervision, writing—review & editing. RS and HAS did methodology, formal analysis, software, supervision, writing—review & editing. All authors approved the final version of the manuscript submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Since it is a systematic review and meta-analysis, this is not applicable.
Consent of publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Table S1:
PRISMA Checklist (2020). Table S2: Inclusion and exclusion criteria. Table S3: The adjusted search terms as per searched electronic databases [as of 17.04.2023]. Table S4: Quality assessment with the use of National Heart, Lung, and Blood Institute (NHLBI) quality assessment tool
Additional file 2. Figure S1: I
nfluence diagnostics. Figure S2: Forest plot showing the pooled estimates after removing poor-quality studies. Figure S3: Forest plot showing the pooled estimates after removing two outlier studies. Figure S4: Leave one out analysis
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Unadkat, S.V., Padhi, B.K., Bhongir, A.V. et al. Association between homocysteine and coronary artery disease—trend over time and across the regions: a systematic review and meta-analysis. Egypt Heart J 76, 29 (2024). https://doi.org/10.1186/s43044-024-00460-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-024-00460-y