Abstract
Background
Prediction of ovarian response prior to the ovarian stimulation cycle is useful in determining the optimal starting dose of recombinant follicle-stimulating hormone (r-FSH). This study was designed to (I) evaluate which of the following parameters (age, AMH, and FSH) can be used as a predictor of ovarian response to GnRH antagonist stimulation protocol, (II) determine the cutoff value of AMH and age for predicting poor and high ovarian response, and (III) investigate the relationship between age, AMH level, and other clinical parameters. It is a retrospective study. A total of 318 women with a mean age of 28.2 ± 5.9 years old were included in this study. Hormone levels (FSH, LH, PRL, E2, and AMH) and the number of collected oocytes were determined. Based on the number of retrieved oocytes, the participants were divided into three groups: poor response (oocytes < 4, n= 51), normal response (oocytes 4–14, n= 192), and high response (oocytes > 14, n= 75).
Results
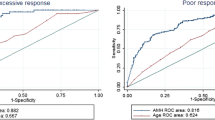
A significant increase has been found in AMH level and number of retrieved oocytes and mature oocytes from low to normal and high ovarian response group (P < 0.001). Also, the age in the poor ovarian response group was significantly greater than normal and high ovarian response groups (P < 0.001). A significant positive correlation has been found between the number of retrieved oocytes and mature oocytes and level of AMH (P < 0.001). The receiver operating characteristic (ROC) curves showed that both AMH and age had the highest accuracy in the prediction of poor ovarian response with a cutoff value < 1.45 and > 31.5 years, respectively. Additionally, the ROC analysis has shown that the AMH had the highest accuracy, followed by age in the prediction of high ovarian response with a cutoff value > 3.55 and < 27.5 years, respectively.
Conclusions
This study demonstrates that AMH level and women’s age may be used as potential predictors of ovarian response to GnRH antagonist stimulation protocol.
Similar content being viewed by others
Background
Around 10 to 18% of couples have a problem with having children [1]. Female infertility is considered a primary cause of about 37% of infertile couples [2]. In females, the fecundity will begin to decline significantly when women age arrives at 30–35 years, and it declines sharply after age 37 years [3]. The decline in female fecundity occurs normally as a result of the continuous process of oocyte atresia [4]. The reduction in fertility associated with the female age is characterized by decreases in the quality and quantity of oocytes, gradual elevation in the level of FSH, and reductions in the level of anti-Müllerian hormone (AMH) and inhibin B levels [3]. Nowadays, intracytoplasmic sperm injection (ICSI) is considered one of the most important ways to overcome infertility problems and one of the effective treatment approaches that are used by infertility clinics [5]. In assisted reproduction technology, the females undergo ovarian stimulation in order to permit retrieval of multiple oocytes during one cycle, and it is occurring through the administration of exogenous gonadotropins [6]. During this period, maintaining the LH and FSH levels above a critical threshold is considered a very necessary step [7]. The GnRH agonist or antagonist protocol can be used to ensure the prevention of a premature spike of LH that would induce ovulation [8]. During ovarian stimulation protocol, the embryologists use several ovarian reserve tests which include the anti-Müllerian hormone, basal FSH, and antral follicle count as a predictor of ovarian response, ICSI outcomes, and occurrence of pregnancy [9]. However, the use of these tests is still limited because they have a low predictive value, show cycle-dependent fluctuations, and lack clear cutoff values [10]. A previous study showed a negative correlation between the number of antral follicles and ovarian aging [11].
Anti-Müllerian hormone is a glycoprotein produced exclusively in the gonads [12]. In female, the AMH arrived at a high peak of around 25 years and then gradually decline until becoming undetectable before menopause [13]. A previous study has noted that the AMH is better in the prediction of ovarian response than age and basal FSH [14]. Other studies showed that the level of AMH cannot be used as an indicator of embryo quality and pregnancy chances [15]. Other studies found a relationship between the high level of AMH hormone before the start of the stimulation protocol and the increase in the risk of ovarian hyperstimulation syndrome (OHSS) [16]. Several studies showed an association between the level of AMH and the follicular pool, and it can be used as an ovarian reserve marker [17]. AMH also can be used to predict poor as well as excessive response in IVF [18]. This study was designed to (I) assess which of the following parameters (age, AMH, and FSH) can be used as a predictor of ovarian response to GnRH antagonist stimulation protocol, (II) determine the cutoff value of AMH and age for predicting poor and high ovarian response, and (III) investigate the relationship between age, AMH level, and other clinical parameters.
Methods
Study population
This retrospective study included three hundred and eighteen women with a mean age of 28.2 ± 5.9 years old; all cases attended to the Al Bassma Fertility Center in the Palestinian Territories between May 2010 and December 2011. All the participants were selected according to the following inclusion criteria: women age between 18 and 48 years old, women undergoing GnRH antagonist protocols, first ICSI cycle, a normal body mass index, women have a regular menstrual cycle, and the male partner has normal semen parameters. In contrast, the women were excluded from the present study depending on the following criteria: cigarette smokers, diabetes mellitus, women using an oral contraceptive, endocrine abnormality, endocrine disorders (polycystic ovarian syndrome or polycystic ovaries), women with a history of ovarian surgery, and women suffering from a recurrent abortion. The calculations of sample size were based on the formula for a cross-sectional study where the EPI-INFO statistical package version 7.2 was used with a 99.9% confidence interval (CI), 80% power, 0.4 ratios, 3.75 risk ratio, 4.9 odds ratio, and 30% outcome in the exposed group. Consequently, the total sample size was 314 persons. The medical records were used by the researcher to gather the general and medical information that included females’ age; body mass index; menstrual history; hormone profile; number of collected oocytes, mature oocytes, immature oocytes, and fertilized oocytes; number of embryos transferred; and the pregnancy results.
Hormone profile and ovarian stimulation
All women included in this study have been undergoing ovarian stimulation by using GnRH antagonist protocols with a recombinant FSH. The ultrasonographic and blood samples were collected from all women on the third day of the menstrual cycle. Briefly, the serum was separated by centrifugation at 3500 rpm for 15 min, and then, all of the following hormones (basal level of E2, FSH, LH, PRL, TSH, and AMH) were measured using the Tosoh Instrument (AIA-360, Tokyo, Japan). The oocyte pickup was scheduled 33–36 h after the administration of 5000 to 10,000 IU of hCG (Pregnyl) depending upon the age of females and the degree of ovarian response. According to the ovarian response, the samples were divided into three groups: a poor response (< 4 oocytes retrieved, n = 51), a normal response (4 to 14 oocytes retrieved, n = 192), and a high response (> 14 oocytes retrieved, n = 75) [19]. Embryo cleavage was evaluated after 16–18 h from ICSI, where the high-quality embryos (grade I or II) were transferred into the uterine cavity after 3 days from ICSI. The embryos were transferred, and all patients received luteal support with vaginal progesterone until a pregnancy test was performed after 2 weeks from embryo transferred. The cases were classified as pregnant women when the level of the β-hCG hormone arrived at more than 5 mIU/mL. For this study, multiple pregnancies were regarded as one pregnancy.
Statistics analysis
All the data were analyzed using IBM SPSS for Windows software package version 24.0 (SPSS, Inc., Chicago, IL, USA). Samples of this study were non-normally distributed (non-parametric) according to the value of the skewness test, kurtosis test, and Z-value. Kruskal–Wallis (H-test) and Mann-Whitney (U-test) were applied to compare the means of quantitative variables among the study groups. Spearman’s test was used to evaluate the correlation coefficient between the clinical parameters. Receiver operating characteristic (ROC) curves were generated for ovarian reserve markers (female age, AMH level, and basal level of FSH) to compare their ability to predict low or high ovarian response. The results in the abovementioned procedures were accepted as statistically significant when P < 0.05.
Results
Clinical parameters of the study population and their ovarian response
Table 1 shows that the age in the poor ovarian response group was significantly greater than normal and high ovarian response groups (P < 0.001). A significant variation has been found in the basal level of E2 and the oocyte fertilization rate among the study groups (P = 0.041 and P < 0.001, respectively). In contrast, the levels of AMH and E2 on hCG day; the number of retrieved oocytes, mature oocytes, immature oocytes, and fertilized oocytes; and number of embryos transferred, as well as the value of ß-hCG, were increased significantly from low ovarian response to normal and high ovarian response (P < 0.001).
Correlation between clinical parameters of the study population
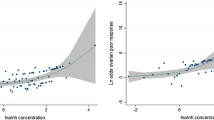
As indicated in Table 2, a positive significant correlation was found between AMH level (r = 0.707, P < 0.001); the number of mature oocytes (r = 0.867, P < 0.001), immature oocytes (r = 0.779, P < 0.001), and fertilized oocytes (r = 0.843, P < 0.001); number of embryos transferred (r = 0.496, P < 0.001) and ß-hCG level (r = 0.333, P < 0.001); and the number of retrieved oocyte. Conversely, a significant negative association was found between the basal level of FSH and the number of retrieved oocyte (r = −0.112, P = 0.046). There was also a significant positive association between the number of mature oocytes (r = 0.558, P < 0.001), immature oocytes (r = 0.599, P < 0.001), and fertilized oocytes (r = 0.573, P < 0.001); number of embryos transferred (r = 0.268, P < 0.001); and ß-hCG level (r = 0.212, P < 0.001) and AMH level. A significant negative correlation has been shown between AMH level and FSH level (r = −0.221, P < 0.001). A significant positive correlation was reported between the number of immature oocytes and fertilized oocytes, number of embryos transferred, and ß-hCG level (r = 0.445, P < 0.001; r = 0. 936, P < 0.001; r = 0. 583, P < 0.001; and r = 0. 374, P < 0.001, respectively) and the number of mature oocytes. In contrast, a significant negative correlation was noted between the number of mature oocytes and the basal level of E2 hormone (r = −0.125, P = 0.025). A significant positive association was found between AMH level (r = 0.236, P < 0.001); the number of retrieved oocyte (r = 0.355, P < 0.001), mature oocytes (r = 0.360, P < 0.001), immature oocytes (r = 0.249, P < 0.001), and fertilized oocytes (r = 0.330, P < 0.001); and number of embryos transferred (r = 0.139, P = 0.013) and E2 level on hCG day.
Predictive value of AMH and age during the different ovarian response
As illustrated in Table 3, the ROC curve analysis in poor response showed that both AMH and age had the highest accuracy (AUC = 0.894, 95% CI = 0.832–0.955, P < 0.001; AUC = 0.675, 95% CI = 0.588–0.761, P < 0.001, respectively) in predicting poor response when compared with the basal FSH level (AUC = 0.529, 95% CI = 0.433–0.626, P = 0.519). The selected cutoff value of AMH and age for prediction of poor response was < 1.45 ng/mL (80.7% sensitivity, 78.4% specificity) and > 31.5 years (52.9% sensitivity, 77.6% specificity), respectively (Figs. 1, 2, and 3). In high response, the ROC curve analysis showed that AMH had the highest accuracy (AUC = 0.888, 95% CI = 0.849–0.928, P < 0.001), followed by age (AUC = 0.613, 95% CI = 0.542–0.684, P = 0.004) and the basal level of FSH (AUC = 0.570, 95% CI = 0.496–0.644, P = 0.075) (Table 3). In the prediction of high ovarian response, the optimum cutoff value of AMH was > 3.55 ng/mL (sensitivity 81.3%, specificity 74.5%), that of age was < 27.5 years (sensitivity 52.1%, specificity 76.0%) (Figs. 4, 5, and 6).
Clinical parameters among the different age groups
As pointed in Table 4, a significant decrease has been found among the different age groups in the basal levels of LH (P = 0.026), AMH (P < 0.001), and E2 on hCG day (P = 0.002); the number of retrieved oocytes (P < 0.001), mature oocytes (P < 0.001), immature oocytes (P < 0.001), and fertilized oocytes (P < 0.001); number of embryos transferred (P < 0.001); and the value of ß-hCG (P < 0.001) where the highest value in the mentioned parameters was observed in the younger women.
Correlation between clinical parameters and women’s age
As shown in Table 5, significant negative correlation has been found between levels of LH (P = 0.005), AMH (P < 0.001), and E2 on hCG day (P = 0.002); numbers of retrieved oocytes (P < 0.001), mature oocytes (P < 0.001), immature oocytes (P < 0.001), and fertilized oocytes (P < 0.001); and number of embryos transferred (P < 0.001), the value of ß-hCG (P < 0.001), and women’s age. Conversely, a significant positive correlation was found between the FSH level (P < 0.011), the oocyte fertilization rate (P < 0.001), and women’s age.
Study population in relation to clinical pregnancy outcome
Table 6 reveals a significant reduction in the age and basal level of E2 of pregnant women compared to non-pregnant women (P < 0.001). A significant increase has been found in AMH level (P = 0.002); the number of retrieved oocytes (P < 0.001), mature oocytes (P < 0.001), immature oocytes (P = 0.013), and fertilized oocytes (P < 0.001); and number of embryos transferred (P < 0.001) of pregnant women compared to non-pregnant women.
Correlations between clinical parameters in pregnant women
As illustrated in Table 7, there is a negative correlation between the AMH level (r = −0.185, P = 0.020); the number of retrieved oocytes (r = −0.232, P = 0.003), mature oocytes (r = −.208, P = 0.009), immature oocytes (r = −0.178, P = 0.025), and fertilized oocytes (r = −0.222, P = 0.005); number of embryos transferred (r = −0.162, P = 0.043); and the age of pregnant women. On the other hand, a positive correlation has been found between the basal FSH level and the age of pregnant women (r = 0.161, P = 0.043). In addition, a significant positive correlation was noted between the basal E2 level; the number of retrieved oocyte, mature oocytes, immature oocytes, and fertilized oocytes (P = 0.035, P < 0.001, P < 0.001, P < 0.001, and P < 0.001, respectively), and the level of AMH. In contrast, a negative significant correlation was found between AMH level and the basal level of FSH (r = −0.196, P = 0.014). A positive significant correlation was noted between the AMH level; the number of retrieved oocytes, mature oocytes, immature oocytes, and fertilized oocytes (P < 0.001, P < 0.001, P < 0.001, P < 0.001, and P < 0.001, respectively); and E2 level on hCG day. A negative significant correlation has been observed between the E2 level on hCG day (P = 0.003) and the age of pregnant women.
Discussion
Despite the success of assisted reproductive technology in overcoming female reproductive problems in several cases, there are still some cases that respond poorly to the stimulation protocol which in turn leads to the ICSI failure. The prediction of ovarian response plays a critical role in the determination of the optimal starting dose from recombinant FSH which leads to increases in the ability of ovarian response and reduces the number of doses needed during stimulation [20]. A previous study indicated that there are several factors, including age, length of the menstrual cycle, basal level of FSH, AMH level, and antral follicle count, that can be used as clinical predictors of oocyte yield and ovarian response during ovarian stimulation [21]. However, there are few studies investigating the ability to predicting ovarian response in GnRH antagonist protocols [22, 23]. To improve the ovarian response and ICSI outcomes, it is necessary to study the association between the AMH level, age, and ovarian response and to estimate the cutoff value of the AMH level and women’s age at which the ovarian response may be poor and high [24]. The present study has found a significant increase among the responder groups in AMH level; E2 level on hCG day; number of retrieved oocytes, mature oocytes, immature oocytes, and fertilized oocytes; number of embryos transferred; and the value of ß-hCG. Besides, a significant reduction was found in the age, basal E2 level, and oocyte fertilization rate among the responder groups. All of the mentioned results are in agreement with a previous study prepared by Seifer and his colleagues who found a relationship between circulating AMH levels and ovarian response to gonadotropin treatment. They showed that women with ≥ 11 oocytes retrieved had serum AMH concentrations 2.5 times higher than those of women with ≤ 6 oocytes retrieved [25]. Other studies supported such findings [19, 26].
The present study showed a significant negative association between the number of retrieved oocytes, AMH level, and the basal level of FSH. Conversely, a significant positive correlation was found between the number of retrieved oocytes, mature oocytes, immature oocytes, and fertilized oocytes; number of embryos transferred; and AMH level. Similar results demonstrated that antral follicle count (AFC) was closely related to serum AMH level on the third day of the cycle in women suffering from infertility problems [27, 28]. A review manuscript reported that five of the studies showed that AFC and AMH had a correlation similar to the number of oocytes retrieved, whereas four other studies indicated that AMH was either less good or better [29].
In the present study, the cutoff values of the age and AMH as a predictor of poor response were > 31.5 years (AUC= 0.675) and < 1.45 ng/ml (AUC= 0.894), respectively. These findings are in line with previous studies that found that the cutoff value of AMH for predicting poor ovarian response is between 0.30 and 1.40 ng/ml [30,31,32]. Another meta-analysis study that included 28 studies of women undergoing ART exhibited that AMH (area under the curve, AUC= 0.78) is a better predictor of poor response to ovarian stimulation than age (AUC= 0.61) [33]. In addition, the La Marca study observed that low AMH cutoff values (0.1 to 1.66 ng/mL) have 76% sensitivities and 79% specificities for the prediction of poor response to gonadotropin stimulation [12]. Nevertheless, these findings disagree with another study that found that the cutoff value for AMH of a poor response from a normal response was 0.1 and 2.97 ng/ml [34]. This variation was supported by Kelsey et al.’s study which reported that the best cutoff value for AMH was 0.7 ng/mL in predicting poor response [13].
The optimum cutoff value of AMH and age of the high response in this study were > 3.55 ng/mL (AUC= 0.888) and < 27.5 years (AUC= 0.613), respectively. The value of AMH cutoff is in agreement with a systematic review of two studies that used AMH to predict high response to gonadotropin stimulation and found that high AMH cutoff values (3.36 to 5.0 ng/mL) have sensitivities and specificities ranging between 53 and 90.5% and 70 and 94.9%, respectively [12, 33]. In contrast, these findings do not match with a previous article found that the cutoff of AMH level in predicting high stimulation was > 4.89 ng/mL (AUC= 0.82, sensitivity= 55%; a specificity = 85%) [32].
Analysis ROC revealed that AMH and women’s age are the most accurate of other tests in predicting ovarian response compared to the basal level of FSH. The AUC for AMH was higher than age and basal FSH, and these findings match with previous studies [35, 36]. In contrast, a previous study found the antral follicle count (AFC) to be better than the AMH level [37]. The variation in the cutoff values of AMH and age for poor and high response prediction is probably resulting from the use of different assay methods, differences in the definition of poor and high response, different study populations, and different stimulation protocol. However, one can say that AMH is overall able to identify a large percentage of expected poor and high responders [21].
The results showed a significant decrease among the age groups in AMH level; the number of retrieved oocytes, mature oocytes, and fertilized oocytes; and number of embryos transferred. Such results are in the line with a previous study that used Chinese women across different age groups and found a significant reduction in AMH level with an increase in the women’s age [38]. A significant negative correlation was found between AMH level; E2 level on hCG day; the number of retrieved oocytes, mature oocytes, and fertilized oocytes; number of embryos transferred; and the women’s age. A previous study recommended that the level of AMH may be considered as the best marker of ovarian aging and the time to menopause [39]. While another study mentioned that the reduction in the fecundity ability was shown in women with very low AMH levels [40].
Finally, the present study observed a significant reduction in the women’s age and basal level of E2 in pregnant women compared to non-pregnant women; a significant increase was found in the AMH level, the number of retrieved oocytes and mature oocytes, number of embryos transferred in pregnant women compared to non-pregnant women. A negative correlation was observed between AMH level, the number of retrieved oocytes and mature oocytes, number of embryos transferred, and the age of pregnant women. These findings match with a previous study that noted that AMH level is associated with the oocyte yield and could be used to predict pregnancy outcomes [41]. In addition, other authors observed a correlation between higher baseline serum AMH levels and higher clinical pregnancy rates [42]. Furthermore, several studies have found that low AMH levels correlate with lower rates of clinical pregnancies and higher cancelation rates, but it has a weak ability to predict the clinical pregnancy [14, 43]. Conversely, another study was unable to observe an association between baseline AMH levels and pregnancy rates [44].
Conclusions
This study demonstrates that AMH level and women’s age were good biomarkers for the prediction of ovarian response to GnRH antagonist stimulation protocol. The AMH value (< 1.45 ng/mL) and women’s age (> 31.5 years) can be considered as potential indicators of poor ovarian response. In contrast, the value of AMH (> 3.55 ng/mL) and the women’s age (< 27.5 years) are considered potential indicators of high ovarian response.
Limitations
Future studies with a large sample size are needed in order to confirm these results.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ICSI:
-
Intracytoplasmic sperm injection
- AMH:
-
Anti-Müllerian hormone
- FSH:
-
Follicle-stimulating hormone
- E2:
-
Estradiol
- LH:
-
Luteinizing hormone
- TSH:
-
Thyroid-stimulating hormone
- PRL:
-
Prolactin
- ß-HCG:
-
Beta human chorionic gonadotropin
References
Weiss RV, Clapauch R (2014) Infertilidade feminina de origem endócrina. Arq Bras Endocrinol Metabol 58(2):144–152. https://doi.org/10.1590/0004-2730000003021
Unuane D, Tournaye H, Velkeniers B, Poppe K (2011) Endocrine disorders & female infertility. Best Pract Res Clin Endocrinol Metab 5(6):861–873
Scheffer JA, Scheffer B, Scheffer R, Florencio F, Grynberg M, Lozano DM (2018) Are age and anti-Müllerian hormone good predictors of ovarian reserve and response in women undergoing IVF? JBRA Assist Reprod 22(3):215–220. https://doi.org/10.5935/1518-0557.20180043
Nelson SM (2013) Biomarkers of ovarian response: current and future applications. Fertil Steril 99(4):963–969. https://doi.org/10.1016/j.fertnstert.2012.11.051
Andersen AN, Goossens V, Gianaroli L, Felberbaum R, De Mouzon J, Nygren KG (2007) Assisted reproductive technology in Europe, 2003. Results generated from European registers by ESHRE. Hum Reprod 22(6):1513–1525
Farquhar C, Marjoribanks J (2018) Assisted reproductive technology: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews (8):CD010537. https://doi.org/10.1002/14651858.CD010537.pub5
Mochtar MH, Van der Veen F, Ziech M, van Wely M, Musters A (2007) Recombinant luteinizing hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles. Cochrane Database of Systematic Reviews (2):CD005070. https://doi.org/10.1002/14651858.CD005070.pub2
Wang Y, Kuang Y, Chen Q, Cai R (2018) Gonadotropin-releasing hormone antagonist versus progestin for the prevention of premature luteinising hormone surges in poor responders undergoing in vitro fertilisation treatment: study protocol for a randomised controlled trial. Trials 19(1):1–6
Hendriks DJ, te Velde ER, Looman CW, Bancsi LF, Broekmans FJ (2008) Expected poor ovarian response in predicting cumulative pregnancy rates: a powerful tool. Reprod BioMed Online 17(5):727–736. https://doi.org/10.1016/S1472-6483(10)60323-9
Bukman A, Heineman MJ (2001) Ovarian reserve testing and the use of prognostic models in patients with subfertility. Hum Reprod Update 7(6):581–590. https://doi.org/10.1093/humupd/7.6.581
Van Disseldorp J, Eijkemans MJ, Klinkert ER, Te Velde ER, Fauser BC, Broekmans FJ (2007) Cumulative live birth rates following IVF in 41- to 43-year-old women presenting with favourable ovarian reserve characteristics. Reprod BioMed Online 14(4):455–463. https://doi.org/10.1016/S1472-6483(10)60893-0
La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, Stabile G, Volpe A (2010) Anti-Müllerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update 16(2):113–130. https://doi.org/10.1093/humupd/dmp036
Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH (2011) A validated model of serum anti-Müllerian hormone from conception to menopause. PLoS One 6(7):e22024. https://doi.org/10.1371/journal.pone.0022024
Tal R, Tal O, Seifer BJ, Seifer DB (2015) Antimüllerian hormone as predictor of implantation and clinical pregnancy after assisted conception: a systematic review and meta-analysis. Fertil Steril 103(1):119–130. https://doi.org/10.1016/j.fertnstert.2014.09.041
Arce JC, La Marca A, Klein BM, Andersen AN, Fleming R (2013) Antimüllerian hormone in gonadotropin releasing-hormone antagonist cycles: prediction of ovarian response and cumulative treatment outcome in good-prognosis patients. Fertil Steril 99(6):1644–1653. https://doi.org/10.1016/j.fertnstert.2012.12.048
Lee TH, Liu CH, Huang CC, Wu YL, Shih YT, Ho HN, Yang YS, Lee MS (2008) Serum anti-Müllerian hormone and estradiol levels as predictors of ovarian hyperstimulation syndrome in assisted reproduction technology cycles. Hum Reprod 23(1):160–167. https://doi.org/10.1093/humrep/dem254
Meczekalski B, Czyzyk A, Kunicki M, Podfigurna-Stopa A, Plociennik L, Jakiel G, Maciejewska-Jeske M, Lukaszuk K (2016) Fertility in women of late reproductive age: the role of serum anti-Müllerian hormone (AMH) levels in its assessment. J Endocrinol Investig 39(11):1259–1265. https://doi.org/10.1007/s40618-016-0497-6
Lee RW, Khin LW, Hendricks MS, Tan HH, Nadarajah S, Tee NW, Loh SF, Tai BC, Chan JK (2020) Ovarian biomarkers predict controlled ovarian stimulation for in vitro fertilisation treatment in Singapore. Singap Med J 61(9):463–468. https://doi.org/10.11622/smedj.2020130
Yassin MM, Sharif FA, Laqqan MM (2013) Anti-Mullerian hormone as a predictor of ovarian reserve and ovarian response in IVF women from Gaza strip. Iran J Reprod Med 11(4):261
Popovic-Todorovic B, Loft A, Bredkjæer HE, Bangsbøll S, Nielsen IK, Andersen AN (2003) A prospective randomized clinical trial comparing an individual dose of recombinant FSH based on predictive factors versus a ‘standard’ dose of 150 IU/day in ‘standard’ patients undergoing IVF/ICSI treatment. Hum Reprod 18(11):2275–2282. https://doi.org/10.1093/humrep/deg472
Baker VL, Gracia C, Glassner MJ, Schnell VL, Doody K, Coddington CC, Shin SS, Marshall LA, Alper MM, Morales AJ, Pavone ME (2018) Multicenter evaluation of the Access AMH antimüllerian hormone assay for the prediction of antral follicle count and poor ovarian response to controlled ovarian stimulation. Fertil Steril 110(3):506–513 Rep
Bosch E, Labarta E, Zuzuarregui J, Iliodromiti S, Nelson SM (2018) Prediction of ovarian response with an automated AMH assay (Elecsys®) in GnRH antagonist cycles. Fertil Steril 110(4):e330. https://doi.org/10.1016/j.fertnstert.2018.07.926
Liu XH, Wu XH, Yang S (2019) Changes and correlations of anti-Müllerian hormone and stem-cell factors in different ovarian reserve patients during GnRH-antagonist protocol and the effects on controlled ovarian hyperstimulation outcomes. Arch Gynecol Obstet 300(6):1773–1783. https://doi.org/10.1007/s00404-019-05332-4
Ron-El R, Raziel A, Strassburger D, Schachter M, Kasterstein E, Friedler S (2000) Outcome of assisted reproductive technology in women over the age of 41. Fertil Steril 74(3):471–475. https://doi.org/10.1016/S0015-0282(00)00697-X
Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM (2002) Early follicular serum Müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril 77(3):468–471. https://doi.org/10.1016/S0015-0282(01)03201-0
Vural B, Cakiroglu Y, Vural F, Filiz S (2014) Hormonal and functional biomarkers in ovarian response. Arch Gynecol Obstet 289(6):1355–1361. https://doi.org/10.1007/s00404-013-3132-1
Umarsingh S, Adam JK, Krishna SB (2020) The relationship between anti-Müllerian hormone (AMH) levels and pregnancy outcomes in patients undergoing assisted reproductive techniques (ART). PeerJ 8:e10390 ADD
Fanchin R, Schonäuer LM, Righini C, Guibourdenche J, Frydman R, Taieb J (2003) Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod 18(2):323–327. https://doi.org/10.1093/humrep/deg042
Huang J, Lin J, Gao H, Wang Y, Zhu X, Lu X, Wang B, Fan X, Cai R, Kuang Y (2019) Anti-Müllerian hormone for the prediction of ovarian response in progestin-primed ovarian stimulation protocol for IVF. Front Endocrinol 10:325 ADD
Saleh H, Moiety F, Agameya AF, Elkassar Y, El Sharakwy RM, Zeidan D, Elmeligy H (2020) Comparison between antral follicle count and anti-Müllerian hormonal level in the prediction of ovarian response and pregnancy outcome in intracytoplasmic sperm injection patients: implications in personalizing ovarian stimulation. Clin Exp Obstet Gynecol 47(2):166–173 ADD
Neves AR, Blockeel C, Griesinger G, Garcia-Velasco JA, La Marca A, Rodriguez I, Drakopoulos P, Alvarez M, Tournaye H, Polyzos NP (2020) The performance of the Elecsys® anti-Müllerian hormone assay in predicting extremes of ovarian response to corifollitropin alfa. Reprod BioMed Online 41(1):29–36 ADD
Zheng H, Chen S, Du H, Ling J, Wu Y, Liu H, Liu J (2017) Ovarian response prediction in controlled ovarian stimulation for IVF using anti-Müllerian hormone in Chinese women: a retrospective cohort study. Medicine 96(13):e6495
Broer SL, Dólleman M, Van Disseldorp J, Broeze KA, Opmeer BC, Bossuyt PM, Eijkemans MJ, Mol BW, Broekmans FJ, Broer SL, Dólleman M (2013) Prediction of an excessive response in in vitro fertilization from patient characteristics and ovarian reserve tests and comparison in subgroups: an individual patient data meta-analysis. Fertil Steril 100(2):420–429. https://doi.org/10.1016/j.fertnstert.2013.04.024
La Marca A, Sunkara SK (2014) Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update 20(1):124–140. https://doi.org/10.1093/humupd/dmt037
Iliodromiti S, Anderson RA, Nelson SM (2015) Technical and performance characteristics of anti-Müllerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update 21(6):698–710. https://doi.org/10.1093/humupd/dmu062
Asada Y, Tsuiki M, Sonohara M, Fukunaga N, Hattori Y, Inoue D, Ito R, Hashiba Y (2019) Performance of anti-Müllerian hormone (AMH) levels measured by Beckman Coulter Access AMH assay to predict oocyte yield following controlled ovarian stimulation for in vitro fertilization. Reprod Med Biol 18(3):273–277. https://doi.org/10.1002/rmb2.12271
Kwee J, Schats R, McDonnell J, Themmen A, de Jong F, Lambalk C (2008) Evaluation of anti-Müllerian hormone as a test for the prediction of ovarian reserve. Fertil Steril 90(3):737–743. https://doi.org/10.1016/j.fertnstert.2007.07.1293
Cui Y, Shi Y, Cui L, Han T, Gao X, Chen ZJ (2014) Age-specific serum antimüllerian hormone levels in women with and without polycystic ovary syndrome. Fertil Steril 102(1):230–236. https://doi.org/10.1016/j.fertnstert.2014.03.032
Freeman EW, Sammel MD, Lin H, Gracia CR (2012) Anti-Mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol 97(5):1673–1680. https://doi.org/10.1210/jc.2011-3032
Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, Baird DD (2017) Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 318(14):1367–1376
Lukaszuk K, Liss J, Kunicki M, Jakiel G, Wasniewski T, Woclawek-Potocka I, Pastuszek E (2014) Anti-Müllerian hormone (AMH) is a strong predictor of live birth in women undergoing assisted reproductive technology. Reprod Biol 14(3):176–181. https://doi.org/10.1016/j.repbio.2014.03.004
Moro F, Tropea A, Scarinci E, Leoncini E, Boccia S, Federico A, Alesiani O, Lanzone A, Apa R (2016) Anti-Müllerian hormone concentrations and antral follicle counts for the prediction of pregnancy outcomes after intrauterine insemination. Int J Gynecol Obstet 133(1):64–68. https://doi.org/10.1016/j.ijgo.2015.08.021
Yao L, Zhang W, Li H, Lin W (2015) The role of serum AMH and FF AMH in predicting pregnancy outcome in the fresh cycle of IVF/ICSI: a meta-analysis. Int J Clin Exp Med 8(2):1755–1767
Lekamge DN, Barry M, Kolo M, Lane M, Gilchrist RB, Tremellen KP (2007) Anti-Müllerian hormone as a predictor of IVF outcome. Reprod BioMed Online 14(5):602–610. https://doi.org/10.1016/S1472-6483(10)61053-X
Acknowledgements
The authors would like to express their gratitude to all the doctors and clinical staff in the Al Basma Fertility Center, Palestinian Territories.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
M. M. L collected and processed the samples. Additionally, he contributed to data analysis, and he was a major contributor in writing the manuscript. M. M. Y performed a review for data analysis, discussion preparation, and the writing of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The current study was approved by the Institutional Ethics Committee (Reference. No. PHRC/HC/03/10), and consent was provided according to the Declaration of Helsinki. Besides, all participants signed an informed approval form to participate in the present study. All samples were analyzed according to the guidelines and standard procedures of Al Basma Fertility Center, Palestinian Territories.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Laqqan, M.M., Yassin, M.M. Predictive factors of ovarian response to GnRH antagonist stimulation protocol: AMH and age are potential candidates. Middle East Fertil Soc J 26, 16 (2021). https://doi.org/10.1186/s43043-021-00062-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-021-00062-7