Abstract
Background
Hereditary transient neonatal hyperparathyroidism (TNHP) is a rare autosomal-recessive condition caused by variants in TRPV6 gene which encodes for a transient maternal–fetal calcium transport channel. This is characterized by interference with placental maternal–fetal calcium transport causing fetal calcium deficiency. It primarily manifests as defective bone mineralization, narrow and bell-shaped thorax, bone fractures and short bones at birth. The current study aimed to describe a novel TRPV6 variant linked with TNHP in an Indian family and the review of literature.
Case presentation
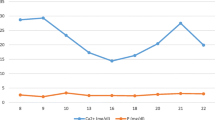
The proband is a term female neonate with fetal growth restriction born to a third-degree consanguineous couple. She was noted to have diffuse defective bone mineralization, narrow and bell-shaped thoracic cavity, short bones and curved ribs without any bone fractures. The first pregnancy was affected with similar features in the fetus and had been terminated. Parental whole-exome sequencing suggested heterozygous missense variant in exon 12 of the TRPV6 gene (c.1585G > A, p.Asp529Asn) in both the parents. The proband required non-invasive respiratory support for ten days in neonatal intensive care unit. She had low calcium and high parathyroid hormone (PTH) and alkaline phosphatase (ALP) levels. She received calcium, phosphorus and vitamin D supplements for three months leading to normalization of serum PTH and ALP levels. Whole-exome sequencing of the proband suggested a homozygous missense variant in exon 12 of the TRPV6 gene (p.Asp529Asn; ENST00000359396.9) that results in the amino acid substitution of asparagine for aspartic acid at codon 529. To the best of author’s knowledge, this is the twelfth case of TNHP reported in literature. The novel variant of TRPV6 gene present in this family has not been reported earlier.
Conclusion
Our finding broadens the genotypic spectrum of TNHP.
Similar content being viewed by others
Background
Calcium metabolism in the body is regulated by Vitamin D-PTH axis. Placental calcium transport is an additional mechanism for fetal calcium metabolism. In humans, higher levels of fetal calcium as compared to the maternal levels are maintained by this placental calcium transporter. This occurs due to energy-dependent transcellular transport of calcium in placenta as compared to predominant paracellular transport of calcium in the intestine [1]. Transient receptor potential cation channel, subfamily V, member 6 (TRPV6) is an important placental calcium transport channel. Mutations in the gene encoding TRPV6 have been associated with fetal calcium deficiency resulting in transient neonatal hyperparathyroidism (TNHP). This is an autosomal-recessive condition. Several hotspots for TRPV6 mutation have been reported. Homozygous and compound heterozygous states for various mutations in TRPV6 explain the different mechanisms of disease onset. This is characterized by diffuse defective bone mineralization, short bones, fetal bone fractures, narrow and bell-shaped thorax and respiratory and feeding difficulties in the neonates [2]. We hereby describe an Indian family in which proband had homozygous missense variant in exon 12 of the TRPV6 gene (c.1585G > A, p.Asp529Asn), and both parents were heterozygous for the same variant. Fewer than a dozen cases of TNHP have been reported, highlighting the scarcity of essential research data regarding the correlation between disease phenotypes and genotypes. This is a novel variant linked with TNHP. Written informed consent was obtained from the parents for publication of this case report and accompanying images and was approved by our hospital.
Case presentation
An early term female neonate was born to a second gravida mother by cesarean delivery due to non-progression of labor and face presentation. The parents were consanguineous (third degree) and short statured (Fig. 1). First pregnancy had been terminated as fetus had narrow thorax, short bones and fetal growth restriction (suspected to have lethal skeletal dysplasia). Genetic evaluation and autopsy were not done. Genetic consultation was obtained during current pregnancy: karyotype was normal, whole-exome sequencing suggested heterozygous missense variant in exon 12 of the TRPV6 gene (c.1585G > A, p.Asp529Asn) in both the parents. Antenatal scan suggested short bones (less than 5th centile), narrow and bell-shaped thorax, fetal growth restriction and severe polyhydramnios (amniotic fluid index (AFI) = 35.5). Mother had Vitamin D deficiency and was treated for the same. The neonate cried immediately after birth and continuous positive airway support (CPAP) was initiated in the delivery room for respiratory distress. Apgar score was 8 and 9 at 1 and 5 min of life, respectively. Skeletal radiographs suggested diffuse osteopenia, metaphyseal dysplasia, generalized demineralization, no obvious bone fractures and no C2/C3 subluxation (Fig. 2A and 2B). Clinical possibility of neonatal hyperparathyroidism and osteogenesis imperfecta were considered, and she was evaluated for the same. Laboratory investigations suggested hypocalcemia, low phosphorus, high ALP, very high PTH and low Vitamin D levels. Based on the heterozygous state of both parents for TRPV6 mutation and clinical, radiographic and laboratory features, possibility of TNPH was considered. She was commenced on calcium infusion, oral phosphorus and Vitamin D supplementation. She received nasal CPAP, a kind of non-invasive respiratory support for 10 days due to retractive breathing efforts. With improvements in respiratory support requirement and decreasing trend in PTH level, she was switched to oral calcium, phosphorus and vitamin D (2000 IU/day). She was discharged in good health and had normal growth and development at 3 months of age. The supplements were discontinued at 3 months of age with normalization of calcium, phosphorus, vitamin D, ALP and PTH levels. Whole-exome sequencing of the proband suggested homozygous missense variant in exon 12 of the TRPV6 gene (c.1585G > A, p.Asp529Asn) confirming the clinical diagnosis of TNHP.
Genetic studies
Whole-exome sequencing of the proband suggested a homozygous missense variant in exon 12 of the TRPV6 gene (chr7:g.142874130C > T; Depth: 77x) that results in the amino acid substitution of asparagine for aspartic acid at codon 529 (p.Asp529Asn; ENST00000359396.9). Same variant was identified in heterozygous state in both the parents. The observed variant lies in the “Ion transport protein” domain of the TRPV6 protein. The p.Asp529Asn variant has not been reported in the 1000 genomes, gnomAD (v3.1), gnomAD (v2.1), topmed and Indian population database (MedVarDb v4.0). The in silico prediction of the variant is damaging by PolyPhen-2 (HumDiv), SIFT and LRT. The reference codon is conserved across species.
Discussion
In this study, we report a case involving an Indian family exhibiting features of TNHP. The proband, a term female neonate born to a consanguineous couple displayed diffuse osteopenia, bowing of ribs, narrow thorax, short bones, respiratory difficulty and fetal growth restriction. Genetic testing identified homozygous missense variant of TRPV6 in the proband, along with same heterozygous variant in both the parents.
TRPV6 variants are associated with TNHP, with total 11 cases documented in the literature across 5 studies (Table 1).
Neonatal hyperparathyroidism can be primary or secondary. Primary neonatal hyperparathyroidism is mostly caused by mutations in calcium sensing receptor (CASR) gene [7]. Secondary neonatal hyperparathyroidism can be caused by mutations in SLC12A1 gene encoding for sodium–potassium–chloride cotransporter-2 (NKCC2), mucolipidosis-II or maternal pseudohypoparathyroidism [8, 9]. Another variety of secondary neonatal hyperparathyroidism is linked with TRPV6 mutations. This variety is transient as it is due to poor placental transport of calcium leading to fetal calcium deficiency and features of hyperparathyroidism including biochemical and skeletal changes. TRPV6 is an energy-dependent calcium transport channel which facilitates active transport of maternal–fetal calcium against the gradient during antenatal period [1]. TRPV6 is a complex of four identical subunits; each subunit comprises of six transmembrane segments (S1–S6) forming an inwardly rectifying calcium channel [10]. This facilitates transcellular maternal–fetal calcium transport. This is the predominant mechanism of calcium transport across the placenta. After birth, paracellular non-energy-dependent calcium transport channel is the major mechanism of calcium transport across the intestine. Hence, the biochemical and skeletal changes in this condition normalize after postnatal calcium, phosphorus and vitamin D supplementation. There are several hotspots of TRPV6 mutations. Transmembrane segments S2–S3 and Ankyrin repeats are the major ones [10]. The biallelic mutations could be homozygous or compound heterozygous leading to loss of function of calcium transport channel. Table 1 displays the various reported variants of TRPV6. Clinical characteristics include antenatal polyhydramnios, narrow thoracic cavity, respiratory and feeding difficulties, diffuse defective bone mineralization, bowing of long bones, fractures, metaphyseal dysplasia and short stature with normal cognitive outcome. Biochemical abnormalities include low to normal calcium, low phosphorus, low vitamin D, high ALP and high PTH levels [2,3,4,5,6]. Postnatal supplementation with calcium, phosphorus and vitamin D (2000 IU/day) leads to gradual normalization of PTH and ALP levels and skeletal changes over a varying period of time (mostly by 1–2 years of age) [2,3,4,5,6]. Understanding the hotspots and variants of TRPV6 could raise the possibility of gene therapy by using small chaperones. Prenatal diagnosis of TRPV6 variant could help in deciding for the continuation of pregnancy with affected fetus, considering the transient nature of the condition as well as normal cognitive outcome in the affected individual. This also raises the possibility of intra-amniotic infusion of calcium in the affected pregnancy. However, this needs confirmation in large studies.
The current report broadens the genotypic spectrum of TNHP linked with TRPV6 variant. It also reviews the reported cases available in literature describing the variable clinical manifestations and time needed to normalize the skeletal changes associated with this condition.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AFI:
-
Amniotic fluid index
- ALP:
-
Alkaline phosphatase
- AR:
-
Ankyrin repeats
- CASR:
-
Calcium sensing receptor
- CPAP:
-
Continuous positive airway pressure
- LRT:
-
Likelihood ratio test
- ND:
-
No details
- PTH:
-
Parathyroid hormone
- SIFT:
-
Sorting intolerant from tolerant
- TNHP:
-
Transient neonatal hyperparathyroidism
- TRPV6:
-
Transient receptor potential cation channel, subfamily V, member 6
References
Salles JP (2016) Bone metabolism during pregnancy. Ann Endocrinol 77(2):163–168
Suzuki Y, Chitayat D, Sawada H, Deardorff MA, McLaughlin HM, Begtrup A et al (2018) TRPV6 variants interfere with maternal-fetal calcium transport through the placenta and cause transient neonatal hyperparathyroidism. Am J Hum Genet 102(6):1104–1114
Yamashita S, Mizumoto H, Sawada H, Suzuki Y, Hata D (2019) TRPV6 gene mutation in a dizygous twin with transient neonatal hyperparathyroidism. J Endocr Soc 3(3):602–606
Suzuki Y, Sawada H, Tokumasu T, Suzuki S, Ninomiya S, Shirai M et al (2020) Novel TRPV6 mutations in the spectrum of transient neonatal hyperparathyroidism. J Physiol Sci 70:1–10
Burren CP, Caswell R, Castle B, Welch CR, Hilliard TN, Smithson SF et al (2018) TRPV6 compound heterozygous variants result in impaired placental calcium transport and severe undermineralization and dysplasia of the fetal skeleton. Am J Med Genet 176(9):1950–1955
Almidani E, Elsidawi W, Almohamedi A, Ahmed IB, Alfadhel A (2020) Case report of transient neonatal hyperparathyroidism: medically free mother. Cureus 12(2)
Egbuna OI, Brown EM (2008) Hypercalcaemic and hypocalcaemic conditions due to calcium-sensing receptor mutations. Best Pract Res Clin Rheumatol 22(1):129–148
Li D, Tian L, Hou C, Kim CE, Hakonarson H, Levine MA (2016) Association of mutations in SLC12A1 encoding the NKCC2 cotransporter with neonatal primary hyperparathyroidism. J Clin Endocrinol Metab 101(5):2196–2200
Unger S, Paul DA, Nino MC, McKay CP, Miller S, Sochett E, Braverman N, Clarke JT, Cole DE, Superti-Furga A (2005) Mucolipidosis II presenting as severe neonatal hyperparathyroidism. Eur J Pediatr 164:236–243
Lee BM, Lee GS, Jung EM, Choi KC, Jeung EB (2009) Uterine and placental expression of TRPV6 gene is regulated via progesterone receptor-or estrogen receptor-mediated pathways during pregnancy in rodents. Reprod Biol Endocrinol 7:1–1
Acknowledgements
The authors acknowledge genetic laboratory for doing the sequencing.
Funding
None.
Author information
Authors and Affiliations
Contributions
C.K., S.V. and S.O. conceptualized the study, gathered clinical data, provided patient care and helped with manuscript drafting, writing and editing. R.P., C.K. and D.D. wrote the manuscript and reviewed relevant literature. N.N.D. and S.P.B. supervised experimental work and helped with manuscript writing and editing. S.P.B., D.D. and R.P. helped with manuscript writing and patient care. S.V. and S.O. did literature search and editing of manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
No personal identifiers have been retained. All procedures performed in the study were in accordance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki Declaration and its later amendments. Formal ethical approval was obtained for the case report.
Consent for publication
Written informed consent was obtained from the parents for publication of this case report and accompanying images.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, C., Vani, S., Deshmukh, N.N. et al. Novel TRPV6 variant linked with transient neonatal hyperparathyroidism. Egypt J Med Hum Genet 25, 72 (2024). https://doi.org/10.1186/s43042-024-00537-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-024-00537-y