Abstract
Background
Coronavirus disease of 2019 (COVID-19) is associated with venous thromboembolism (VTE), not only during hospitalization but also after discharge, raising concerns about anticoagulant (AC) use for post-discharge COVID-19 patients. We aimed to systematically review the current literature on the possible benefits or risks regarding extended thromboprophylaxis.
Main body
We searched related databases from December 1, 2019, to October 6, 2022, including studies on the necessity, duration, and selection of the ideal AC regarding extended thromboprophylaxis for post-discharge COVID-19 patients. The screening of the selected databases led to 18 studies and 19 reviews and guidelines. Studies included 52,927 hospitalized COVID-19 patients, with 19.25% receiving extended thromboprophylaxis. VTE events ranging from 0 to 8.19% (median of 0.7%) occurred in a median follow-up of 49.5 days. All included studies and guidelines, except four studies, recommended post-discharge prophylaxis after an individual risk assessment indicating high thrombotic and low bleeding risk. Studies used risk assessment models (RAMs), clinical evaluation, and laboratory data to identify COVID-19 patients with a high risk of VTE. IMPROVE-DD was the most recommended RAM. Direct oral anticoagulants (DOACs) and low molecular weight heparins (LMWHs) were the most used AC classes.
Conclusions
Post-discharge prophylaxis for COVID-19 patients is recommended after an individual assessment. The IMPROVE-DD model can help predict VTE risk. After distinguishing patients who need post-discharge AC therapy, DOACs for 30–35 days and LMWHs for 40–45 days can be the drug of choice. Further studies, particularly the results of the ongoing randomized controlled trials (RCTs), are required. Also, to properly handle such patients, every physician should consider lifestyle modification in addition to pharmacological treatment for post-discharge VTE prophylaxis.
Similar content being viewed by others
Background
In December 2019, the Coronavirus disease 2019 (COVID-19) outbreak led to a pandemic [1]. As of November 1, 2022, over 627 million confirmed cases have resulted in more than 6.5 million fatalities worldwide [2]. A significant number of venous thromboembolism (VTE) events have been observed in COVID-19 patients, likely due to endothelium damage, immobility, weakness, and prolonged inflammation [3]. VTE, defined as presenting pulmonary embolism (PE) or deep vein thrombosis (DVT), is a common medical concern associated with potentially fatal complications [4].
Due to different study designs, the prevalence of VTE in hospitalized COVID-19 patients is variable. A previous meta-analysis review, including nearly 2000 COVID-19 patients, reported that the weighted mean prevalence of VTE among Intensive care unit (ICU) and non-ICU patients was 31.3% [5]. In other studies, the VTE pooled prevalence was 17%, with a fourfold higher VTE rate in ICU patients [3, 6]. Due to the reduction in mortality rate and high incidence of VTE in COVID-19 patients, current guidelines recommend using in-hospital thromboprophylaxis for all hospitalized patients, especially critically ill patients [7]. However, even after the disease’s acute phase, patients can still experience VTE after hospital dismissal. In the recent systematic reviews, post-discharge VTE pooled prevalence was reported to be around 1.16–1.8%, suggesting a higher rate than other medically ill patients [8, 9]. 80% of VTE cases occur 30–45 days after hospital discharge [10]. Hence, the appropriate early thromboprophylaxis for COVID-19 discharged patients is essential.
The question to be discussed is the necessity, duration, and selection of the ideal anticoagulant (AC) in post-discharge COVID-19 patients. Several reviews and studies provided evidence regarding the possible benefits of post-discharge AC therapy; for instance, The MICHELLE randomized controlled trial (RCT) studied the necessity and duration of extended thromboprophylaxis using oral ACs [11]. However, as the American Society of Hematology guideline states, studies with a high level of evidence have spoken little about this issue, and the need for systematic review studies to summarize data and provide high-level evidence is required [12]. Furthermore, there are other ongoing RCTs underway, including Post-hospital Thromboprophylaxis RCT (NCT04650087), Hero-19 (NCT04542408), and XACT (NCT04640181), from which no findings have yet been published.
Eventually, still there remains the possibility of COVID-19 pandemic recurrence in the recent future, the spread of new variants, and even similar pandemics [13]. As a result, the question regarding post-discharge thromboprophylaxis in COVID-19 patients remains highly relevant. This practical systematic review seeks to provide a recommendation for physicians based on guidelines, reviews, RCTs, and other current evidence-based data, regarding extended thromboprophylaxis in hospitalized COVID-19 patients without VTE diagnosis at discharge time.
Main text
Protocol and registration
This systematic review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and is registered on PROSPERO (Registration Number: CRD42022365107) [14].
Eligibility criteria
We included peer-reviewed observational studies, RCT studies, and reviews, especially guidelines and position papers reporting the necessity, type, and duration of VTE thromboprophylaxis in post-discharge COVID-19 patients. We excluded conference papers, conference abstracts, erratums, retracted papers, correspondence papers, book titles, and meta-analyses. We also excluded studies carried out on animal or cellular models. Studies had to be available in English.
Search strategy
We searched PubMed, EMBASE, Web of Science, Scopus, Cochrane, and clinicaltrials.gov from December 1, 2019, to October 6, 2022. We also screened all the review's reference lists by hand-searching. To find relevant literature for the systematic search, we used the search query provided in Additional file 1: Appendix A.1.
Study selection
We initially screened titles and abstracts of studies for duplication and relevance. The full text of all potentially relevant studies was then independently studied by two authors (R. A. and B. D.) to determine the final study selection. Resolution of disagreement was resolved by consensus and the third author's final decision (M. KA.).
Data extraction
The following data were extracted by two authors (R. A. and B. D.) from eligible articles: Study characteristics (study titles, authors, year of publication, publication study type, and study site country), population characteristics (number of patients, gender, and age), percentage of patients in the ICU setting, post-discharge thromboprophylaxis name, dosage, and recommended duration of the used AC, risk assessment tool, post-discharge events (thromboembolic events and major bleedings), and duration of follow-ups.
Risk of bias assessment
Two authors (R. A. and B. D.) assessed the risk of bias and quality of individually selected studies using the Newcastle–Ottawa Quality Assessment Scale (NOS) for cohort studies [15], adapted NOS for cross-sectional studies [16], and the Jadad scale [17] for the RCT studies (Additional file 1: Appendix A.2). NOS and adopted NOS assess the risk of bias within domains, including the study groups' selection, comparability, and the ascertainment of the outcome of interest. The quality of studies was graded using the star system with a maximum possible score of 9 for NOS and 10 for adopted NOS. The Agency for Healthcare Research and Quality (AHRQ) standard was used to convert the NOS (good, fair, and poor) [18]. Thresholds for converting the Adopted NOS (very good, good, satisfactory, and unsatisfactory) were based on a study by Herzog et al. [16]. The Jadad scale evaluated the randomization, blinding, and description of withdrawals with a maximum score of 5. Based on a study by Falagas et al. [19], an RCT with a score of 2 and above was considered a good quality study.
Data analysis
We used a qualitative analysis and presented the findings with a descriptive approach, odds ratio (OR) with a 95% confidence interval (CI), and risk ratio (RR) with a 95% CI based on the included studies and the summative nature of this systematic review. A meta-analysis and statistical calculations were not performed because the studies' design and reporting differed.
Results
Search results
Our initial search in the selected databases yielded 4897 titles, including 37 studies that met the eligibility criteria. The detailed search process is depicted in Fig. 1. 18 out of 37 studies, including eight retrospective cohorts, seven prospective cohorts, two cross-sectional studies, and only one RCT (Table 1). Studies were conducted worldwide, including nine from The United States, two from Russia, and the other six from Norway, Brazil, Spain, Belgium, Singapore, Iran, and England. 19 out of 37 studies were guidelines and reviews, including 14 guidelines, four position papers, and one state-of-the-art review (Table 2). Six Guidelines and reviews were International; the others were from The United States, England, Brazil, Italy, Algeria, Scotland, and Germany.
Risk of bias assessment
The systematic review included 18 studies. Four cohorts were of good quality, and the other 11 were studies with lower quality scores, mainly due to comparability issues. One of the cross-sectional studies was of good quality, and the other was of satisfactory quality. The RCT was of good quality. The majority of included studies (12/18) were of low quality, and the others (6/18) were of high quality (Additional file 1: Appendix A.2).
Characteristics of patients and included studies
The major characteristics of included studies are summarized in Table 1. This systematic review included 18 studies with a total of 52,927 patients. Thirteen studies reported the mean age ranging from 40 to 68.6 years. The follow-up period of the included studies was different, ranging from 30 to 393 days after hospital discharge (median of 49.5 days). All the studies reported the rate of thromboembolic events in their follow-up duration, with a total of 1182 VTE events ranging from 0 to 8.19% (median of 0.7%). All but three studies reported that the PE ratio is equal to or greater than DVT. Eight studies reported the rate of post-discharge bleeding ranging from 0 to 3.7%. (median of 0%). Only one study did not use ACs after hospital discharge for any patients, and the others used AC for a total of 10,088 patients (19.25% of all) [20]. Ten studies reported the rate of ICU patients ranging from 7.8 to 54%, where the highest rate of ICU patients was in the MICHELLE study with the highest ratio of post-discharge VTE events [11]. Also, the major characteristics of included guidelines and reviews are summarized in Table 2.
Post-discharge thromboprophylaxis: necessity, evaluation, and AC selection
Based on the results of the included guidelines and studies, there are controversial views on post-discharge thromboprophylaxis. All the guidelines but one [21], and most studies (11/18), were in favor of this matter, but after an individual risk assessment; indicated in post-discharge COVID-19 patients with high VTE risk, low bleeding risk, and no known contraindications (Tables 1 and 2). Li et al. reported a reduced risk post-discharge VTE in patients who received the therapeutic AC at discharge (OR: 0.18 and 95% CI 0.04–0.75); however, the association of post-discharge prophylactic AC with post-discharge VTE was insignificant [22]. As the only published good-quality RCT, the MICHELLE trial study investigated the VTE and bleeding outcomes in the rivaroxaban and control group at day 35. Post-discharge thromboprophylaxis reduced the risk of VTE events by 67% in the rivaroxaban group (95% CI 0.12–0.90), and no major bleeding occurred [11]. Nevertheless, three cohorts and one cross-sectional study implied no role for post-discharge thromboprophylaxis [20, 23,24,25]. Additionally, three cohort studies did not provide a definite opinion on this matter [26,27,28].
Studies used risk assessment models (RAMs), clinical evaluation, and laboratory data to identify COVID-19 patients with high post-discharge thrombotic risk. Almost half of the guidelines (9/19) used RAMs which all mentioned The International Medical Prevention Registry on Venous Thromboembolism (IMPROVE). IMPROVE is a RAM consisting of seven variables, including the previous episode of VTE (3 points), known thrombophilia (2 points), current paralysis or paresis of lower-limb extremity (2 points), Current cancer (2 points), ICU/CCU stay (1 point), immobilization (1 point), and age > 60 years (1 point), categorizing COVID-19 patients into low (0–1 score), moderate (2–3 score), and high VTE risk (≥ 4 scores) [29]. Two high-quality studies, including Courtney et al. and Ramacciotti et al., reported a significant association between a higher IMPROVE VTE risk score and receiving extended thromboprophylaxis [11, 30]. Accordingly, in the MICHELLE trial study, with increasing the modified IMPROVE VTE risk score from 2–3 to ≥ 4, the RR increased by 27% in a way that patients with IMPROVE VTE score ≥ 4 or 2–3 with a D-dimer > 500 ng/mL were suitable for receiving extended thromboprophylaxis [11]. The IMPROVE-DD RAM with eight variables, including the D-dimer (2 points), has a similar cut-off as IMPROVE for high-risk VTE patients, [31]. In a prospective cohort CORE-19 registry, Giannis et al. demonstrated that the IMPROVE-DD RAM score ≥ 4 was significantly associated with an increased risk of VTE, arterial thromboembolism, and mortality in post-discharge COVID-19 patients (OR: 3.64 with 95% CI 2.91–4.55) [32]. Padua Prediction Score (PPS) (4/37) and the Caprini model (2/37) were used less in the included studies. Moreover, most included studies (6/18) and guidelines (10/19) used clinical evaluation as an important factor for assessing the VTE risk. 6/18 studies and 5/19 guidelines mentioned lab data, especially D-dimer (Tables 1 and 2).
Direct oral anticoagulants (DOACs) and low molecular weight heparins (LMWHs) have been used more than other AC classes in the reviewed studies, with 9/18 included studies and 8/19 guidelines suggesting LMWH; 8/18 included studies, and 9/19 guidelines suggesting DOACs. Unfractionated heparin (UFH) and vitamin K antagonist both with 3/18 included studies but none of the guidelines mentioned any of these two AC classes. Cohort studies have reported a post-discharge thromboprophylaxis of 7–28 days for LMWHs and 30–35 days for DOACs, while in guideline studies, the range is between 14 and 45 days for both AC classes (Tables 1 and 2).
Discussion
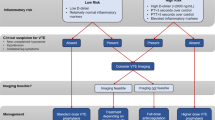
COVID-19 disease seems to be associated with a higher risk of VTE incidence, especially in more severe cases [8]. This practical systematic review aimed to determine the need to receive thromboprophylaxis and whether post-discharge thromboprophylaxis improves outcomes, including decreasing VTE events accompanying low bleeding risks. Then, identifying high-risk VTE patients and post-discharge thromboprophylaxis management, including the type of drug, dosage, and medication duration, will be discussed. Figure 2 provides a pragmatic approach for managing post-discharge thromboprophylaxis in COVID-19 patients without VTE diagnosis at discharge time based on the available evidence.
Suggested algorithm for post-discharge thromboprophylaxis in COVID-19 patients. COVID-19 = coronavirus disease of 2019; VTE = venous thromboembolism; IMPROVE-DD = International Medical Prevention Registry on Venous Thromboembolism and D-dimer; ICU = intensive care unit; CCU = cardiac care unit; AC = anticoagulant; DOAC = direct oral anticoagulants; P.O = per os; LMWH = low molecular weight heparin; S.C = subcutaneous
Should COVID-19 patients receive post-discharge VTE thromboprophylaxis?
While several observational cohort studies, RCTs, and guidelines studied thromboprophylaxis during and after hospitalization, the role of post-discharge VTE thromboprophylaxis remains controversial [12, 22, 33, 34]. Most guidelines recommend against routinely continuing VTE prophylaxis after hospital discharge [34, 35]. Still, they suggest an individual risk assessment and using ACs after discharge in patients with high thrombotic risk, low bleeding risk, and no contraindications (Table 2).
Likewise, most of the included studies in this systematic review agreed with post-discharge VTE thromboprophylaxis if the patient's risk assessment indicated a high-risk situation for VTE. In between, three good-quality cohort studies reported a significant association between post-discharge VTE risk reduction and extended thromboprophylaxis [22, 30, 36]. As in the study by Li et al., this risk reduction was stated to be 82%; although, in the Courtney et al. study, the chance of bleeding increased significantly with post-discharge AC [22, 30]. The MICHELLE trial provided valuable information about post-discharge VTE thromboprophylaxis. The results showed that AC therapy in high-risk patients after discharge reduces the VTE events, and the risk of bleeding will remain unchanged [11].
Three cohort studies and one cross-sectional study suggested against using extended thromboprophylaxis due to their results that only the Eswaran et al. study was of Good quality and the others were of studies with lower quality scores [20, 23, 25, 37]. It is worth saying that these four studies had the lowest average age among the included studies. Tan et al. included patients with few comorbidities and the IMPROVE VTE score of 0 or 1 in 91.3% of all patients [20]. Stawiarski et al. evaluated patients with low D-dimer levels and moderate COVID-19 disease [23]. These three poor-quality studies had few ICU-admitted patients, which has been proven important in increasing the risk of VTE after discharge [20, 23, 37]. The Eswaran et al. study found no correlation even after adjusting for possible confounders such as age and ICU admission [25]. This matter can be attributed to the lack of accurate follow-up and AC thromboprophylaxis in high-risk patients, which may have led to a low incidence of VTE.
Recommendation
Due to the inflammatory state and the chance of post-discharge recurrence of VTE in COVID-19 patients, we suggest that the physicians decide on extended thromboprophylaxis based on individual assessment of VTE and bleeding risk.
Which COVID-19 patients should receive post-discharge thromboprophylaxis? Tools, lab data, and clinical evaluation
Predicting VTE risk, identifying hospitalized patients with COVID-19 at high VTE risk, and discriminating who may benefit from post-discharge thromboprophylaxis with a low risk of major bleeding remains a critical clinical issue [38]. Several tools and models, including the Caprini model, the IMPROVE VTE RAM, the modified IMPROVE RAM, the IMPROVE-DD RAM, the PPS, and the Wells model have been used in COVID-19 patients to assess the need for thromboprophylaxis. IMPROVE RAM was the most applied RAM among the studies to assess the VTE risk in post-discharge COVID-19 patients, and the other RAMs were less used by studies or recommended by guidelines. In a study by Goldin et al. in 9407 patients, the IMPROVE VTE RAM without D-dimer demonstrated a sensitivity of 83.9% and specificity of 29.2% [31]. MICHELLE RCT used modified IMPROVE RAM assigned to COVID-19 patients with IMPROVE score of ≥ 4 or 2–3 with an elevated D-dimer (> 2 times the upper limit of normal or as stated in MICHELE RCT with a D-dimer > 500 ng/mL) for patients with increased risk of VTE [11, 39]. For this reason, IMPROVE-DD eliminates the need for separate grouping using a D-dimer and increases validity scores to a sensitivity of 97.1% and specificity of 21.5% simultaneously [31]. Furthermore, various guidelines have also suggested the IMPROVE RAM, which is either the IMPROVE-VTE RAM with D-dimer or IMPROVE-DD itself (Table 2).
Tsaplin et al. [40] used the original Caprini score (2005 version) and eight modified versions to predict VTE frequency. Among the four modifications used to predict the risk of symptomatic VTE 6 months after discharge, all the versions demonstrated high sensitivity and specificity, especially Caprini with D-dimer and Caprini with COVID-19 risk scores with a sensitivity of 75% and a specificity of 81%. However, the original Caprini score correlates significantly with the VTE risk with the cut-off score of seven [40]. More studies are needed to evaluate the modified versions of the Caprini score. A retrospective cohort study also validated Caprini and IMPROVE RAM as a practical RAM independent of each other [39].
Not all VTE risk assessments are based on models and scores but on the patient's lab data and clinical evaluations. Lab data including D-dimers > two times upper the normal limit (threshold adjusted according to age) [11, 23, 30, 41,42,43,44], and pre-discharge C-reactive protein (CRP) level > 10mg/dl [22, 42] are important factors having significant association with increasing the risk of VTE [33]. In this regard, Li et al. reported a 3.76-fold (95% CI 1.86–7.57) and 3.02-fold (95% CI 1.45–6.29) higher risk of VTE with patient's peak D-dimer levels greater than 3μg/mL and pre-discharge CRP levels greater than 10mg/dL, respectively [22].
Clinical evaluations have long been essential, with easy access to assess the thrombosis risk. Prolonged immobilization [41, 43,44,45,46], advanced age (> 70–75 years) [43, 44, 47, 48], previous history of VTE [22, 43,44,45,46, 48, 49], active cancer [30, 41, 43,44,45,46, 48, 49], known thrombophilia [44, 45, 48, 49], and chronic heart or respiratory failure [21, 23, 47, 48] are the most important factors increasing the VTE risk that will be examined during the clinical evaluation. Some clinical risk factors are not included in IMPROVE-DD RAM. However, they are mentioned in the included studies, including obesity, use of estrogen, family history of VTE, comorbid chronic inflammatory or autoimmune condition, chronic kidney disease (CKD), recent major surgery (e.g., orthopedic procedure), and atrial fibrillation (Tables 1 and 2). Pregnancy is a controversial indication; two included studies reported pregnancy as an indication [10, 30], while the ISTH guideline [50], due to the risk of bleeding, has reported it as a contraindication, demonstrating greater consideration during the risk of bias assessment.
Recommendation
Clinical evaluation and laboratory data are practical factors in AC thromboprophylaxis. The most important clinical risk factors are prolonged immobilization, advanced age, previous history of VTE, active cancer, known thrombophilia, and chronic heart or respiratory failure. In this regard, IMPROVE-DD RAM is designed based on most of the mentioned risk factors and has shown good efficiency in assessing high-risk VTE events in COVID-19 patients without VTE diagnosis at discharge time.
Post-discharge VTE AC thromboprophylaxis in patients with COVID-19: which and how?
The choice of medications, dosing, and duration of thromboprophylaxis should be based on high-quality, evidence-based data and guideline recommendations. Recommended drug medication to prevent thrombosis can be placed in four popular classes of ACs, including LMWHs, DOACs, UFH, and vitamin K antagonists. Several studies recommended DOACs as a post-discharge thromboprophylaxis agent. Three high-quality studies, including the MICHELLE trial, recommended rivaroxaban 10mg daily for 30–35 days. Alternatively, apixaban 2.5mg BID and dabigatran 110mg BID can be used as the choices of DOACs [11, 25, 36]. Also, ISTH, the anticoagulation forum, the VAS, and the health system anticoagulation task force guidelines favored rivaroxaban 10mg daily for 30–42 days [35, 50,51,52]. The VAS guideline also recommended betrixaban 80mg daily for 40 days [52].
Several cohort and guideline studies recommended LMWHs, especially enoxaparin. In this regard, Quiros Ambel et al. provided a protocol in which patients in the absence of hemorrhagic risk and high risk of thrombosis should receive weight or albumin/creatinine ratio (ACR) adjusted LMWH (enoxaparin or bemiparine) for 4–6 weeks [27]. Patients weighted ≤ 50 kg or elderly patients with ACR < 30 ml/min should receive 2500 IU sc/day of bemiparine or 20mg sc/day of enoxaparin, patients weighted 51–80 kg should receive 40mg sc/day of enoxaparin or 3500IU sc/day of bemiparine. Finally, patients who weighed 81-100 kg and > 100 kg were suggested to receive 60mg sc/day of enoxaparin and 80mg sc/day of enoxaparin, respectively. In the same direction, Engelen et al. suggested enoxaparin 0.5 mg/kg daily for 14 days, and Giannis et al. used any dose of enoxaparin < 80 mg daily [42, 47]. In addition, the health system anticoagulation task force guideline recommends enoxaparin 40mg Qday subcutaneously for 6 weeks as an alternative over DOACs [51]. Generally, apart from Li et al., all other included studies emphasize the preference for prophylactic dosage over therapeutic dosage [22]. Regarding the selection of the recommended duration for extended prophylaxis, the included studies have suggested a shorter duration than the guidelines [33, 42, 47]. However, the majority of the guidelines have suggested 40–45 days [41, 43, 51, 52]. Finally, due to limitations, such as INR checks for warfarin and the need for injection for UFH and fondaparinux, the two classes of drugs, LMWH and DOACs, seem to be more acceptable.
Recommendation
If a COVID-19 patient needs extended thromboprophylaxis, we suggest oral AC medications such as DOACs, especially rivaroxaban 10mg daily for 30–35 days, and subcutaneous AC drugs such as the LMWH family, especially weight-adjusted enoxaparin, for 40–45 days. Depending on the specialist's evaluation and the persistence of VTE risk factors, an individual risk assessment should be repeated, and, if necessary, the length of thromboprophylaxis should be continued.
Role of lifestyle modification
The immune system and hemostasis have a close relationship, with each system protecting the host and preventing the spread of foreign diseases [53]. In patients with COVID-19, immunothrombosis has been hypothesized as a pathogenic mechanism in which endothelial dysfunction, hypercoagulability, and activation of innate immune cells contribute to the observed prothrombotic condition [54]. In addition, several environmental factors can affect a person's immune system. In order to have a healthy lifestyle and thus a better immunity system, we can refer to [E(e)SEEDi], which includes five fundamental items: "External and internal environment—Sleep—Emotion—Exercise—Diet" Interventions, also known as magic polypill [55].
Modifications such as communication with loved ones, washing hands, 7–9 h of sleep at night, control of obstructive sleep apnea, decreasing anxiety and depression, maintaining a healthy weight by exercise, anti-inflammatory/antioxidant diet, quitting smoking and reducing alcohol consumption are beneficial E(e)SEEDi for every COVID-19 patients [55].
Cardiovascular events, including VTE, are closely related to a person's lifestyle, and E(e)SEED imbalance can reduce the body's immunity and, as a result, increase the risk of cardiovascular events. In this regard, in addition to pharmacological treatment in post-discharge VTE prophylaxis, every physician should consider lifestyle modification to manage such patients thoroughly [55].
Limitation
The limitations of this study include the use of only one published RCT and other related clinical trial studies are ongoing and have not yet been published. For this reason, most of the data presented in this practical systematic review are from cohort studies and guidelines. Due to the rapid rate of newly published articles on patients with COVID-19 about post-discharge thromboprophylaxis, relevant studies may have been published since the end of our search date.
Conclusions
COVID-19 disease is associated with a hypercoagulable state that has increased VTE risk. Since COVID-19 coagulopathy persists after the acute phase of the disease, extended thromboprophylaxis remains controversial. Based on this systematic review, which included studies and guidelines, after a risk/benefit assessment, post-discharge AC therapy can be reasonable in high-risk patients. Clinical characteristics and laboratory data accompanying RAMs, particularly IMPROVE-DD, can help predict VTE risk. After distinguishing patients who need post-discharge AC therapy, DOACs for 30–35 days and LMWHs for 40–45 days can be the drug of choice. Further studies, particularly the results of the ongoing RCTs, are required to choose better the type of AC, dosage, and duration of prophylaxis. In addition, lifestyle modification is also an aspect to consider when deciding to use AC for post-discharge COVID-19 patients.
Availability of data and materials
Not applicable.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- VTE:
-
Venous thromboembolism
- PE:
-
Pulmonary embolism
- DVT:
-
Deep vein thrombosis
- ICU:
-
Intensive care unit
- AC:
-
Anticoagulant
- RCT:
-
Randomized controlled trial
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- NOS:
-
Newcastle–ottawa scale
- AHRQ:
-
Agency for healthcare research and quality
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- RR:
-
Risk ratio
- RAM:
-
Risk assessment model
- IMPROVE:
-
The international medical prevention registry on venous thromboembolism
- CCU:
-
Cardiac care unit
- PPS:
-
Padua prediction score
- DOAC:
-
Direct oral anticoagulants
- LMWH:
-
Low molecular weight heparins
- UFH:
-
Unfractionated heparin
- CRP:
-
C-reactive protein
- CKD:
-
Chronic kidney disease
- ACR:
-
Albumin/creatinine ratio
- IQR:
-
Interquartile range
- n/a:
-
Not available
- DTI:
-
Direct thrombin inhibitor
- BMI:
-
Body mass index
References
Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273
WHO Coronavirus (COVID-19) Dashboard (1 November 2022) WHO. https://covid19.who.int/
Dobesh PP, Trujillo TC (2020) Coagulopathy, venous thromboembolism, and anticoagulation in patients with COVID-19. Pharmacotherapy 40:1130–1151. https://doi.org/10.1002/phar.2465
Cade JF (1982) High risk of the critically ill for venous thromboembolism. Crit Care Med 10:448–450
Di Minno A, Ambrosino P, Calcaterra I, Di Minno MND (2020) COVID-19 and venous thromboembolism: a meta-analysis of literature studies. Semin Thromb Hemost 46(07):763–771. https://doi.org/10.1055/s-0040-1715456
Jiménez D, García-Sanchez A, Rali P, Muriel A, Bikdeli B, Ruiz-Artacho P, Le Mao R, Rodríguez C, Hunt BJ, Monreal M (2021) Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest 159:1182–1196
Nasiri MJ, Haddadi S, Tahvildari A, Farsi Y, Arbabi M, Hasanzadeh S, Jamshidi P, Murthi M, Mirsaeidi M (2020) COVID-19 clinical characteristics, and sex-specific risk of mortality: systematic review and meta-analysis. Front Med. https://doi.org/10.3389/fmed.2020.00459
Zuin M, Engelen MM, Barco S, Spyropoulos AC, Vanassche T, Hunt BJ, Vandenbriele C, Verhamme P, Kucher N, Rashidi F (2022) Incidence of venous thromboembolic events in COVID-19 patients after hospital discharge: a systematic review and meta-analysis. Thromb Res 209:94–98
Mansory EM, Abu-Farhaneh M, Iansavitchene A, Lazo-Langner A (2022) Venous and arterial thrombosis in ambulatory and discharged COVID-19 Patients: a systematic review and meta-analysis. TH Open 6:e276–e282
Patell R, Bogue T, Koshy A, Bindal P, Merrill M, Aird WC, Bauer KA, Zwicker JI (2020) Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood 136:1342–1346
Ramacciotti E, Agati LB, Calderaro D, Aguiar VCR, Spyropoulos AC, de Oliveira CCC, Dos Santos JL, Volpiani GG, Sobreira ML, Joviliano EE (2022) Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet 399:50–59
Cuker A, Tseng EK, Nieuwlaat R, Angchaisuksiri P, Blair C, Dane K, Davila J, DeSancho MT, Diuguid D, Griffin DO, Kahn SR, Klok FA, Lee AI, Neumann I, Pai A, Righini M, Sanfilippo KM, Siegal D, Skara M, Terrell DR, Touri K, Akl EA, Al Jabiri RN, Al Jabiri YN, Barbara AM, Bognanni A, Bou Akl I, Boulos M, Brignardello-Petersen R, Charide R, Chan M, Colunga-Lozano LE, Dearness K, Darzi AJ, Hussein H, Karam SG, Kolb P, Mansour R, Morgano GP, Morsi RZ, Muti-Schünemann G, Nadim MK, Noori A, Philip BA, Piggott T, Qiu Y, Benitez YR, Schünemann F, Stevens A, Solo K, Wiercioch W, Mustafa RA, Schünemann HJ (2022) American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: July 2021 update on postdischarge thromboprophylaxis. Blood Adv 6:664–671. https://doi.org/10.1182/bloodadvances.2021005945
Wang C, Han J (2022) Will the COVID-19 pandemic end with the Delta and Omicron variants? Springer
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88:105906
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2000) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford
Herzog R, Álvarez-Pasquin M, Díaz C, Del Barrio JL, Estrada JM, Gil Á (2013) Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 13:1–17
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Viswanathan M, Ansari M, Berkman N, Chang S, Hartling L, McPheeters M, Treadwell J (2012) Assessing the risk of bias of individual studies in systematic reviews of health care interventions. Agency for healthcare research and quality methods guide for comparative effectiveness reviews. AHRQ Methods for Effective Health Care
Falagas ME, Siempos II, Bliziotis IA, Panos GZ (2006) Impact of initial discordant treatment with β-lactam antibiotics on clinical outcomes in adults with pneumococcal pneumonia: a systematic review. Mayo Clin Proc 81(12):1567–1574. https://doi.org/10.4065/81.12.1567
Tan JY, Tan CW, Wong WH, Cheong MA, Lee LH, Kalimuddin S, Low JGH, Ng HJ (2021) Post-hospitalization venous thromboembolism in COVID-19 patients: evidence against routine post-hospitalization prophylactic anticoagulation. Int J Lab Hematol. https://doi.org/10.1111/ijlh.13633
NICE (2022) COVID-19 rapid guideline: managing COVID-19. https://www.guidelines.co.uk/infection/nice-managing-covid-19-guideline/455939.article. Accessed 10 July 2022
Li P, Zhao W, Kaatz S, Latack K, Schultz L, Poisson L (2021) Factors associated with risk of postdischarge thrombosis in patients with COVID-19. JAMA Netw Open 4:e2135397. https://doi.org/10.1001/jamanetworkopen.2021.35397
Stawiarski K, Loutoo A, Velardi L, Zarich S (2021) D-dimer driven deep vein thrombosis prophylaxis strategy for hospitalized patients with COVID-19. Thromb Res 201:151–153
Rashidi F, Barco S, Kamangar F, Heresi GA, Emadi A, Kaymaz C, Jansa P, Reis A, Rashidi A, Taghizadieh A (2021) Incidence of symptomatic venous thromboembolism following hospitalization for coronavirus disease 2019: prospective results from a multi-center study. Thromb Res 198:135–138
Eswaran H, Jarmul JA, Shaheen AW, Meaux D, Long T, Saccoccio D, Moll S (2021) Vascular thromboembolic events following COVID-19 hospital discharge: incidence and risk factors. Res Pract Thromb Haemost 5:292–295. https://doi.org/10.1002/rth2.12485
Parks AL, Auerbach AD, Schnipper JL, Bertram A, Jeon SY, Boyle B, Fang MC, Gadrey SM, Siddiqui ZK, Brotman DJ (2022) Venous thromboembolism (VTE) prevention and diagnosis in COVID-19: practice patterns and outcomes at 33 hospitals. PLoS ONE 17:e0266944
Ambel HQ, Crespo-Robledo P, Juaristi KA, Plo-Seco I, Simón JJM, Fernández EP, Encinas MP (2021) Effectiveness of antithrombotic prophylaxis in hospitalised patients with SARS-CoV-2 infection. Eur J Hosp Pharm. https://doi.org/10.1136/ejhpharm-2021-002877
Spyropoulos AC, Crawford JM, Chen Y-W, Ashton V, Campbell AK, Milentijevic D, Peacock WF (2022) Occurrence of thromboembolic events and mortality among hospitalized COVID-19 patients: large observational cohort study of electronic health records. TH Open 06:e408
Spyropoulos AC, Cohen SL, Gianos E, Kohn N, Giannis D, Chatterjee S, Goldin M, Lesser M, Coppa K, Hirsch JS (2021) Validation of the IMPROVE-DD risk assessment model for venous thromboembolism among hospitalized patients with COVID-19. Res Pract Thrombos Haemost 5:296–300
Courtney LA, Trujillo TC, Saseen JJ, Wright G, Palkimas S (2022) Evaluation of the clinical impact of thromboprophylaxis in patients with COVID-19 following hospital discharge. Ann Pharmacother. https://doi.org/10.1177/10600280211064306
Goldin M, Lin SK, Kohn N, Qiu M, Cohen SL, Barish MA, Gianos E, Diaz A, Richardson S, Giannis D (2021) External validation of the IMPROVE-DD risk assessment model for venous thromboembolism among inpatients with COVID-19. J Thromb Thrombol 52:1032–1035
Giannis D, Allen SL, Tsang J, Flint S, Pinhasov T, Williams S, Tan G, Thakur R, Leung C, Snyder M, Bhatia C, Garrett D, Cotte C, Isaacs S, Gugerty E, Davidson A, Marder GS, Schnitzer A, Goldberg B, McGinn T, Davidson KW, Barish MA, Qiu M, Zhang M, Goldin M, Matsagkas M, Arnaoutoglou E, Spyropoulos AC (2021) Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood 137:2838–2847. https://doi.org/10.1182/blood.2020010529
Salisbury R, Iotchkova V, Jaafar S, Morton J, Sangha G, Shah A, Untiveros P, Curry N, Shapiro S (2020) Incidence of symptomatic, image-confirmed venous thromboembolism following hospitalization for COVID-19 with 90-day follow-up. Blood Adv 4:6230–6239. https://doi.org/10.1182/bloodadvances.2020003349
NIH (2022) Antithrombotic therapy in patients with COVID-19. https://www.covid19treatmentguidelines.nih.gov/. Accessed 26 Sept 2022
Barnes GD, Burnett A, Allen A, Ansell J, Blumenstein M, Clark NP, Crowther M, Dager WE, Deitelzweig SB, Ellsworth S (2022) Thromboembolic prevention and anticoagulant therapy during the COVID-19 pandemic: updated clinical guidance from the anticoagulation forum. J Thromb Thrombol 54:197
Motloch LJ, Jirak P, Mirna M, Fiedler L, Davtyan PA, Lakman IA, Gareeva DF, Tyurin AV, Gumerov RM, Matskeplishvili ST (2022) Early antithrombotic post-discharge therapy using prophylactic DOAC or dipyridamole improves long-term survival and cardiovascular outcomes in hospitalized COVID-19 survivors. Front Cardiovasc Med 9:916156
Rashidi F, Barco S, Kamangar F, Heresi GA, Emadi A, Kaymaz C, Jansa P, Reis A, Rashidi A, Taghizadieh A, Rezaeifar P, Moghimi M, Ghodrati S, Mozafari A, Foumani AA, Tahamtan O, Rafiee E, Abbaspour Z, Khodadadi K, Alamdari G, Boodaghi Y, Rezaei M, Muhammadi MJ, Abbasi M, Movaseghi F, Koohi A, Shakourzad L, Ebrahimi F, Radvar S, Amoozadeh M, Fereidooni F, Naseari H, Movalled K, Ghorbani O, Ansarin K (2021) Incidence of symptomatic venous thromboembolism following hospitalization for coronavirus disease 2019: Prospective results from a multi-center study. Thromb Res 198:135–138. https://doi.org/10.1016/j.thromres.2020.12.001
Spyropoulos AC, Weitz JI (2020) Hospitalized COVID-19 patients and venous thromboembolism: a perfect storm. Am Heart Assoc
Spyropoulos AC, Lipardi C, Xu J, Peluso C, Spiro TE, De Sanctis Y, Barnathan ES, Raskob GE (2020) Modified IMPROVE VTE risk score and elevated D-dimer identify a high venous thromboembolism risk in acutely ill medical population for extended thromboprophylaxis. TH open 4:e59–e65
Tsaplin S, Schastlivtsev I, Zhuravlev S, Barinov V, Lobastov K, Caprini JA (2021) The original and modified Caprini score equally predicts venous thromboembolism in COVID-19 patients. J Vasc Surg Venous Lymphat Disord 9(1371–1381):e1374
Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian CD, Ageno W, Madjid M, Guo Y (2020) COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol 75:2950–2973
Engelen MM, Vandenbriele C, Balthazar T, Claeys E, Gunst J, Guler I, Jacquemin M, Janssens S, Lorent N, Liesenborghs L, Peerlinck K, Pieters G, Rex S, Sinonquel P, Van der Linden L, Van Laer C, Vos R, Wauters J, Wilmer A, Verhamme P, Vanassche T (2021) Venous thromboembolism in patients discharged after COVID-19 hospitalization. Semin Thromb Hemost 47(04):362–371. https://doi.org/10.1055/s-0041-1727284
Chekkal M, Deba T, Hadjali S, Lamara H, Oulaa H, Zouai K, Hariti G (2020) Prevention and treatment of COVID-19-associated hypercoagulability: recommendations of the Algerian society of transfusion and hemobiology. Transfus Clin Biol 27:203–206
Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, Levi M, Samama CM, Giannis D (2020) Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 18:1859
Yelin D, Moschopoulos CD, Margalit I, Gkrania-Klotsas E, Landi F, Stahl J-P, Yahav D (2022) ESCMID rapid guidelines for assessment and management of long COVID. Clin Microbiol Infect 28:955
Marietta M, Ageno W, Artoni A, De Candia E, Gresele P, Marchetti M, Marcucci R, Tripodi A (2020) COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfus 18:167
Giannis D, Allen SL, Tsang J, Flint S, Pinhasov T, Williams S, Tan G, Thakur R, Leung C, Snyder M (2021) Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood J Am Soc Hematol 137:2838–2847
Orsi FA, De Paula EV, Santos FdO, Teruchkin MM, Campêlo DHC, Mello TT, Chindamo MC, Macedo AVS, Rocha AT, Ramacciotti E (2020) Guidance on diagnosis, prevention and treatment of thromboembolic complications in COVID-19: a position paper of the Brazilian Society of Thrombosis and Hemostasis and the Thrombosis and Hemostasis Committee of the Brazilian Association of Hematology, Hemotherapy and Cellular Therapy. Hematol Transfus Cell Therapy 42:300–308
Linnemann B, Bauersachs R, Grebe M, Klamroth R, Müller O, Schellong S, Lichtenberg M (2020) Venous thromboembolism in patients with COVID-19 (SARS-CoV-2 infection)–a position paper of the German Society of Angiology (DGA). Vasa. https://doi.org/10.1024/0301-1526/a000885
Schulman S, Sholzberg M, Spyropoulos AC, Zarychanski R, Resnick HE, Bradbury CA, Broxmeyer L, Connors JM, Falanga A, Iba T (2022) ISTH guidelines for antithrombotic treatment in COVID-19. J Thromb Haemost 20:2214–2225
Watson RA, Johnson DM, Dharia RN, Merli GJ, Doherty JU (2020) Anti-coagulant and anti-platelet therapy in the COVID-19 patient: a best practices quality initiative across a large health system. Hosp Pract 48:169–179
Gerotziafas GT, Catalano M, Colgan M-P, Pecsvarady Z, Wautrecht JC, Fazeli B, Olinic D-M, Farkas K, Elalamy I, Falanga A (2020) Guidance for the management of patients with vascular disease or cardiovascular risk factors and COVID-19: position paper from VAS-European independent foundation in angiology/vascular medicine. Thromb Haemost 120:1597–1628
Engelmann B, Massberg S (2013) Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 13:34–45
Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, Muenchhoff M, Hellmuth JC, Ledderose S, Schulz H (2020) Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation 142:1176–1189
Hu C-S (2020) Analysis of COVID-19 cases and public measures in China. SN Compr Clin Med 2:1306–1312
Vaughn VM, Ratz D, McLaughlin ES, Horowitz JK, Flanders SA, Middleton EA, Grant PJ, Kaatz S, Barnes GD (2022) Eligibility for posthospitalization venous thromboembolism prophylaxis in hospitalized patients with COVID-19: a retrospective cohort study. J Am Heart Assoc 11:e025914
Tholin B, Fiskvik H, Tveita A, Tsykonova G, Opperud H, Busterud K, Mpinganzima C, Garabet L, Ahmed J, Stavem K (2022) Thromboembolic complications during and after hospitalization for COVID-19: incidence, risk factors and thromboprophylaxis. Thrombosis Update 6:100096
Ferreira C, da Rocha AP, Nunes S, de Oliveira MS, Prado CCL, Cristina V, Veiga C, Vidal ÁT, Xavier RM, Zavascki AP (2022) Brazilian Guidelines for the pharmacological treatment of patients hospitalized with COVID-19. Rev Bras Ter Intensiva 34:1–12
Patti G, Lio V, Cavallari I, Gragnano F, Riva L, Calabrò P, Di Pasquale G, Pengo V, Rubboli A (2020) Questions and answers on practical thrombotic issues in SARS-CoV-2 infection: a guidance document from the italian working group on atherosclerosis, thrombosis and vascular biology. Am J Cardiovasc Drugs 20:559–570
SIGN (2020) COVID-19 position statement: The prevention and management of thromboembolism in hospitalised patients with COVID-19-related disease. https://www.sign.ac.uk/media/1691/sg_prevention_of_thromboembolism_in_hospitalised_patients.pdf. Accessed 16 Jul 2020
Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, Holley AB, Jimenez D, Le Gal G, Rali P (2020) Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest 158:1143–1163
Zhai Z, Li C, Chen Y, Gerotziafas G, Zhang Z, Wan J, Liu P, Elalamy I, Wang C (2020) Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost 120:937–948
Acknowledgements
The authors thank Dr. Maryam Ravan and Dr. Mehrdad Farrokhi for critically reading the manuscript.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: MKA; methodology: RAB, MKA, BD; investigation and literature search: RAB, BD; data curation: RAB, BD; writing—original draft: RAB, BD, CMR; writing—review and editing: MKA, CMR; visualization: RAB; supervision and Project administration: RAB, MKA.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1
. Appendix A.1. Search strategy. Appendix A.2. Risk of bias assessment of included studies based on Newcastle-Ottawa Scale (NOS), adopted NOS, and Jadad scale.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amani-Beni, R., Kermani-Alghoraishi, M., Darouei, B. et al. A systematic review on post-discharge venous thromboembolism prophylaxis in patients with COVID-19. Egypt Heart J 75, 72 (2023). https://doi.org/10.1186/s43044-023-00400-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-023-00400-2