Abstract
Background
Right atrial pressure plays a critical role as a hemodynamic parameter in diagnosing pulmonary hypertension and other cardiac diseases, as well as guiding the treatment and prognosis of various cardiac disorders. If there is no obstruction between the inferior or superior vena cava (SVC) as central veins and the right atrium, the pressures in these veins could be considered equal to the right atrial pressure. This study aimed to examine the correlation between echocardiographic methods for estimating right atrial pressure and invasive measurements of central venous pressure (CVPi) in infants and children with congenital heart disease during the 48 h after cardiac surgery and to establish regression equations for echocardiographic estimation of central venous pressure (CVPe).
Results
We prospectively enrolled 43 infants and children, ranging in age from 6 months to 16 years, including 20 males and 23 females. We found a significant correlation between CVPi and the ratio of the maximal diameter of IVC to the maximal diameter of the descending aorta ratio (IVCmax/DAOmax) (r = 0.529, P < 0.001), SVCS/D velocity ratio (SVCS/D) (r = 0.462, P = 0.006), right atrial vertical diameter (RAVD) (r = 0.409, P = 0.01), area (r = 0.384, P = 0.014), and tricuspid valve A wave acceleration rate (TVAAR) (r = 0.315, P = 0.048). Multiple regression analysis yielded an equation for estimating central venous pressure using four parameters related to the IVC, SVC, tricuspid valve, and right atrium. The equation is as follows: estimated CVP = 4.36 + (2.35 × IVCmax/DAOmax) + (1.06 × SVCS/D) + (0.059 × RAVD) + (0.001 × TVAAR). This equation is strongly correlated with CVPi (Pearson r = 0.698, P = 0.002).
Conclusions
The estimation of central venous pressure through a multi-parametric equation that included the ratio of the maximal diameter of the inferior vena cava to the maximal diameter of the descending aorta, the ratio of S to D velocity of the superior vena cava, the vertical diameter of the right atrium, and the acceleration rate of the A wave of the tricuspid valve demonstrated a robust correlation with invasively measured central venous pressure. To assess the accuracy of predicted pressures by this equation, further investigations are required to apply this innovative multi-parametric formula to a prospective population of pediatric patients with congenital heart disease.
Similar content being viewed by others
Background
Right atrial pressure plays a critical role as a hemodynamic parameter in diagnosing pulmonary hypertension and other cardiac diseases, as well as guiding the treatment and prognosis of various cardiac disorders [1,2,3,4,5]. In the absence of any obstruction between the inferior or superior vena cava and the right atrium, it is reasonable to assume that the pressures in these central veins are equal to that of the right atrium [6]. Limited data exist regarding the performance of different echocardiographic methods for estimating right atrial pressure in infants and children with congenital heart disease, especially within the first 48 h following cardiac surgery in the intensive care unit setting. The objectives of this study were to assess the correlation between echocardiographic methods for estimating right atrial pressure and invasive measurements of central venous pressure in infants and children with congenital heart disease during the 48-h postoperative period and to establish regression equations for the echocardiographic estimation of central venous pressure in this particular context.
Methods
Study design and study population
We conducted a prospective observational study between 2021 and 2022, enrolling infants and children who had undergone cardiac surgery for congenital heart disease within the first 48 h after the operation, provided that specific conditions were met. These conditions included invasive monitoring of central venous and arterial pressures through catheterization of the internal jugular vein and femoral artery, absence of obstruction between the inferior vena cava (IVC) and the right atrium, exclusion of Glenn bidirectional shunt or Fontan operation as surgical procedures, extubated with spontaneous breathing, presence of an adequate acoustic window for echocardiographic examination, and maintenance of stable hemodynamic status in the patient. During echocardiography, all patients were awake and in a calm state.
Measurement of central venous pressure
The central venous pressure was measured using Arrow pediatric three-lumen central venous catheter (Arrow International LLC, Morrisville, NC 27560 USA) inserted into the internal jugular vein and Bioptimal disposable pressure monitoring kit (Biosensors International, Shanghai International Holding Corp. 20,537 Hamburg, Germany). The positioning accuracy of the central venous catheter was assessed by examining the chest X-ray of every patient [4].
The measurements were standardized.
Echocardiographic examination
The echocardiographic examinations were performed in the intensive care unit using the Philips Affinity 70 C echocardiography machine (Philips Healthcare, USA), equipped with probes operating at 5 and 8 megahertz (MHz) frequencies.
All echocardiographic examinations were conducted by a senior and well-trained fellow of pediatric cardiology, with the patient positioned in a supine posture. Standard echocardiographic images were acquired from various windows, including subcostal, apical, parasternal, and suprasternal views.
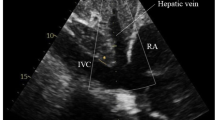
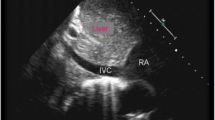
In the right ventricular focused four-chamber view, measurements of Doppler variables related to the tricuspid valve and dimensions of the right atrium were obtained in accordance with established guidelines [7,8,9]. Doppler evaluation of the hepatic vein and inferior vena cava, as well as the measurement of the maximum and minimum diameter of the inferior vena cava at a proximal point to the junction of the hepatic vein, and descending aorta at the same level, was conducted in the subcostal window, following the previously described methodology. The superior vena cava was examined from the suprasternal window [10,11,12] (Fig. 1). The measurements were standardized.
A The heart and the connections of the right atrium to the surrounding vessels. B The Doppler profile of the inferior vena cava, superior vena cava, and hepatic vein. C The measurement of maximal and minimal diameters of the inferior vena cava. D The pulse-wave Doppler of the tricuspid valve flow. E The tissue Doppler of the lateral annulus of the tricuspid valve (IVC inferior vena cava, SVC superior vena cava, HV hepatic vein, TV tricuspid valve, PW pulse Doppler, TD tissue Doppler)
The diameters of the inferior vena cava and right atrial volume were indexed by the body surface area. The ratio between the minimum and maximum diameters of the inferior vena cava (IVC) to the minimum and maximum diameters of the descending aorta (DAO) at the same level and inferior vena cava collapsibility and distensibility indices is calculated using the following formulas [13]:
\(\frac{{{\text{IVC}}_{{{\text{max}}}} }}{{{\text{DAO}}_{{{\text{max}}}} }} = \frac{{\text{Maximal diameter of inferior vena cava}}}{{\text{Maximal diameter of descending aorta}}}\)
\(\frac{{{\text{IVC}}_{{{\text{min}}}} }}{{{\text{DAO}}_{{{\text{min}}}} }} = \frac{{\text{Minimal diameter of inferior vena cava}}}{{\text{Minimal diameter of descending aorta}}}\)
\({\text{IVC collapsibility index}} = \frac{{{\text{IVC maximal size in inspiration}} - {\text{IVC minimal size in expiration }}}}{{\text{IVC maximal size in inspiration}}}\)
\({\text{IVC distensibility index}} = \frac{{{\text{IVC maximal size in inspiration}} - {\text{IVC minimal size in expiration }}}}{{\text{IVC minimal size in inspiration}}}\)
Statistical analysis
The data distribution was evaluated using the Shapiro–Wilk test to assess its normality. Descriptive statistics, including the mean, standard deviation, median, interquartile range, minimum, and maximum, were provided for continuous variables. The presentation of categorical variables included the absolute counts and corresponding percentages.
The correlation between invasively measured central venous pressure and numerical echocardiographic variables was evaluated using linear regression, and Pearson r correlation coefficient and R square values were reported. The Chi-square or Fisher’s exact test assessed the association between categorical variables. The statistical analysis was conducted using IBM SPSS Statistics for Windows, version 27 (IBM Corp., Armonk, NY, USA). A significance level of P < 0.05 was used to determine statistical significance.
Ethical considerations
Informed parents or guardians’ consent was obtained. This study received approval from the Institutional Research Ethics Committees. The study was conducted in accordance with the ethical guidelines outlined in the 2013 Declaration of Helsinki [14].
Results
We prospectively enrolled 43 infants and children, ranging in age from 6 months to 16 years. The study included 20 males and 23 females. The diagnoses of the patients are shown in Fig. 2. Table 1 represents a descriptive analysis of the basic characteristics and variables. Tables 2 and 3 provide a descriptive analysis of the measured and calculated variables for the following structures: inferior and superior vena cava, hepatic vein, tricuspid valve, and right atrium.
Correlation between inferior vena cava variables and invasively measured central venous pressure (CVPi) (Table 4)
There was a significant and moderate correlation between the ratio of the maximum size of the IVC to the maximum size of the descending aorta at the same level in the subcostal view and CVPi (r = 0.529, P value < 0.001). Similarly, a significant correlation was observed between the minimal diameters of these two vessels and invasively measured central venous pressure (r = 0.448, P value = 0.004). Additionally, a weak correlation was found between the maximum size of the IVC, measured during inspiration, and the CVPi (r = 0.333, P value = 0.031). The corresponding regression equations are shown in Table 4.
Correlation between superior vena cava variables and invasively measured central venous pressure (CVPi) (Table 5)
A significant and moderate correlation was observed between the ratio of S velocity to D velocity (r = 0.462, P value = 0.006). Table 5 displays the regression equation corresponding to the aforementioned relationship.
Correlation between hepatic vein variables and invasively measured central venous pressure (CVPi) (Table 6)
We did not observe any significant relationship between the Doppler variables of the hepatic vein and CVPi. Similarly, there was no significant correlation between a hepatic vein systolic filling fraction greater than 55% or a hepatic vein systolic velocity to diastolic velocity ratio of less than or equal to 1 and CVPi (P values of 0.091 and 0.689, respectively).
Correlation between tricuspid valve variables and invasively measured central venous pressure (CVPi) (Table 7)
Among the Doppler and tissue Doppler variables of the tricuspid valve, which included E/e′, only the tricuspid valve A wave acceleration rate (TVAAR) exhibited a weak correlation with CVPi (r = 0.315, P value = 0.048). The corresponding regression equation can be found in Table 7.
Correlation between right atrial variables and invasively measured central venous pressure (CVPi) (Table 8)
A moderate correlation was observed between the major axis of the right atrial vertical axis and CVPi (r = 0.409, P value = 0.010). Similarly, a weak relationship was found between the right atrial area and CVPi (r = 0.384, P value = 0.014). The corresponding regression equations are displayed in Table 8.
Introduction of a multivariable equation for estimation of central venous pressure (Fig. 3)
A scatterplot depicting the correlation between invasively measured central venous pressure and the ratio of the maximal diameter of the inferior vena cava (IVC) to the maximal diameter of the descending aorta. B Scatterplot illustrating the correlation between invasively measured central venous pressure and the superior vena cava S/D velocity ratio. C Scatterplot showing the correlation between invasively measured central venous pressure and the right atrial vertical axis. D Scatterplot demonstrating the correlation between invasively measured central venous pressure and the tricuspid valve A wave acceleration rate. E Scatterplot illustrating the correlation between invasively measured central venous pressure and multiple variables, including the ratio of the maximal diameter of the inferior vena cava (IVC) to the maximal diameter of the descending aorta, superior vena cava S/D velocity ratio, right atrial vertical axis, and tricuspid valve A wave acceleration rate
Using the most robust parameter related to IVC, SVC, tricuspid valve, and right atrium, we performed a multiple regression analysis and obtained the following multi-parametric equation.
With a strong correlation with CVPi (Pearson r = 0.698, P value = 0.002):
Discussion
The right atrial cavity is anatomically connected to three vessels: the inferior vena cava (IVC), superior vena cava (SVC), and coronary sinus (CS). In a partially analogous manner to communicating vessels, the transmission of pressure from the right atrium to the IVC, SVC, and CS is anticipated to be influenced by several factors. These factors encompass the relative positioning of these vessels in relation to the right atrial cavity, the distance from the mid-right atrium, the discrepancy in the angle between the flow direction within these vessels and the right atrial cavity, and the size of the openings that establish the connection between these vessels and the right atrium (Fig. 1).
Among these vessels, the hepatic vein stands out as the furthest from the mid-right atrium and demonstrates a significantly distinct flow direction compared to the direction of flow within the right atrium. In our analysis, we did not identify any significant correlation between invasively measured central venous pressure and the hepatic vein systolic to diastolic velocity ratio or hepatic vein systolic filling fraction, as presented in Table 6. In contrast, Nagueh et al. conducted a study involving 35 adult patients with a wide range of diagnoses, including Wolff–Parkinson–White, mediastinal tumor, dilated cardiomyopathy, and other diseases. They demonstrated a correlation coefficient of 0.86 between hepatic vein systolic filling fraction (HVSFF) and mean right atrial pressure or central venous pressure [15]. The mean RA pressure for their patients varied from 2 to 28 mmHg. Discrepancies in the findings may be attributed to disparities in patient age, patient composition, right atrial pressures, and Doppler profile of hepatic veins in infants and children.
In contrast to the hepatic vein, we found a significant and moderate correlation between invasively measured central venous pressure and the ratio of the maximal and minimal dimensions of the inferior vena cava (IVC) to the maximal and minimal dimensions of the descending aorta, respectively. The utilization of ratios proves to be more reliable in the pediatric population. Additionally, within the initial 24 h following cardiothoracic surgery, Krastins and colleagues reported elevated intra-abdominal pressure in 66.67% of pediatric patients [16]. Kaptein and colleagues noted that the dimensions of the IVC and IVC collapsibility could be influenced by various factors, including increased intra-abdominal pressure and tricuspid regurgitation [17]. Therefore, the unique postoperative environment presents additional challenges when utilizing IVC size as a surrogate for right atrial pressure, primarily due to the impact of elevated intrabdominal pressure on the thin-walled IVC [16,17,18].
However, our analysis did not reveal any statistically significant correlation between the collapsibility index of the inferior vena cava (IVC) and the invasively measured central venous pressure (CVPi). This lack of correlation may be attributed to limited respiratory excursion in pediatric patients following cardiac surgery, which can be influenced by factors such as pain, diaphragmatic paresis, or other confounding variables affecting IVC collapsibility [14]. Similarly, in a study conducted by Kishiki et al., which included 60 children with congenital heart disease, no significant correlation was found between the inferior vena cava collapsibility index (IVCCI) and mean right atrial pressure [18]. However, they did observe a significant and moderate correlation between IVCCI and mean right atrial pressure when measured using three-dimensional echocardiography.
We also observed a moderate correlation between the S/D velocity ratio of the superior vena cava and CVPi. The superior vena cava acts as a conduit vessel situated higher than the right atrium and, given the effects of gravity, might be expected to be less responsive to pressure variations within the right atrial cavity compared to the inferior vena cava, which is located lower than the right atrium. In contrast to the study of o Murayama et al., we did not find any significant relationship between S/D < 1.9 and elevated central venous pressure (or mean right atrial pressure) [19]. There is a question regarding the role of the relative positions of the superior vena cava (SVC) and inferior vena cava (IVC) in relation to the right atrial cavity. The SVC, positioned as a conduit vessel above the right atrial cavity, raises uncertainty about its responsiveness to changes in right atrial pressure compared to the inferior vena cava, which benefits from gravity due to its lower position relative to the right atrial cavity. However, no studies have been conducted to address this question thus far. Furthermore, in the postoperative environment of the cardiac intensive care unit (CICU), greater variations in preload and afterload of the right heart are anticipated, potentially impacting the Doppler pattern of the superior vena cava.
This study showed that the right atrial vertical axis (major axis) and the right atrial area had a moderate correlation with CVPi. Patel et al. demonstrated a correlation between the three-dimensional right atrial volume index and the mean right atrial pressure [20]. The lack of relationship of the horizontal axis may implicate the importance of choosing a dimension that is in alignment with the direction of flow within the right atrium may be a more sensitive variable to serve as a surrogate for mean right atrial pressure.
Limitations
The main limitations of this study are its relatively small sample size and the lack of invasively measured mean right atrial pressure. However, it is important to note that a majority of similar previous and concurrent studies have also enrolled a similar or even smaller number of patients [21,22,23]. With regard to the latter limitation, it can be assumed that mean right atrial pressure is equal to central venous pressure since there was no obstruction observed between the superior vena cava and the right atrium.
Additionally, our measurements were taken at a time when the patients exhibited stable hemodynamic states. We refrained from repeating these measurements under varying loading conditions, such as hypovolemia or volume overload, for two primary reasons: to eliminate potential confounding factors and to prevent imposing extra stress on postoperative children who were not in a state of hemodynamic stability. Therefore, to evaluate the impact of different loading conditions on the results, further study is recommended.
Conclusions
This study introduced a novel multi-parametric regression equation that demonstrates a strong correlation with invasively measured central venous pressure in 43 infants and children with congenital heart disease during the initial 48 h following cardiac surgery in the cardiac intensive care unit. The equation incorporates the maximal dimension of the inferior vena cava relative to the maximal dimension of the descending aorta at the same level, the right atrial vertical axis, the S/D ratio of the pulse Doppler of superior vena cava, and the acceleration rate of the A wave of the tricuspid valve (estimated CVP = 4.36 + (2.35 × IVCmax/DAOmax) + (1.06 × SVCS/D) + (0.059 × RAVD) + (0.001 × TVAAR).
Conducting future studies applying this formula to a prospective population of pediatric patients with congenital heart disease is crucial in order to determine the mean difference between the predicted and actual pressures.
Availability of data and materials
The datasets used in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ASD:
-
Atrial septal defect
- BT:
-
Blalock–Taussig
- CS.:
-
Coronary sinus
- CVP:
-
Central venous pressure
- CVPe :
-
Estimated (predicted) central venous pressure
- CVPi :
-
Invasively measured central venous pressure
- HV:
-
Hepatic vein
- HVSFF:
-
Hepatic vein systolic filling fraction
- IQR:
-
Interquartile range
- IVC:
-
Inferior vena cava
- IVCCI:
-
Inferior vena cava collapsibility index
- IVCDI:
-
Inferior vena cava distensibility index
- IVCmax :
-
Maximal dimension of inferior vena cava
- IVCmin :
-
Minimal dimension of inferior vena cava
- LPA:
-
Left pulmonary artery
- PDA:
-
Patent ductus arteriosus
- RA:
-
Right atrium
- RPA:
-
Right pulmonary artery
- RV:
-
Right ventricle
- SD:
-
Standard deviation
- SPSS:
-
Statistical Package for Social Sciences
- SVC:
-
Superior vena cava
- SVCS/D:
-
Ratio of S wave to D wave of superior vena cava
- TVAAR:
-
Acceleration rate of A wave of the tricuspid valve
References
Lawlor M, Ng VG, Ahmed S, Dershowitz L, Brener M, Kampaktsis P, Pitts A, Vahl TP, Nazif T, Leon MB et al (2023) Right atrial pressure in pulmonary hypertension assessment in tricuspid regurgitation. J Am Coll Cardiol 81(8, Supplement):1970
Miranda WR, Oh JK (2017) Effusive-constrictive pericarditis. Cardiol Clin 35(4):551–558
Nagata R, Harada T, Omote K, Iwano H, Yoshida K, Kato T, Kurosawa K, Nagai T, Anzai T, Obokata M (2022) Right atrial pressure represents cumulative cardiac burden in heart failure with preserved ejection fraction. ESC Heart Fail 9(2):1454–1462
Magder S (2017) Right atrial pressure in the critically ill: How to measure, what is the value, what are the limitations? Chest 151(4):908–916
Al-Qadi MO, Holbrook J, Ford HJ, Ceppe A, LeVarge BL (2023) Prognostic value of respiratory variation in right atrial pressure in patients with precapillary pulmonary hypertension. Chest. https://doi.org/10.1016/j.chest.2023.03.030
Beigel R, Cercek B, Luo H, Siegel RJ (2013) Noninvasive evaluation of right atrial pressure. J Am Soc Echocardiogr 26(9):1033–1042
Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T (2010) Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the pediatric measurements writing group of the American society of echocardiography pediatric and congenital heart disease council. J Am Soc Echocardiogr 23(5):465–495
Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, Pignatelli RH, Rychik J (2006) Task force of the pediatric council of the american society of E, pediatric council of the American society of E: guidelines and standards for performance of a pediatric echocardiogram: a report from the task force of the pediatric council of the American society of echocardiography. J Am Soc Echocardiogr 19(12):1413–1430
Sun Z-Y, Li Q, Li J, Zhang M-W, Zhu L, Geng J (2023) Echocardiographic evaluation of the right atrial size and function: relevance for clinical practice. Am Heart J Plus: Cardiol Res Pract 27:100274
Fadel BM, Kazzi B, Mohty D (2023) Ultrasound imaging of the superior vena cava: a state-of-the-art review. J Am Soc Echocardiogr 36(5):447–463
Toma M, Giovinazzo S, Crimi G, Masoero G, Balbi M, Montecucco F, Canepa M, Porto I, Ameri P (2021) Multiparametric versus Inferior vena cava-based estimation of right atrial pressure. Front Cardiovasc Med 8:632302
Lee KS, Abbas AE, Khandheria BK, Lester SJ (2007) Echocardiographic assessment of right heart hemodynamic parameters. J Am Soc Echocardiogr 20(6):773–782
Yildizdas D, Aslan N (2020) Ultrasonographic inferior vena cava collapsibility and distensibility indices for detecting the volume status of critically ill pediatric patients. J Ultrason 20(82):e205–e209
World Medical A (2013) World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310(20):2191–2194
Nagueh SF, Kopelen HA, Zoghbi WA (1996) Relation of mean right atrial pressure to echocardiographic and Doppler parameters of right atrial and right ventricular function. Circulation 93(6):1160–1169
Krastins J, Straume Z, Auzins J (2010) Intraabdominal pressure in children after cardiothoracic surgery. Acta Chirurgica Latviensis 9(1):28–32
Kaptein MJ, Kaptein EM (2021) Inferior vena cava collapsibility index: clinical validation and application for assessment of relative intravascular volume. Adv Chronic Kidney Dis 28(3):218–226
Kishiki K, Fujimaki H, Terada M, Kobayashi T, Ueda T, Hamamichi Y, Inage A, Ishii T, Yoshikawa T, Yazaki S et al (2018) Assessment of right atrial pressure in children with congenital heart disease: simultaneous three-dimensional echocardiography and catheterization study. J Am Coll Cardiol 71(11, Supplement):A578
Murayama M, Kaga S, Okada K, Iwano H, Nakabachi M, Yokoyama S, Nishino H, Tsujinaga S, Chiba Y, Ishizaka S et al (2022) Clinical utility of superior vena cava flow velocity waveform measured from the subcostal window for estimating right atrial pressure. J Am Soc Echocardiogr 35(7):727–737
Patel AR, Alsheikh-Ali AA, Mukherjee J, Evangelista A, Quraini D, Ordway LJ, Kuvin JT, Denofrio D, Pandian NG (2011) 3D echocardiography to evaluate right atrial pressure in acutely decompensated heart failure correlation with invasive hemodynamics. JACC Cardiovasc Imaging 4(9):938–945
Simonson JS, Schiller NB (1988) Sonospirometry: a new method for noninvasive estimation of mean right atrial pressure based on two-dimensional echographic measurements of the inferior vena cava during measured inspiration. J Am Coll Cardiol 11(3):557–564
Natori H, Tamaki S, Kira S (1979) Ultrasonographic evaluation of ventilatory effect on inferior vena caval configuration. Am Rev Respir Dis 120(2):421–427
Patel SG, Woolman P, Li L, Craft M, Danford DA, Kutty S (2017) Relation of right atrial volume, systemic venous dimensions, and flow patterns to right atrial pressure in infants and children. Am J Cardiol 119(9):1473–1478
Acknowledgements
Not applicable
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
EMR did conceptualization, methodology, validation, data analysis, original draft writing, review, editing, and supervision. NP done echocardiographic examinations, data collection, data entry, and original draft writing. HR, AZ and BG gave resources, original draft writing, review, and editing. . All authors have read and approved the final manuscript before submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study received approval from the Research Ethics Committees of Tehran University of Medical Sciences (ID: IR.TUMS.CHMC.REC.1401.105). The study was conducted in accordance with the ethical guidelines outlined in the 2013 Declaration of Helsinki.
Consent to participate
Informed written consent to participate in the study was obtained from the parents or legal guardians of patients under 16 years old and from patients who were 16 years old or older.
Consent for publication
Not applicable.
Competing interests
None of the authors have any conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Malakan Rad, E., Parizadeh, N., Radmehr, H. et al. Correlation between echocardiographic estimation of right atrial pressure and invasive measurement of central venous pressure in postoperative pediatric patients with congenital heart disease: a prospective observational study. Egypt Heart J 76, 25 (2024). https://doi.org/10.1186/s43044-024-00456-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-024-00456-8