Abstract
Polycystic ovary syndrome (PCOS) is the most common gynecological endocrine disorders affecting up to 10% of all females in their reproductive age, and its cause of onset is still elusive. A spectrum of recent research reflected diverse associations between increased plasma level of anti-Mullerian hormone (AMH) and different clinical features of PCOS. Since AMH levels reflect the pool of growing follicles that potentially can ovulate, it can be stated that serum AMH levels can be used to assess the “functional ovarian reserve,” rather mentioning it as the “ovarian reserve.” AMH also appears to be a premier endocrine parameter for the assessment of atrophied ovarian follicular pool in response to age of individuals. AMH hinders the follicular development as well as the follicular recruitment and ultimately resulting in follicular arrest which is the key pathophysiologic condition for the onset of PCOS. Furthermore, FSH-induced aromatase activity remains inhibited by AMH that aids emergence of other associated clinical signs of PCOS, such as excess androgen, followed by insulin resistance among the PCOS individuals. Given the versatile association of AMH with PCOS and scarcity in literature explaining the underling mechanisms how AMH relates with PCOS, this review article will discuss the roles of AMH in the pathogenesis of PCOS which may introduce a new era in treatment approach of PCOS.
Similar content being viewed by others
Introduction
Polycystic ovary syndrome (PCOS) is the most common gynecological endocrine disorders affecting up to 10% of all females in their reproductive age [1, 2]. In the year of 1935, Stein and Leventhal first pointed out the PCOS condition among seven patients suffering from amenorrhea, infertility, and hirsutism [3]. The disease is mainly characterized by an excess availability of androgen and ovarian dysfunctions [4]. Several research articles indicate that PCOS is the most common endocrine and metabolic disorder in women of reproductive age [5,6,7]. The presence of any two of the conditions, i.e., the presence of oligo or anovulation, clinical or biochemical androgen excess or polycystic ovarian morphology detected by ultrasonography can be used as an identifying tool for the diagnosis of PCOS [8]. Insulin resistance, obesity and impaired gonadotropin release [3] is also correlated with PCOS. Apart from hormonal imbalance, genetic and environmental factors are also responsible for the disease [9]. Empirical studies carried out in pregnant rhesus monkeys treated with androgens showed morphological changes within ovary which are similar to PCOS and several PCOS associated symptoms in their female off-springs [10].
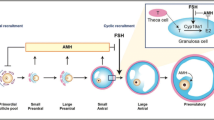
Anti-Müllerian hormone (AMH) known as Müllerian-inhibiting substance (MIS) is a homodimeric glycoprotein in nature belonging to the superfamily of transforming growth factor-β [11]. The “p” arm (small) of chromosome 19 contains the gene for AMH [12]. By considering its structure, AMH is also linked with other members of transforming growth β (TGF-β)-like inhibin and factor-bone morphogenetic proteins (BMP), etc., which are also regulator of ovarian folliculogenesis [13]. Besides that, they show wide range of functions due to their extensive expression as ligand, whereas the expression of AMH is limited to the primary sex organs only and thus probably exerting its action only on the reproductive organs [14]. Emerging evidences reveal that the serum level of AMH is 2–threefold increase in women suffering from PCOS compared to a normo-ovulatory control woman [15, 16]. AMH released from the granulosa cells of the ovarian follicles is a prime factor responsible for folliculogenesis in the ovaries [17]. In spite of substantial investigations and research work, the role of AMH remains elusive in PCOS, i.e., whether AMH is a significant marker of PCOS or a component accountable for PCOS. Henceforth, the aim of the present review article is to enumerate the physiological cross-talk between AMH and PCOS besides discussing relevant studies highlighting the possible roles of AMH in the complex pathophysiology of PCOS (Fig. 1).
AMH and its receptors in ovary
AMH is synthesized as a pro-hormone. After its secretion, it participates in various biochemical pathways to produce a transforming growth factor-β-like functionally active C-terminal disintegrates which are noncovalently-attached [18, 19]. Ovarian granulosa cells are the only source of AMH [20], its concentration is inversely proportional with age, and it becomes faint after the menopause [21]. But its minute alteration in concentration can be seen during the menstrual cycle which is not so much significant [22,23,24]. Sertoli cells also express AMH in male reproductive system which inhibits the development of Müllerian ducts during the embryonic life and thus acts as a marker for the sexual differentiation besides differences in two different reproductive tracts [25,26,27,28]. When there is no influence of AMH, the Müllerian ducts give rise to the development of fallopian tube, uterus, cervix, and the upper one third of the vagina, and any alterations in AMH levels or its receptors may disrupt the development of female reproductive system [29]. The appearance of AMH usually be observed from the primary follicle stage during folliculogenesis; meanwhile, as this phase is FSH-dependent, the peak level of AMH expression is also observed from pre-antral and small antral follicles developed through the folliculogenesis and then gradually decreases along with the size of the follicle viz. the absolute absence of expression will be seen in the follicles’ diameter with more than 8 mm [20, 30]. This pattern of AMH expression has been illustrated in several studies by comparing the expression of AMH-mRNA and AMH concentration respectively in the isolated human granulosa cells (GC) and in the follicular fluid [31, 32]. The expression of ovarian AMH and mRNA of anti-Müllerian hormone receptor type II (AMHRII) were explained by Baarends et al. [33] in in vivo adult rat model; also, it was observed that both the expressions were attenuated by the action of FSH and estrogen during the differentiation of antral follicles and which is thought to be a crucial event required for follicular selection [17, 34] as it is well-known that AMH hinders the follicular development. For the support of this last statement, an increased rate of folliculogenesis was observed with the involvement of multiple numbers of growing follicles in AMH knock-out animal [35, 36]. AMH-induced lack of FSH sensitivity was observed in granulosa cells, and it was also confirmed in vivo in AMH deficient mice [36]. In primates, declined follicular growth or development resulted by AMH due to depletion in FSH as well as cAMP-induced aromatase action indicating that AMH attenuated the mRNA expression of aromatase and mRNA expression of luteinizing hormone (LH) receptor-stimulated with cAMP and FSH, respectively [37]. In human granulosa cells, downregulation of aromatase mRNA expression and reduced synthesis of estradiol were obtained after the administration of AMH [34, 38]. Regarding the same, during the culture of the follicles, AMH was also responsible to lower the development of initially growing follicles [20]. Anti-Müllerian hormone receptor type I (AMHRI) and anti-Müllerian hormone receptor type II (AMHRII) are the two different types of transmembrane receptor proteins responsible for the AMH actions which are nothing, but the serine–threonine-specific kinase proteins and generally SMAD proteins [receptor-regulated Smads (R-Smad) and common Smad (Smad4)] [18] are the cytoplasmic effectors for these two receptors. These receptors are located on reproductive organs and on the Mullerian ducts [39]. Abundant expression of AMH and its receptor usually observed on the granulosa cells of follicles entered in preantral and small antral phases [40]. Establishment of expression of AMHRII mRNA in theca cells indicates a chance of intercellular AMH signaling control during the folliculogenesis [41]. Overall, AMH inhibits premature recruitment of the follicles and follicular maturation of the follicle during the folliculogenesis interestingly, and AMH will be suppressed if the follicles become large antral follicles followed by increased FSH (follicle-stimulating hormone) sensitivity leads to greater production of estrogen followed by two other physiological process, i.e., selection of follicle and successive release of ovum as seen in the normal ovary.
Role of AMH as a marker of ovarian reserve
The term “ovarian reserve” denotes the quantitative and qualitative measures of standing oocytes in both the ovaries, viz senile ovary can be defined as age related dwindle of ovarian reserve. Consequently, the remaining count of primordial follicles considered as a foremost marker to determine the ovarian reserve [42] which is burdensome to determine directly, although the width of the standing primordial follicular pool appears to be associated with the count of follicles that throw oneself into the pool of growing follicles [43,44,45] and these growing follicles are the only sources of AMH. Thus, circulating AMH level appears to indicate the size of the standing pool of primordial follicle as seen in different studies [46,47,48,49]. Nevertheless, there are no such clear evidences regarding the correlation between AMH level and oocyte quality, whereas the age-related declination of follicular pool was observed to be an explanation of diminished oocyte quality [50, 51]. Along with the number of primordial follicles, some endocrine parameters like FSH, estradiol, and inhibin B and ultrasonographic parameters like count of antral follicles and determination of ovarian volume are also considered as markers of ovarian reserve. Estimation of those parameters are directly or indirectly related with measurement of antral follicular pool. Quantitative assessment of antral follicle can be done directly by ultrasonographic estimation, whereas the estimation of inhibin B and estradiol levels during early follicular phase can be considered as indirect quantitative measurement of these antral follicles. On the other hand, both inhibin-B and estradiol are considered as strong predictive marker of antral follicular pool indirectly, as they usually control the FSH level by a negative feedback loop. Likewise, age-related declination of oocyte quantity leads to decreased levels of inhibin B and estradiol causes increase in FSH level [52]. But AMH is determined as a better marker forever for the quantitative assessment of oocyte/follicle pool than those three endocrine markers because of its stable plasma concentration even between the cycles. However, FSH, estradiol and inhibin B show fluctuations [53, 54] between the cycles. Henceforth, AMH demonstrates the sustained non-cyclic development of the follicles. Thus, comparatively less influence of AMH levels can be observed by such circumstances that deaden the later FSH-dependent phases of follicular growth as seen during pregnancy [55] or hormonal contraception [56, 57] or hormonal therapy with gonadotropin-releasing hormone (GnRH) agonist [58]. Moreover, regarding the ovarian reserve, the exact cutoff value of AMH is lacking till date; but multiple longitudinal evidences has described that AMH appeared to be as best hormonal parameter to determine the ovarian aging [59, 60] and can be used as a predictive marker for the onset of menopause [61,62,63]. Interestingly, in case of fertile or infertile women, stress or any other psychological conditions are not related to AMH level. However, the link in between obesity and AMH remains controversial [64,65,66,67,68] and probably that same arguments were also observed in the relationships between the AMH and body mass index (BMI) [69, 70]. On the other hand, as PCOS or IR (insulin resistance) is now a day being predicted by several anthropometric parameters like waist to height ratio (WHtR), waist to hip ratio (WHR), and waist circumference (WC) [71]; thus, in near future, it is a loop that increased AMH level might be predicted through these anthropometric parameters in near future.
Role of AMH in PCOS pathogenesis
Nowadays, PCOS has become the most frequent and concerning worldwide health issue among the women of reproductive age [72]. PCOS ovaries account for a huge presence of follicles with the diameter of up to 7 mm [73, 74], signifying the limited follicular growth at the moment when synthesis of AMH is highest. However, numerous observations explained that serum AMH level must be increased among the PCOS individuals as compared to control ovaries [59, 75, 76]. Moreover, the follicular fluid concentration of AMH was observed as five-folds greater among the anovulatory PCOS individuals when compared with ovulatory individuals [76]; it is because of sharp increased synthesis of AMH from each granulosa cells (approximately seventy-five-folds) in polycystic ovary (PCO) as compared with normal ovarian granulosa cells [34] as the granulosa cells of PCO may express increased AMH mRNA [77, 78]. These evidences conclude that increased AMH is not only due to the greater follicular count followed by greater granulosa cell density, but also due to increased synthesis in each granulosa cell causes excess AMH availability among the PCOS individuals. Thus, AMH was found to be associated to predict the harshness of PCO state including its diagnostic criteria like oligo/amenorrhea, hyperandrogenism, and polycystic ovarian morphology [79,80,81,82] conferring brace to the concept that, beside a biomarker, AMH has important contributions for the pathogenesis of PCOS too.
Role of AMH in alteration of gonadotropin functions
Several studies reported, about 50% of women suffering from PCOS has an elevated level of luteinizing hormone (LH) without any metabolic impairment [83, 84]. Few studies have reported as well in this regard and augmented GnRH secretion might result from failure of negative feedback following exposure of the prenatal hypothalamus to androgens [85]. On the other hand, the mean FSH level was found to be lower in comparison to the controls. Until now, the particular reason for such incidence remains elusive. Literature review revealed that in the past, a higher ratio of LH/FSH was used as a diagnostic criterion. Later on, it was found to be very much insensitive and was rejected. Moreover, it was found that AMH is responsible for the impairment of gonadotropin function.
Converging evidences propose that AMH and luteinizing hormone (LH) concentrations among the patients suffering from PCOS are positively correlated [86] irrespective of androgen and FSH concentrations [87,88,89]. However, there lies a controversy regarding this relationship/association. Several in vitro (from luteinized GCs) studies [90, 91] opined that LH can stimulate both release and expression of AMH, whereas according to in vivo studies, AMH synthesis starts in the primary follicles and increases before the release of LHR though expression of GC delays luteinizing hormone receptor (LHR) [20]. Conversely, empirical studies have also shown that AMH has extra gonadal effects as well as aids in the stimulation of GnRH neurons. Several research articles denoted that in murine and adult humans, 50% of GnRH neurons have AMH type 2 receptor [92]. Moreover, in vivo and in vitro studies proposed AMH stimulates the pulsatile release of GnRH-dependent LH via central action. The electrophysiological trials indicated exogenous AMH amplifies/augments GnRH neurons’ neuronal activity. Besides those, the AMHR2 is also distributed in the hypothalamic region and which is supposed to synergistically steers the synthesis and secretion of GnRH from the hypothalamic neuronal cells [93]. As the release of GnRH is steered by the hypothalamic neurons, the yield and pulsatility of LH in the anterior pituitary is elevated. Furthermore, AMH shows its effects on the pituitary level [94, 95] as well as control the functions of gonadotropic cells. The expression of AMHR2 gene in gonadotropic cells in both human and mouse were observed to be activated by GnRH. Converging evidences demonstrated that the release of GnRH at an elevated frequency (one pulse per 30 min) raised the expression of AMHR2 by the gonadotropic cells, whereas poor frequency (one pulse in every 2 h) has no consequence in primates [95, 96]. The relationship between GnRH pulsatility and activation of pituitary AMHR2 in humans, particularly individuals suffering from PCOS till date remain vague. Thus, still it is difficult to make any possible interlink between extra gonadal action of AMH and pathophysiological onset of PCOS in this regard. Apart from the high LH, low circulating FSH is found to be associated with PCOS and it is well known to all and subsequently alterations of physical morphology (e.g., high BMI, low WHtR), high WC or WHR, besides the intra-ovarian morphology like the presence of small diameter follicles usually found in PCOS individuals. AMH is associated with PCOS; however, the relationship between AMH and FSH levels is yet to be established; still, several works have been done in this perspective [97]. Again, congenital gonadotropic impairments lead to a decline in the AMH level, whereas it rises under the influence of exogenous FSH [98]. Henceforward, we can conclude that the relationship between AMH and FSH is obscure and it varies depending upon the disease condition. Additionally, activation of pulse frequency of GnRH and an increased AMH level raises the release of LH and decline in the FSH [99]. In other words, AMH is responsible for neuronal and hormonal dysregulation of PCOS; however, no human experiments have been found so far to prove the fact.
Role of AMH in irregular ovulation
It is now clear that, AMH restricts the folliculogenesis in the ovaries by fading the circulating levels and functions of FSH [100, 101]. But several studies reported that the grade of irregular ovulation is also associated with circulating AMH levels. Regarding the same, a group of researchers recommended that PCOS can be classified into individuals with ovulation and the individuals with anovulation, and the second one classification was made on the basis of circulating AMH as they were observed with eighteen times greater AMH levels than the PCOS individuals with ovulation (normo-ovulatory) [100]. Moreover, irrespective to polycystic ovarian condition, patients with anovulation were found with increased AMH levels (which was also found to be associated with the duration of menstrual cycle) as compared with normo-ovulatory individuals [102]. Probably, the two different reasons can be marked for the same, the increased number of antral follicles with small diameter (2 mm to 5 mm approximately) and increased level of AMH are positively correlated; parallelly, the follicular arrest usually seen among PCOS patients due to the inhibition of FSH action by the increased AMH levels and signifies the negative correlation between these two hormones [16, 103]. The count of the same size follicles was found to be positively associated with seriousness of menstrual irregularity among the PCOS women, and their association was found strongly among the individuals with amenorrhea [88, 104]. Eventually, increased level of AMH was also observed the adolescent individuals with oligomenorrhoea as compared to control subjects [105,106,107,108]. Besides those, AMH concentration was observed as a predictor for amenorrhea among the patients suffering from high circulating AMH [109,110,111]. These observations recommend that increased small antral follicles with diameters of 2–5 mm are common in the patients of PCOS suffering from anovulation too, probably responsible to build an intra-ovarian AMH reach condition which diminishes the FSH action during the follicular section and thus resulting as anovulation among the PCOS individuals. Some invitro studies as well as some studies on animal models have also confirmed the role of AMH on follicular development and expressions of PCOS characteristics. Although it is believed that the prognosis of anovulation is related to increased AMH level in PCOS, but the exact reason of the elevation of AMH is still in dark. However, the features which are firmly correlated to the pathogenesis of PCOS includes elevated LH level, hyperandrogenism, metabolic syndrome, and/or insulin resistance may be involved. In this regard, it has been seen that LH level and androgen concentration is correlated with AMH levels according to several research works [79,80,81, 112]. LH is believed to be in behind the elevated production of AMH from granulosa cells of polycystic ovaries only but not from normo-physiologic ovaries [91]. Furthermore, increased AMH expression was observed without any change of AMHRII appearance in the GCs of PCOS individuals with oligo/anovulation in response to LH, but this observation was not found among the ovulatory PCOS individuals or among control groups possess less AMHRII appearance in their granulosa cells [113, 114] which indicate the effect of LH on increased AMH level followed by AMH dominated restrictions in follicular growth. In addition to this, androgens regulate the FSH-dependent initial growth stages of follicles [115, 116] which might amplify an excess production of AMH. However, the fact remains controversial. Studies done by Carlsen et al. did not show any remarkable change in AMH concentration in PCOS while suppressing androgens for six months with administration of dexamethasone. He carried out a 6-month study through androgen suppression, and it did not show any alterations in the AMH level [117, 118]. Contrariwise, insulin resistance (IR), hyperinsulinemia, and homeostatic model assessment (HOMA-IR) were found to be associated with AMH concentration with positive correlation among the individuals suffering from PCOS [119,120,121]. Again, another study showed a positive relationship in between serum fasting insulin and AMH level in women irrespective of PCOS condition [122, 123]. According to few studies’ opinion, such alterations may be due to two different causes: either insulin typically affects granulosa cells which probably alters the synthesis and release of AMH or an augmented yield of androgen in hyperinsulinemia condition in PCOS might be responsible [123,124,125]. However, more research needs to be carried out to find out the correlation between insulin and AMH level as well as the rise in insulin-dependent AMH in PCOS individuals. Genetic factors might be another reason which can be responsible for the over-expression of AMH among the individuals with PCOS. The involvement of activin receptor-like kinase-2 (ALK2) and its receptor in follicle development during PCO morphology was investigated by Kevenaar et al., and they found a significant association between activin A receptor type 1 (ACVR1), serum AMH, and folliculogenesis among PCOS individuals suggesting the probable involvement of ALK2 pathway responsible for irregular ovulation in patients with PCOS [126]. A growing body of evidences denoted the dramatic as well as vital role of AMH for the conversion of primary follicles from primordial follicles in both control and PCOS individuals. AMH immunostaining is found to be lesser in the in primordial follicles of the anovulatory PCOS women in contrast to normo-ovulatory PCOS patients [30]. Remarkably, in anovulatory and normo-ovulatory PCOS women, the appearing strength of AMH on immunohistochemical method remained the same in both the follicles, i.e., pre-antral and antral follicles [30]. Thus, anovulatory PCOS women are consisting of low-grade hindrance of AMH on primordial follicles followed by early folliculogenesis resulting as aggregation of multiple pre-antral and/or small follicles subsequently causes excess synthesis and secretion of AMH correlating a loop of vicious cycle in positive feedback mechanism.
AMH and hyperandrogenism
Mounting evidences revealed that theca interna cells produce androgens and aromatase enzyme converts it to estrogen in the granulosa cells [127, 128]. Again, LH activates steroidogenesis and thereby yields androgens from the theca interna cells. Emerging studies denoted that in PCOS women, an increased serum level of AMH is positively correlated with serum androgens such as testosterone and androstenedione levels [122, 129, 130], which might be responsible for hyperandrogenism in women with PCOS. Literature review depicted that a decline in the aromatase activity in granulosa cells in polycystic ovaries might be a cause for AMH induced hyperandrogenism [96, 131]. Various researches tried to highlight the accurate role of AMH on CYP19 in granulosa cells and emerged a crucial fall in FSH induced estradiol synthesis through AMH-induced aromatase (CYP19) inhibition in granulosa cells [132,133,134]. Such relationship might be a cause for the interrelationship of an increased AMH level and poor level of estradiol in PCOS [134]. Chang et al. reported a similar decline in aromatase mRNA expression followed by estrogen production due to reduced FSH in response to AMH which in turn causes an elevated level of androgens consecutively and such incident indicates the paracrine action of AMH on theca interna cells resulting in alteration of normal ovarian physiology and proceedings of PCO condition [135]. Moreover, AMH-mediated hindrance of FSH-dependent aromatase activity might be responsible for the irregular development of follicles in PCOS. Thus, AMH may be responsible for hyperandrogenism in women suffering from PCOS although, associated other factors may also be directed for the hormonal alteration in PCOS.
Conclusions
This review has concisely explained the association of AMH with the pathophysiology and clinical observations of PCOS. To summarize, AMH represses follicular developments, recruitments, and cause anovulation. The key underlying mechanisms may include AMH-mediated hyperandrogenism and IR in women with PCOS. Increased level of AMH may even attribute to failure in basic treatment outcomes for PCOS through weight reduction, ovulation induction, etc. However, several studies have reported that AMH levels can be managed by drugs like clomiphene citrate or metformin, along with receptor level modifications. Extensive studies are still required to fully understand the detailed role of AMH in the etiopathology of PCOS which will also show future path to treat the disease clinically.
Availability of data and materials
Not applicable.
Abbreviations
- AMH:
-
Anti-Müllerian hormone
- MIS:
-
Müllerian-inhibiting substance
- TGF-β:
-
Transforming growth β
- BMP:
-
Bone morphogenetic proteins
- AMHRII:
-
Anti-Müllerian hormone receptor type II
- AMHRI:
-
Anti-Müllerian hormone receptor type I
- SMAD:
-
Suppressor of mothers against decapentaplegic
- FSH:
-
Follicle-stimulating hormone
- GnRH:
-
Gonadotropin-releasing hormone
- IR:
-
Insulin resistance
- WHtR:
-
Waist to height ratio
- WHR:
-
Waist to hip ratio
- WC:
-
Waist circumference
- PCO:
-
Polycystic ovary
- LH:
-
Luteinizing hormone
- LHR:
-
Luteinizing hormone receptor
- GC:
-
Granulosa cells
- HOMA-IR:
-
Homeostatic model assessment for insulin resistance
- ALK2:
-
Activin receptor-like kinase-2
- CYP19:
-
Aromatase enzyme complex
References
Wolf WM, Wattick RA, Kinkade ON, Olfert MD (2018) Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Env Res Pub Health 15(11):2589
Escobar-Morreale HF (2018) Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 14(5):270–284
Azziz R, Adashi EY, Stein, (2016) Leventhal: 80 years on. Am J Obstet Gynecol 214(2):247 (e1-24 7.e11)
Rosenfield RL (2020) Current concepts of polycystic ovary syndrome pathogenesis. Curr Opinion Pediat 32(5):698
Bhattacharya K, Sengupta P, Dutta S, Chaudhuri P, Das Mukhopadhyay L, Syamal AK (2021) Waist-to-height ratio and BMI as predictive markers for insulin resistance in women with PCOS in Kolkata, India. Endocrine 72(1):86–95
Bachelot A (2016) Polycystic ovarian syndrome: clinical and biological diagnosis. Ann Biol Clin (Paris) 74(6):661–667
Hassan MF, Sengupta P, Dutta S (2021) Assisted reproductive technologies for women with polycystic ovarian syndrome. Biomed Pharmacol J 14:1305–1309
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81(1):19–25
Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI (2006) Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab 91(6):2100–2104
Abbott DH, Tarantal AF, Dumesic DA (2009) Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol 71(9):776–784
Yu X, Li Z, Zhao X, Hua L, Liu S, He C, Yang L, Davis JS, Liang A (2022) Anti-Müllerian hormone inhibits FSH-induced cumulus oocyte complex in vitro maturation and cumulus expansion in mice. Animals 12(9):1209
Josso N, Picard JY (2022) Genetics of anti-Müllerian hormone and its signaling pathway. Best Prac Res Clin Endocrinol Metab 25:101634
Knight PG, Glister C (2006) TGF-beta superfamily members and ovarian follicle development. Reproduction 132(2):191–206
Rifkin D, Sachan N, Singh K, Sauber E, Tellides G, Ramirez F (2022) The role of LTBPs in TGF beta signaling. Dev Dyn 251(1):95–104
Ramezani Tehrani F, Rahmati M, Mahboobifard F, Firouzi F, Hashemi N, Azizi F (2021) Age-specific cut-off levels of anti-Müllerian hormone can be used as diagnostic markers for polycystic ovary syndrome. Reprod Biol Endocrinol 19(1):76
Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S et al (2003) Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab 88(12):5957–5962
Visser JA, de Jong FH, Laven JS, Themmen AP (2006) Anti-Müllerian hormone: a new marker for ovarian function. Reproduction 131(1):1–9
Olumide OB, Godwin AI, Titilayo JO, Christian IO, Etukudoh NS, Uchejeso OM, Temitope ST, Dutta S, Sengupta P. Assessment of serum anti-Müllerian hormone (AMH) as an independent marker for oligozoospermia and non-obstructive azoospermia in infertile Nigerian men. Biomed Pharmacol J 2022.
Peigné M, Pigny P, Pankhurst MW, Drumez E, Loyens A, Dewailly D et al (2020) The proportion of cleaved anti-Müllerian hormone is higher in serum but not follicular fluid of obese women independently of polycystic ovary syndrome. Reprod Biomed Online 41(6):1112–1121
Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA et al (2004) Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 10(2):77–83
Bertone-Johnson ER, Manson JE, Purdue-Smithe AC, Steiner AZ, Eliassen AH, Hankinson SE et al (2018) Anti-Müllerian hormone levels and incidence of early natural menopause in a prospective study. Hum Reprod 33(6):1175–1182
La Marca A, Stabile G, Artenisio AC, Volpe A (2006) Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod 21(12):3103–3107
Gorkem U, Togrul C (2019) Is there a need to alter the timing of anti-Müllerian hormone measurement during the menstrual cycle? Geburtshilfe Frauenheilkd 79(7):731–737
Kissell KA, Danaher MR, Schisterman EF, Wactawski-Wende J, Ahrens KA, Schliep K et al (2014) Biological variability in serum anti-Müllerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod 29(8):1764–1772
Rajpert-De Meyts E, Jørgensen N, Graem N, Müller J, Cate RL, Skakkebaek NE (1999) Expression of anti-Müllerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab 84(10):3836–3844
Rey R (2005) Anti-Müllerian hormone in disorders of sex determination and differentiation. Arq Bras Endocrinol Metabol 49(1):26–36
Chaudhuri GR, Das A, Kesh SB, Bhattacharya K, Dutta S, Sengupta P et al (2022). Obesity and male infertility: multifaceted reproductive disruption. Middle East Fertil Soc J;27:8. https://doi.org/10.1186/s43043-022-00099-2
Sengupta P, Dutta S, Karkada IR, Chinni SV (2021) Endocrinopathies and male infertility. Life (Basel) 12(1):10
Lemcke RA, Stephens CS, Hildebrandt KA, Johnson PA (2018) Anti-Müllerian hormone type II receptor in avian follicle development. Biol Reprod 99(6):1227–1234
Stubbs SA, Hardy K, Da Silva-Buttkus P, Stark J, Webber LJ, Flanagan AM et al (2005) Anti-müllerian hormone protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. J Clin Endocrinol Metab 90(10):5536–5543Predictive factors of ovarian response to GnRH antagonist
Andersen CY, Schmidt KT, Kristensen SG, Rosendahl M, Byskov AG, Ernst E (2010) Concentrations of AMH and inhibin-B in relation to follicular diameter in normal human small antral follicles. Hum Reprod 25(5):1282–1287
Jeppesen JV, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K et al (2013) (2013) Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod 19(8):519–527
Gültiken N, Yarim M, Aslan S, Gürler H, Yarim GF, Tuncay M, İnal S, Schäfer-Somi S (2022) Expression of anti-Müllerian hormone and its type 2 receptor in the ovary of pregnant and cyclic domestic cats. Animals 12(7):877
Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S et al (2007) Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab 92(1):240–245
Almeida FR, Costermans NG, Soede NM, Bunschoten A, Keijer J, Kemp B, Teerds KJ (2018) Presence of anti-Müllerian hormone (AMH) during follicular development in the porcine ovary. PLoS ONE 13(7):e0197894
Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM et al (2001) Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 142(11):4891–4899
Clemente ND, Goxe B, Rémy JJ, Cate R, Josso N, Vigier B et al (1994). Inhibitory effect of AMH upon the expression of aromatase and LH receptors by cultured granulosa cells of rat and porcine immature ovaries. Endocrine (United Kingdom).
Grossman MP, Nakajima ST, Fallat ME, Siow Y (2008) Müllerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil Steril 89(5 Suppl):1364–1370
La Marca A, Volpe A (2006) Anti-Müllerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol (Oxf) 64(6):603–610
Zhao Z, Guo F, Sun X, He Q, Dai Z, Chen X, Zhao Y, Wang J (2018) BMP15 regulates AMH expression via the p38 MAPK pathway in granulosa cells from goat. Theriogenology 118:72–79
Almeida FRCL, Costermans NGJ, Soede NM, Bunschoten A, Keijer J, Kemp B et al (2018) Presence of anti-Müllerian hormone (AMH) during follicular development in the porcine ovary. PLoS ONE 13(7):e0197894
Nakamura S, Tanaka IB III, Komura J, Tanaka S (2022) Premature menopause and obesity due to oocyte loss in female mice chronically exposed to low dose-rate γ-rays. Rad Protect Dosim 198(13–15):926–933
Schuh SM, Kadie J, Rosen MP, Sternfeld B, Pera RA, Cedars MI (2019) Links between age at menarche, antral follicle count, and body mass index in African American and European American women. Fertil Steril 111(1):122–131
Grynberg M, Labrosse J, Bennani Smires B, Sifer C, Peigne M, Sonigo C (2021) Could hormonal and follicular rearrangements explain timely menopause in unilaterally oophorectomized women? Hum Reprod 36(7):1941–1947
Iwase A, Sugita A, Hirokawa W, Goto M, Yamamoto E, Takikawa S (2013) Anti-Müllerian hormone as a marker of ovarian reserve following chemotherapy in patients with gestational trophoblastic neoplasia. Eur J Obstet Gynecol Reprod Biol 167(2):194–198
Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, Groome NP et al (2006) Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology 147(7):3228–3234
Appt SE, Clarkson TB, Chen H, Adams MR, Christian PJ, Hoyer PB et al (2009) Serum antimüllerian hormone predicts ovarian reserve in a monkey model. Menopause 16(3):597–601
Vignali M, Mabrouk M, Ciocca E, Alabiso G, Barbasetti di Prun A et al (2015) Surgical excision of ovarian endometriomas: does it truly impair ovarian reserve? Long term anti-Müllerian hormone (AMH) changes after surgery. J Obstet Gynaecol Res 41(11):1773–1778
Iwase A, Osuka S, Goto M, Murase T, Nakamura T, Takikawa S et al (2018) Clinical application of serum anti-Müllerian hormone as an ovarian reserve marker: a review of recent studies. J Obstet Gynaecol Res 44(6):998–1006
Pellestor F, Andréo B, Arnal F, Humeau C, Demaille J (2003) Maternal aging and chromosomal abnormalities: new data drawn from in vitro unfertilized human oocytes. Hum Genet 112(2):195–203
Laqqan MM, Yassin MM (2021) Predictive factors of ovarian response to GnRH antagonist stimulation protocol: AMH and age are potential candidates. Middle East Fertil Soc J;26: 16. https://doi.org/10.1186/s43043-021-00062-7
Burger HG, Dudley EC, Hopper JL, Shelley JM, Green A, Smith A et al (1995) The endocrinology of the menopausal transition: a cross-sectional study of a population-based sample. J Clin Endocrinol Metab 80(12):3537–45
de Vet A, Laven JSE, de Jong FH, Themmen APN, Fauser BCJM (2019) Reprint of: Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 112(4 Suppll):e183–e188
Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ (2006) Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab 91(10):4057–4063
La Marca A, Giulini S, Orvieto R, De Leo V, Volpe A (2005) Anti-Müllerian hormone concentrations in maternal serum during pregnancy. Hum Reprod 20(6):1569–1572
Kulshrestha R, Barman SS, Bhattacharya S, Chakrabarty A, Bhattacharya K (2018) Emergency contraception: a quick lesson. Int J Res Pharm Sci 10(1):8–9
Li HW, Wong CY, Yeung WS, Ho PC, Ng EH (2011) Serum anti-müllerian hormone level is not altered in women using hormonal contraceptives. Contraception 83(6):582–585
Mohamed KA, Davies WA, Lashen H (2006) Antimüllerian hormone and pituitary gland activity after prolonged down-regulation with goserelin acetate. Fertil Steril 86(5):1515–1517
Mulders AG, Laven JS, Eijkemans MJ, de Jong FH, Themmen AP, Fauser BC (2004) Changes in anti-Müllerian hormone serum concentrations over time suggest delayed ovarian ageing in normogonadotrophic anovulatory infertility. Hum Reprod 19(9):2036–2042
van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH et al (2005) Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril 83(4):979–987
Tehrani FR, Solaymani-Dodaran M, Azizi F (2009) A single test of antimullerian hormone in late reproductive-aged women is a good predictor of menopause. Menopause 16(4):797–802
Biniasch M, Laubender RP, Hund M, Buck K, De Geyter C (2021) Intra- and inter-cycle variability of anti-Müllerian hormone (AMH) levels in healthy women during non-consecutive menstrual cycles: the BICYCLE study. Clin Chem Lab Med 60(4):597–605
Broer SL, Eijkemans MJ, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP et al (2011) Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab 96(8):2532–2539
Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF 3rd (2007) Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril 87(1):101–106
Moy V, Jindal S, Lieman H, Buyuk E (2015) Obesity adversely affects serum anti-müllerian hormone (AMH) levels in Caucasian women. J Assist Reprod Genet 32(9):1305–1311
Luo E, Zhang J, Song J, Feng D, Meng Y, Jiang H et al (2021) Serum anti-Müllerian hormone levels were negatively associated with body fat percentage in PCOS patients. Front Endocrinol (Lausanne) 12:659717
Olszanecka-Glinianowicz M, Madej P, Owczarek A, Chudek J, Skałba P (2015) Circulating anti-Müllerian hormone levels in relation to nutritional status and selected adipokines levels in polycystic ovary syndrome. Clin Endocrinol (Oxf) 83(1):98–104
Ou M, Xu P, Lin H, Ma K, Liu M (2021) AMH is a good predictor of metabolic risk in women with PCOS: a cross-sectional study. Int J Endocrinol 2021:9511772
Simões-Pereira J, Nunes J, Aguiar A, Sousa S, Rodrigues C, Sampaio Matias J et al (2018) Influence of body mass index in anti-Müllerian hormone levels in 951 non-polycystic ovarian syndrome women followed at a reproductive medicine unit. Endocrine 61(1):144–148
Albu D, Albu A (2019) The relationship between anti-Müllerian hormone serum level and body mass index in a large cohort of infertile patients. Endocrine 63(1):157–163
Liu T, Wang Q, Huang W, Tan J, Liu D, Pei T et al (2019) Anthropometric indices to predict insulin resistance in women with polycystic ovary syndrome in China. Reprod Biomed Online 38(1):101–107
Velusami D, Sivasubramanian S (2018) Sympathovagal imbalance and neurophysiologic cognitive assessment using evoked potentials in polycystic ovary syndrome in young adolescents - a cross-sectional study. J Basic Clin Physiol Pharmacol 30(2):233–237
Franks S, Mason H, Willis D (2000) Follicular dynamics in the polycystic ovary syndrome. Mol Cell Endocrinol 163(1–2):49–52
Franks S, McCarthy MI, Hardy K (2006) Development of polycystic ovary syndrome: involvement of genetic and environmental factors. Int J Androl 29(1):278–85 (discussion 286-90)
Hsu JY, James KE, Bormann CL, Donahoe PK, Pépin D, Sabatini ME (2018) Müllerian-inhibiting substance/anti-Müllerian hormone as a predictor of preterm birth in polycystic ovary syndrome. J Clin Endocrinol Metab 103(11):4187–4196
Das M, Gillott DJ, Saridogan E, Djahanbakhch O (2008) Anti-Mullerian hormone is increased in follicular fluid from unstimulated ovaries in women with polycystic ovary syndrome. Hum Reprod 23(9):2122–2126
Catteau-Jonard S, Jamin SP, Leclerc A, Gonzalès J, Dewailly D, di Clemente N (2008) Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab 93(11):4456–4461
Du DF, Li XL, Zheng SH (2016) Expression of anti-Müllerian hormone in two rat models of polycystic ovary syndrome. J Obstet Gynaecol Res 42(12):1761–1767
Homburg R, Ray A, Bhide P, Gudi A, Shah A, Timms P et al (2013) The relationship of serum anti-Mullerian hormone with polycystic ovarian morphology and polycystic ovary syndrome: a prospective cohort study. Hum Reprod 28(4):1077–1083
Lin YH, Chiu WC, Wu CH, Tzeng CR, Hsu CS, Hsu MI (2011) Antimüllerian hormone and polycystic ovary syndrome. Fertil Steril 96(1):230–235
Tal R, Seifer DB, Khanimov M, Malter HE, Grazi RV, Leader B (2014) Characterization of women with elevated antimüllerian hormone levels (AMH): correlation of AMH with polycystic ovarian syndrome phenotypes and assisted reproductive technology outcomes. Am J Obstet Gynecol 211(1):59.e1–8
Łebkowska A, Kowalska I (2017) Anti-Müllerian hormone and polycystic ovary syndrome. Endokrynol Pol 68(1):74–78
Rosenfield RL, Ehrmann DA (2016) The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev 37(5):467–520
Huang CC, Tien YJ, Chen MJ, Chen CH, Ho HN, Yang YS (2015) Symptom patterns and phenotypic subgrouping of women with polycystic ovary syndrome: association between endocrine characteristics and metabolic aberrations. Hum Reprod 30(4):937–946
Walters KA, Gilchrist RB, Ledger WL, Teede HJ, Handelsman DJ, Campbell RE (2018) New perspectives on the pathogenesis of PCOS: neuroendocrine origins. Trends Endocrinol Metab 29(12):841–852
Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BC (2004) Anti-Müllerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab 89(1):318–323
Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S (2016) Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum Reprod Update 22(6):709–724
Dewailly D, Pigny P, Soudan B, Catteau-Jonard S, Decanter C, Poncelet E et al (2010) Reconciling the definitions of polycystic ovary syndrome: the ovarian follicle number and serum anti-Müllerian hormone concentrations aggregate with the markers of hyperandrogenism. J Clin Endocrinol Metab 95(9):4399–4405
Catteau-Jonard S, Pigny P, Reyss AC, Decanter C, Poncelet E, Dewailly D (2007) Changes in serum anti-mullerian hormone level during low-dose recombinant follicular-stimulating hormone therapy for anovulation in polycystic ovary syndrome. J Clin Endocrinol Metab 92(11):4138–4143
Taieb J, Grynberg M, Pierre A, Arouche N, Massart P, Belville C et al (2011) FSH and its second messenger cAMP stimulate the transcription of human anti-Müllerian hormone in cultured granulosa cells. Mol Endocrinol 25(4):645–655
Dilaver N, Pellatt L, Jameson E, Ogunjimi M, Bano G, Homburg R et al (2019) The regulation and signalling of anti-Müllerian hormone in human granulosa cells: relevance to polycystic ovary syndrome. Hum Reprod 34(12):2467–2479
Cimino I, Casoni F, Liu X, Messina A, Parkash J, Jamin SP et al (2016) Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun 7:10055
Barbotin AL, Peigné M, Malone SA, Giacobini P (2019) Emerging roles of anti-Müllerian hormone in hypothalamic-pituitary function. Neuroendocrinology 109(3):218–229
Garrel G, Racine C, L’Hôte D, Denoyelle C, Guigon CJ, di Clemente N et al (2016) Anti-Müllerian hormone: a new actor of sexual dimorphism in pituitary gonadotrope activity before puberty. Sci Rep 6:23790
Silva MSB, Giacobini P (2021) New insights into anti-Müllerian hormone role in the hypothalamic-pituitary-gonadal axis and neuroendocrine development. Cell Mol Life Sci 78(1):1–16
Dewailly D, Barbotin AL, Dumont A, Catteau-Jonard S, Robin G (2020) Role of anti-Müllerian hormone in the pathogenesis of polycystic ovary syndrome. Front Endocrinol (Lausanne) 11:641
Wiweko B, Maidarti M, Priangga MD, Shafira N, Fernando D, Sumapraja K et al (2014) Anti-mullerian hormone as a diagnostic and prognostic tool for PCOS patients. J Assist Reprod Genet;31(10):1311–6.
Bry-Gauillard H, Larrat-Ledoux F, Levaillant JM, Massin N, Maione L, Beau I et al (2017) Anti-Müllerian hormone and ovarian morphology in women with isolated hypogonadotropic hypogonadism/Kallmann syndrome: effects of recombinant human FSH. J Clin Endocrinol Metab 102(4):1102–1111
Le MT, Le VNS, Le DD, Nguyen VQH, Chen C, Cao NT (2019) Exploration of the role of anti-Mullerian hormone and LH/FSH ratio in diagnosis of polycystic ovary syndrome. Clin Endocrinol (Oxf) 90(4):579–585
Pellatt L, Rice S, Mason HD (2010) Anti-Müllerian hormone and polycystic ovary syndrome: a mountain too high? Reproduction 139(5):825–833
Singer T, Barad DH, Weghofer A, Gleicher N (2009) Correlation of antimüllerian hormone and baseline follicle-stimulating hormone levels. Fertil Steril 91(6):2616–2619
Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS (2005) Serum anti-Müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod 20(7):1820–1826
Stracquadanio M, Ciotta L, Palumbo MA (2018) Relationship between serum anti-Mullerian hormone and intrafollicular AMH levels in PCOS women. Gynecol Endocrinol 34(3):223–228
Dewailly D, Catteau-Jonard S, Reyss AC, Maunoury-Lefebvre C, Poncelet E, Pigny P (2007) The excess in 2–5 mm follicles seen at ovarian ultrasonography is tightly associated to the follicular arrest of the polycystic ovary syndrome. Hum Reprod 22(6):1562–1566
Park AS, Lawson MA, Chuan SS, Oberfield SE, Hoeger KM, Witchel SF et al (2010) Serum anti-mullerian hormone concentrations are elevated in oligomenorrheic girls without evidence of hyperandrogenism. J Clin Endocrinol Metab 95(4):1786–1792
Caanen MR, Peters HE, van de Ven PM, Jüttner AMFM, Laven JSE, van Hooff MHA et al (2021) Anti-Müllerian hormone levels in adolescence in relation to long-term follow-up for presence of polycystic ovary syndrome. J Clin Endocrinol Metab 106(3):e1084–e1095
Pinola P, Morin-Papunen LC, Bloigu A, Puukka K, Ruokonen A, Järvelin MR et al (2014) Anti-Müllerian hormone: correlation with testosterone and oligo- or amenorrhoea in female adolescence in a population-based cohort study. Hum Reprod 29(10):2317–2325
Lauritsen MP, Bentzen JG, Pinborg A, Loft A, Forman JL, Thuesen LL et al (2014) The prevalence of polycystic ovary syndrome in a normal population according to the Rotterdam criteria versus revised criteria including anti-Mullerian hormone. Hum Reprod 29(4):791–801
Cui Y, Shi Y, Cui L, Han T, Gao X, Chen ZJ (2014) Age-specific serum antimüllerian hormone levels in women with and without polycystic ovary syndrome. Fertil Steril 102(1):230-236.e2
Tal R, Tal O, Seifer BJ, Seifer DB (2015) Antimüllerian hormone as predictor of implantation and clinical pregnancy after assisted conception: a systematic review and meta-analysis. Fertil Steril 103(1):119–30.e3
Aleyasin A, Aghahoseini M, Mokhtar S, Fallahi P (2011) Anti-mullerian hormone as a predictive factor in assisted reproductive technique of polycystic ovary syndrome patients. Acta Med Iran 49(11):715–720 (PMID: 22131240)
Lie Fong S, Laven JSE, Duhamel A, Dewailly D (2017) Polycystic ovarian morphology and the diagnosis of polycystic ovary syndrome: redefining threshold levels for follicle count and serum anti-Müllerian hormone using cluster analysis. Hum Reprod 32(8):1723–1731
Qi X, Pang Y, Qiao J (2016) The role of anti-Müllerian hormone in the pathogenesis and pathophysiological characteristics of polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 199:82–87
Pierre A, Peigné M, Grynberg M, Arouche N, Taieb J, Hesters L et al (2013) Loss of LH-induced down-regulation of anti-Müllerian hormone receptor expression may contribute to anovulation in women with polycystic ovary syndrome. Hum Reprod 28(3):762–769
Weil S, Vendola K, Zhou J, Bondy CA (1999) Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab 84(8):2951–2956
Orisaka M, Miyazaki Y, Shirafuji A, Tamamura C, Tsuyoshi H, Tsang BK, Yoshida Y (2021) The role of pituitary gonadotropins and intraovarian regulators in follicle development: a mini-review. Reprod Med Biol 20(2):169–175
Carlsen SM, Vanky E, Fleming R (2009) Anti-Müllerian hormone concentrations in androgen-suppressed women with polycystic ovary syndrome. Hum Reprod 24(7):1732–1738
Parahuleva N, Pehlivanov B, Dimitrakova E, Malinova M, Mladenova M (2012) Anti-Mullerian hormone- its role in the pathogenesis of the polycystic ovary syndrome. Akush Ginekol (Sofiia) 51(6):22–26
La Marca A, Malmusi S, Giulini S, Tamaro LF, Orvieto R, Levratti P et al (2004) Anti-Müllerian hormone plasma levels in spontaneous menstrual cycle and during treatment with FSH to induce ovulation. Hum Reprod 19(12):2738–2741
Elgindy EA, El-Haieg DO, El-Sebaey A (2008) Anti-Müllerian hormone: correlation of early follicular, ovulatory and midluteal levels with ovarian response and cycle outcome in intracytoplasmic sperm injection patients. Fertil Steril 89(6):1670–1676
La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V (2004) Mullerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertil Steril 82(4):970–972
Nardo LG, Yates AP, Roberts SA, Pemberton P, Laing I (2009) The relationships between AMH, androgens, insulin resistance and basal ovarian follicular status in non-obese subfertile women with and without polycystic ovary syndrome. Hum Reprod 24(11):2917–2923
Skałba P, Cygal A, Madej P, Dąbkowska-Huć A, Sikora J, Martirosian G et al (2011) Is the plasma anti-Müllerian hormone (AMH) level associated with body weight and metabolic, and hormonal disturbances in women with and without polycystic ovary syndrome? Eur J Obstet Gynecol Reprod Biol 158(2):254–259
Park HT, Cho GJ, Ahn KH, Shin JH, Kim YT, Hur JY et al (2010) Association of insulin resistance with anti-Mullerian hormone levels in women without polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf) 72(1):26–31
Caglar GS, Kahyaoglu I, Pabuccu R, Demirtas S, Seker R (2013) Anti-Mullerian hormone and insulin resistance in classic phenotype lean PCOS. Arch Gynecol Obstet 288(4):905–910
Kevenaar ME, Themmen AP, van Kerkwijk AJ, Valkenburg O, Uitterlinden AG, de Jong FH et al (2009) Variants in the ACVR1 gene are associated with AMH levels in women with polycystic ovary syndrome. Hum Reprod 24(1):241–249
Ashraf S, Rasool SUA, Nabi M, Ganie MA, Masoodi SR, Amin S (2021) Impact of rs2414096 polymorphism of CYP19 gene on susceptibility of polycystic ovary syndrome and hyperandrogenism in Kashmiri women. Sci Rep 11(1):12942
Aghaie F, Khazali H, Hedayati M, Akbarnejad A (2018) The effects of exercise on expression of CYP19 and StAR mRNA in steroid-induced polycystic ovaries of female rats. Int J Fertil Steril 11(4):298–303
Cassar S, Teede HJ, Moran LJ, Joham AE, Harrison CL, Strauss BJ et al (2014) Polycystic ovary syndrome and anti-Müllerian hormone: role of insulin resistance, androgens, obesity and gonadotrophins. Clin Endocrinol (Oxf) 81(6):899–906
Sova H, Unkila-Kallio L, Tiitinen A, Hippeläinen M, Perheentupa A, Tinkanen H et al (2019) Hormone profiling, including anti-Müllerian hormone (AMH), for the diagnosis of polycystic ovary syndrome (PCOS) and characterization of PCOS phenotypes. Gynecol Endocrinol 35(7):595–600
Sacchi S, D’Ippolito G, Sena P, Marsella T, Tagliasacchi D, Maggi E, Argento C, Tirelli A, Giulini S, La Marca A (2016) The anti-Müllerian hormone (AMH) acts as a gatekeeper of ovarian steroidogenesis inhibiting the granulosa cell response to both FSH and LH. J Assist Reprod Genet 33(1):95–100
Tchoudakova A, Callard GV (1998) Identification of multiple CYP19 genes encoding different cytochrome P450 aromatase isozymes in brain and ovary. Endocrinology 139(4):2179–2189
Signorile PG, Petraglia F, Baldi A (2014) Anti-mullerian hormone is expressed by endometriosis tissues and induces cell cycle arrest and apoptosis in endometriosis cells. J Exp Clin Cancer Res 33(1):46
Chang HM, Klausen C, Leung PC (2013) Antimüllerian hormone inhibits follicle-stimulating hormone-induced adenylyl cyclase activation, aromatase expression, and estradiol production in human granulosa-lutein cells. Fertil Steril 100(2):585–92.e1
Ingraham HA, Hirokawa Y, Roberts LM, Mellon SH, McGee E, Nachtigal MW et al (2000) Autocrine and paracrine Müllerian inhibiting substance hormone signaling in reproduction. Recent Prog Horm Res 55:53–67
Acknowledgements
Not applicable
Funding
None.
Author information
Authors and Affiliations
Contributions
KB, PS, and SD designed and planned the research. IS, DS, CB, GRC, SB, SSB, KB, SD, PS, and AKS wrote the article and made the final revisions. The final corrections and adjustments have been made by KB and PS. The authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhattacharya, K., Saha, I., Sen, D. et al. Role of anti-Mullerian hormone in polycystic ovary syndrome. Middle East Fertil Soc J 27, 32 (2022). https://doi.org/10.1186/s43043-022-00123-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-022-00123-5