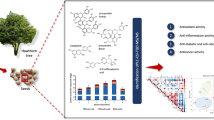
Abstract
Currently, teas and herbal infusions represent an important part of the human diet. Most contain phenolic compounds with high antioxidant activity, usually associated with human health protective functions. This attribute defines teas and infusions as nutraceutical foods. In Argentina, several native species are traditionally used for medical purposes. Some of those species are Larrea cuneifolia, Larrea nitida, Grindelia chiloensis, Pteromonnina dictyocarpa, Mandevilla laxa, and Monttea aphylla. The objectives of this study were to analyze the antioxidant power and the scavenging capacity of infusions obtained from those six medicinal plants, to characterize the phenolic profile, and to study in vitro their safety or cytotoxicity. Additionally, the potential use of two infusions as antioxidant additives in a food model was evaluated. The results indicated that the analyzed plant species are rich in phenolic acids (e.g., caffeic, ferulic, and chlorogenic acid), and flavonoids (e.g., quercetin and kaempferol), with high antioxidant power. The infusion blend obtained with G. chiloensis and L. cuneifolia exhibited the highest value of antioxidant capacity measured with the FRAP technique (193.4 μg EAA/mg DW). On the other hand, L. cuneifolia infusion showed the greatest antioxidant capacity determined by FRAP (131.9 ± 5.2 μg EAA/mg DW) and DPPH assays (0.453 mL/mg s). Additionally, L. cuneifolia infusion showed the highest phenolic content (232.8 μg GAE/mg DW) and flavonoid content (153.3 μg QE/mg DW). None of the infusions showed toxicity in mammalian cells, except for G. chiloensis. Furthermore, the L. cuneifolia and L. nitida infusions showed a high inhibitory effect on lipid oxidation in ground beef (55% and 51% at 4 days of storage, respectively). The results suggest that the studied infusions are safe and a rich source of antioxidants, which supports their use in traditional medicine. However, further exhaustive studies of G. chiloensis infusion are needed to ensure its safety, as it has shown cytotoxicity. Besides, it is worthwhile to advance the study of L. cuneifolia and L. nitida as sources of dietary antioxidants, due to their high antioxidant power and ability to protect against lipid peroxidation.
Graphical Abstract

Similar content being viewed by others
Introduction
In recent years, the consumption of herbal teas and infusions from a great variety of plants has become popular. This fact has been related to their attractive taste and flavor and their high content of antioxidants with potential health benefits (Poswal et al. 2019). This becomes relevant in a context in which more people take care of their health and know that diet affects it. For example, tea from Camellia sinensis is the second most widely consumed beverage in the world after water, and the medical benefits of its phytochemicals are well described (Chaudhary et al. 2023; Luo et al. 2023). In fact, epigallocatechin-3-gallate, which represents more than 50% of the total polyphenols present in green tea, has completed phase II and phase III clinical trials for the treatment of different types of cancer (Tuli et al. 2023). In addition to the popular green and black teas, infusions can be made with different plant tissues, such as leaves, flowers, roots, and barks, among others, and could improve diet quality. Dietary antioxidants are capable of curbing the signals that lead to oxidative stress by increasing natural defenses and preventing oxidative damage and associated diseases (Feng et al. 2023; Martemucci et al. 2022). Abundant experimental and epidemiologic evidence provides a convincing argument that polyphenol antioxidants from infusions can reduce cancer risk and prevent atherosclerosis, coronary heart disease, and other diseases (Liu et al. 2023).
The factors that cause oxidative stress can be present in the air, food, medications, cigarette smoke, insecticides in foods of plant origin, solar radiation, and cell phone radiation, among others (Leni et al. 2020; Vatner et al. 2020; Zheng et al. 2020). Compelling evidence suggests that oxidative stress, resulting from an elevation in reactive oxygen species (ROS) and nitrogen species (NOS), or a decline in antioxidant defenses, disrupts the integrity and functionality of lipids, proteins, and DNA (Moloney & Cotter 2018). This can lead to aging and numerous diseases, including cancer, Parkinson's disease, Alzheimer's disease, atherosclerosis, liver injury, rheumatoid arthritis, type 2 diabetes, and kidney failure, among others (Eguchi et al. 2021; Ionescu-Tucker & Cotman 2021; O’Flaherty 2020; Pisoschi et al. 2021).
The search for antioxidants based on ethnobotanical knowledge and the floristic richness of a country can result in new active principles not detected in any other way. Within the Argentinean vascular flora, distributed in 183 families and 10006 species, approximately 1182 species are used in traditional Argentine medicine (Palchetti et al. 2023; Zuloaga et al. 2019). In Argentina, including the Patagonia region, a wide array of plant species boasting medicinal properties has been documented for their potential in addressing various health issues, such as liver, intestinal, muscular, and renal conditions, among others (Bonilla Bonilla et al. 2023; Carabajal et al. 2021). Nonetheless, in many of these species, the composition of phytochemicals or the mechanisms involved in these effects have not been investigated. Consequently, it becomes imperative to rigorously assess both the efficacy and safety of these medicinal plants.
Phenolic compounds, such as tannins, anthocyanins, and flavonoids, have antioxidant properties, in many cases closely related to anti-inflammatory action, the main mechanism involved in the pathogenesis of the aforementioned diseases (Chandrasekara & Shahidi 2018; Liu et al. 2023). Among the species employed in Argentine folk medicine to address inflammatory disorders, we found several noteworthy candidates for in-depth investigation. These include Monttea aphylla (Miers) Berthan & Hooker (Scrophulariaceae), Mandevilla laxa (Ruiz & Pav.) Woodson (Apocynaceae), Larrea cuneifolia Cav. (Zygophyllaceae), Larrea nitida Cav. (Zygophyllaceae), Pteromonnina dictyocarpa (Griseb.) B Eriksen (Polygalaceae) and Grindelia chiloensis (Corn.) Cabrera (Asteraceae). Notably, the chemical composition and toxicity profiles of these species remain, at least in part, uncharted territory.
According to available bibliographic data, the infusion of M. aphylla is traditionally employed in the treatment of liver and kidney diseases (Azar 2008; Ladio & Lozada 2009; Muiño 2011), and G. chiloensis is used to interrupt diarrhea and as an anti-inflammatory agent for generalized pain (Muiño 2011). Besides, L. cuneifolia and L. nitida are used as decongestants, for joint pain, as a disinfectant, for gastric conditions, among others (Muiño 2011; Musaubach & Plos 2015); M. laxa is used as a purgative (Barboza et al. 2009), and P. dictyocarpa as an antidiarrheal and digestive (Goleniowski et al. 2006). Based on these ethnomedicinal uses, it becomes meaningful to evaluate the chemical composition, antioxidant capacity, and cytotoxicity of these species with potential anti-inflammatory activity.
Given the great scientific, medicinal, and industrial interest in natural antioxidants, the objective of this study was to analyze and compare the chemical composition and antioxidant properties of infusions from native plant species traditionally used for medicinal purposes. In addition, this work aims to evaluate the biological activity of the infusions, with methodologies that ensure their possible use as nutraceutical additives. Additionally, the use of two infusions as food preservatives was also examined.
Materials and methods
Chemical reagents
Dulbecco's modified essential medium (DMEM), antibiotics, reagents, and standards for HPLC were purchased from Sigma–Aldrich. HPLC grade solvents were purchased from Fisher Scientific Co. Fetal bovine serum was purchased from Natocor (www.natocor.com.ar). Milli-Q water was used in all experiments. PES 0.22 μm sterile filter units were obtained from GVS, USA.
Plant material
The stems and leaves of M. aphylla, L. cuneifolia, and L. divaricata were collected in February between 2017 and 2022 near Ramos Mexía Reservoir (39°15′19.8"S, 68°44′48.8"W; Neuquén, Argentina). Leaves of G. chiloensis were collected in October between 2016 and 2022 in the area near the National University of Comahue campus (38°56′22.4"S, 68°3′20.26"W Neuquén, Argentina), and leaves of P. dictyocarpa and M. laxa were collected in February 2016 in Sierras of Córdoba (31°02′45.5"S, 64°27′22.5"W, Córdoba, Argentina). All the above species were washed for 30 min and then dried in an environment at 20 °C with < 30% humidity and protected from light for 20 days. Once dry, they were crushed with a coffee grinder. Samples were labeled and stored in a dry place in plastic bags.
Infusion preparation
Infusions of each species were prepared with different volumes of water, depending on the water absorption capacity of the grinding. Infusions of P. dictyocarpa (PD) and M. laxa (ML) were prepared using 12 g of plant material in 250 mL of boiled water; those of G. chiloensis (GC) with 12 g of plant material in 150 mL of boiled water; and those of M. aphylla (MA), L. cuneifolia (LC) and L. nitida (LN) with 12 g of plant material in 50 mL of boiled water. On the other hand, two infusions blends of species used in traditional medicine for anti-inflammatory purposes were prepared by mixing 1:1 v/v G. chiloensis and M. aphylla (GC + MA) and G. chiloensis and L. cuneifolia (GC + LC). The infusions were regularly prepared with water at 100 °C, subsequently cooled, and left to macerate for 24 h at room temperature. Then, infusions were filtered through a 10-μm pore nylon filter and stored in a freezer at -80 °C until use. For each spectrophotometric determination, the infusions were diluted to ensure that the obtained values fell within the range of the respective standard curves.
Ferric reducing antioxidant power (FRAP) assay
The Benzie and Strain (Benzie & Strain 1996) protocol with minor modifications (Gallia et al. 2020) was employed to study the FRAP activity of the infusions. An aliquot of 50 μL of the sample was added to 950 μL of FRAP reagent. FRAP reagent was prepared by mixing 2.5 mL of 10 mM TPTZ (2,4,6-tri(2-pyridyl)-s-triazine) solution in 40 mM hydrochloric acid with 2.5 mL of 20 mM FeCl3.6H2O and 25 mL of 300 mM sodium acetate buffer pH 3.6. The reaction mixture was incubated at 37 °C in the dark for 7 min. After that, the absorbance was measured at 595 nm in a T60UV–Visible Spectrophotometer (PG Instruments Limited), and the results were expressed as ascorbic acid equivalents per milligram of dry weight (μg AAE/mg DW).
Free radical scavenging capacity (DPPH assay)
The free radical scavenging ability of the infusions was tested by the DPPH (2,2-diphenyl-1-picryl-hydrazyl) radical scavenging assay, based on the method described by Krishnan et al. (2015), with some modifications. A DPPH radical (0.8 mM) solution in 95% ethanol was prepared. A 50-μL aliquot of infusion was mixed with 125 μL of DPPH radical solution and 625 μL of 1:1 distilled water and ethanol solution. The mix was shaken vigorously, and the absorbance was recorded against a blank of ethanol without DPPH. The kinetics of the reactions were monitored at 515 nm in a T60UV–visible spectrophotometer until no further decrease in absorbance was observed. The percentage of inhibition of DPPH was calculated according to the following expression: DP = [(A0–At)/A0]*100, where A0 is the initial absorbance of DPPH (0% inhibition), and At the time “t”, absorbance after the infusion mixture has been produced. Data were expressed using an index known as antiradical efficiency (AE) described by Sanchez-Moreno et al. (1998), with some modifications. This index consists of two parameters: the EC40, which is the concentration of antioxidants necessary to inhibit the initial amount of the radical by 40%, and the tEC40, which is the time that this concentration of antioxidants inhibits 40% of the radical. With these two parameters, the following index was built: AE = 1/(EC40 x tEC40).
Determination of total phenolic content (TPC)
The total phenolic contents of the infusions were measured by the Folin–Ciocalteu method according to Gallia et al. (2020). Briefly, 50 μL of infusion was mixed with 625 μL of sodium carbonate (20%, w/v), 200 μL of ultrapure water, and 125 μL of Folin–Ciocalteu reagent (1 N). After 30 min in the dark, the absorbance was measured at 760 nm in a T60UV–visible spectrophotometer. TPC was expressed as μg of gallic acid equivalents per milligram of dry weight (μg GAE/mg DW).
Determination of total flavonoid content (TFC)
The total flavonoid contents of the infusions were determined by the aluminum chloride colorimetric method according to Gallia et al. (2020). The infusions (50 μL) were mixed with a solution containing 30 μL of 10% sodium nitrite, 60 μL of 20% aluminum chloride hexahydrate, 200 μL of 1 N NaOH, and 660 μL of Milli-Q water. The absorbance of each sample was recorded at 510 nm in a T60UV–visible spectrophotometer and compared with those obtained from a standard curve made with quercetin. TFC was expressed as quercetin equivalents per milligram of dry weight (μg QE/mg DW).
Analysis of phenolic compounds by high-performance liquid chromatography (HPLC)
The determination of phenolic compounds by HPLC was carried out in GC, LN, LC, and MA infusions and the two blends. ML and PD infusions were not considered because they presented the lowest levels of TPC. The infusions were freeze-dried at -80 °C for 46 h using an Beta 2–8 LDplus freeze-dryer (Martin Christ, Gefriertrocknungsanlagen GmbH, Osterode, Germany) until achieving proper drying, and then the residue was dissolved in ultrapure methanol. All samples, performed in duplicate, were filtered through a 0.22 μm filter unit before injection. The phenolic profile was determined by an HPLC system (Agilent 1260, Quat Pump VL, ALS, TCC, DAD, RID, with openLAB Chem Station Software), using a ZORBAX Eclipse XDB-C18 column (4.6 × 250 mm; 5 μm, Agilent), maintained at 25 °C, with a flow rate of 0.5 mL/min and an injection volume of 5 μL. The mobile phase consisted of 1% phosphoric acid (solvent A) and methanol (solvent B). The linear gradient elution was as follows: 0%–15% B from 0 to 10 min, 15%–30% B from 10 to 20 min, 30%–45% B from 20 to 30 min, 45%–60% B from 30 to 40 min, 60%–75% B from 40 to 50 min and 75%–90% B from 50 to 60 min. Identification of peaks was carried out by comparison of their retention times with those obtained by injecting the following standards under the same conditions: catechin, epicatechin, myricetin, kaempferol, quercetin, resveratrol, rutin and gallic, ellagic, tannic, chlorogenic, syringic, caffeic, p-coumaric, and ferulic acids. All the calibration curves presented an R2 (Coefficient of Determination) greater than 0.999. The software used for data processing was openLAB Chem Station, Agilent.
Cell culture and cytotoxicity assay
To test the cytotoxicity of the infusions, two commercial cell lines were used: VERO (African green monkey Cercopithecus aethiops kidney cells) and MCF-7 (derived from a human breast adenocarcinoma). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/mL penicillin-G, and 40 μg/mL gentamycin sulfate and incubated at 37 °C in a 5% CO2 atmosphere (Gallia et al. 2020).
VERO or MCF-7 cells were seeded onto 96-well tissue culture test plates at 6000 cells per well. At 24 h post-seeding, the medium was replaced with fresh medium containing herbal infusion at a final concentration of 50, 100, 150, 200, or 400 μg AAE/mg DW or with fresh medium only (control). Viable cells were stained with crystal violet according to Soria et al. (2008). The absorbance of each well was read at 630 nm with a Rayto RT-2100C micro ELISA reader (Rayto Life and Analytical Sciences Co., Ltd. China). The viability percentage was defined as the relative absorbance of treated versus control cells (100%).
Antioxidant activity against lipid oxidation in meat samples
Preparation of the raw meat samples
The infusions with greater capacity were selected to evaluate lipid oxidation in raw ground beef. Treatments consisted of meat mixed with LC or LN infusions, and distilled water was used as a control. Infusions or water were manually mixed with the meat at a ratio of 1.5 mL/g of meat, and then, samples were kept at 4 °C for 2.5 h. After that, infusions or water were filtered away from the ground beef, and each treatment was wrapped in stoppered glass jars and stored for 7 days at 4 ± 1 °C. Two replicates of the two treatments and control were carried out. The TBARS content was evaluated on days 1, 4, 7, and 11 in each sample by duplicate.
Lipid peroxidation
The thiobarbituric acid reactive substance (TBARS) concentration was determined using the method described by Burri et al. (2019) with modifications. Briefly, 1 g of sample was homogenized with a glass-Teflon homogenizer, and then 4 mL of a solution containing 11.7 mL water and 0.3 mL 25% TCA was added and mixed. After that, the homogenate was mixed with 0.33 mL 96% ethanol to precipitate proteins and vortexed vigorously for 60 s. Then, the sample was incubated in a water bath at 40 °C for 5 min, and centrifuged at 3000 rpm for 10 min. An aliquot of 400 μL of the supernatant was mixed with 50 μL of butylated hydroxytoluene (0.22%, in ethanol), 500 μL 0.72% (w/v) thiobarbituric acid solution and, 500 μL 25% (w/v) TCA solution. The mixture was vortexed and incubated in a water bath at 95 °C for 30 min. After the mixture was cooled in cold water, 1450 μL chloroform: acetic acid solution (2:1) was added, and then, the mixture was vortexed and centrifuged at 2500 rpm for 15 min. The absorbance of the organic phase was measured at 532 nm in a T60UV–visible spectrophotometer, and the results were expressed as μg malondialdehyde/kg meat using a standard curve of 1,1,3,3-tetrametoxypropane (TMP).
Results and discussion
Antioxidant capacity
The antioxidant capacity of the analyzed infusions measured by FRAP assay is shown graphically in Fig. 1, while the results of scavenging capacity obtained with DPPH assay are shown in Fig. 2. Both assays were used in combination to obtain a more comprehensive assessment of antioxidant's power, since FRAP test is more sensitive to compounds that can reduce Fe ions and DPPH assay is more sensitive to compounds that can donate hydrogen atoms to free radicals (Nwachukwu et al. 2021). As shown in Fig. 1, the infusion blend obtained with G. chiloensis and L. cuneifolia (GC + LC; 1:1, v:v) exhibited the highest value of antioxidant capacity measured with the FRAP technique. This value was 2.4 times greater than the sum of the values obtained by the infusions of each species separately (considering that each species contributes 50% in the blend). However, this infusion showed an AE lesser than the sum of the values corresponding to the infusions of each species separately (Fig. 2). Besides, the antioxidant capacity and scavenging capacity of the G. chiloensis and M. aphylla blend (GC + MA; 1:1, v:v) were significantly lesser than GC + LC blend. Regarding simple infusions, L. cuneifolia (LC) infusion showed the greatest antioxidant power determined by both methods, with values of 131.93 ± 5.23 μg EAA/mg DW and 0.4534 mL/mg s, respectively. These results are consistent with the high antioxidant power found in infusions and ethanolic extracts of L. cuneifolia leaves in other recent studies (Carabajal et al. 2017; Lorenzo et al. 2019). Furthermore, its high antioxidant capacity could explain the high values of antioxidant capacity observed in the GC + LC blend. In the present study, the antioxidant capacity of the LC infusion determined by FRAP assay was followed by that corresponding to the L. nitida (LN) and M. aphylla (MA) infusions (Fig. 1). However, the latter showed lower values of AE than Larrea infusions, suggesting the presence of different types of antioxidants. The infusions of G. chiloensis (GC), P. dictyocarpa (PD) and M. laxa (ML) showed similar values of antioxidant capacity measured by FRAP assay, though, PD and ML infusions had higher scavenging capacity, denoted by higher values of AE, than GC infusion (Figs. 1 and 2). It should be noted that this is the first report on the antioxidant capacity of ML infusion. A previous study on PD infusion (Borneo et al. 2009) has revealed a value of 12.27 μg EAA/mg DW using the FRAP technique, five times lower than the one obtained in the present work (58.19 μg EAA/mg DW). Regarding GC infusion, its antioxidant capacity can be attributed to the presence of phenolic compounds found in this study and described by other authors (Gastaldi 2018).
Antioxidant capacity, measured by FRAP assay, of infusions obtained from Grindelia chiloensis (GC), Larrea cuneifolia (LC), Larrea nitida (LN), Monttea aphylla (MA), Pteromonnina dictyocarpa (PD), Mandevilla laxa (ML). Blends: G. chiloensis and M. aphylla (GC + MA); G. chiloensis and L. cuneifolia (GC + LC). The values are expressed as μg AAE/mg DW and represent the mean ± SD of three assays. Different letters indicate significant differences (Tukey, p < 0.05)
Antiradical efficiency (AE) evaluated by DPPH assay of infusions obtained from Grindelia chiloensis (GC), Larrea cuneifolia (LC), Larrea nitida (LN), Monttea aphylla (MA), Pteromonnina dictyocarpa (PD), Mandevilla laxa (ML). Blends: G. chiloensis and M. aphylla (GC + MA); G. chiloensis and L. cuneifolia (GC + LC). The values are expressed as mL/mg s, and represent the mean ± SD of three assays. Different letters indicate significant differences (Tukey, p < 0.05)
The evidence presented in this section about infusions and blends of Argentinian species, is highly relevant when evaluated alongside other studies. These studies reported AE values ranging from 0.060 to 0.097 mL/mg s for red fruits such as strawberries, raspberries, blackberries, and blueberries (Gramza et al. 2019); and antioxidant capacity values of 13.67, 9.14, 3.68, and 10.56 μg EAA/mg DW for acai berries, mango, pineapple, and red cabbage, respectively (Li et al. 2012; Paz et al. 2015).
Phytochemical analysis of infusions
Phenolics and flavonoids are commonly known as the major phytochemical compounds with antioxidant capacity, so the total phenolic and flavonoid contents were analyzed in the infusions (TPC and TFC, respectively). Both Larrea infusions showed the highest values of TPC and TFC (Figs. 3 and 4), which is closely associated with their great antioxidant power, observed in Figs. 1 and 2. In particular, LC showed a significantly higher content of TPC and TFC than LN. TPC value found in LC infusion was similar to the one reported in ethanolic extracts by Lorenzo et al. (2019), but was higher than the one found in infusions obtained using a lower mass-to-volume ratio (Carabajal et al. 2017). Further, Moreno et al. (2018) found values of TPC and TFC in LC and LN alcoholic extracts similar to those reported in the present work, as well as a significantly higher TPC in LC extract than LN. In this sense, it is important to highlight that obtaining metabolites of interest using water as a non-toxic solvent is a priority to achieve sustainable extraction processes (Lajoie et al. 2022).
Total phenolic content of infusions obtained from Grindelia chiloensis (GC), Larrea cuneifolia (LC), Larrea nitida (LN), Monttea aphylla (MA), Pteromonnina dictyocarpa (PD), Mandevilla laxa (ML). Blends: G. chiloensis and M. aphylla (GC + MA); G. chiloensis and L. cuneifolia (GC + LC). The values are expressed as μg GAE/mg DW and represent the mean ± SD of two assays. Different letters indicate significant differences (Tukey, p < 0.05)
Total flavonoid content of infusions obtained from Grindelia chiloensis (GC), Larrea cuneifolia (LC), Larrea nitida (LN), Monttea aphylla (MA), Pteromonnina dictyocarpa (PD), Mandevilla laxa (ML). Blends: G. chiloensis and M. aphylla (GC + MA); G. chiloensis and L. cuneifolia (GC + LC). The values are expressed as μg QE/mg DW and represent the mean ± SD of two assays. Different letters indicate significant differences (Tukey, p < 0.05)
Considering all the extracts analyzed in this study, GC infusion showed intermediate values of TPC and TFC (101.31 μg GAE/mg DW and 36.27 μg QE/mg DW, respectively), but they were higher than those found by other author in infusions obtained using a lower mass-to-volume ratio (Gastaldi 2018). In the present study, both blends containing GC reflected values of TPC and TFC corresponding to the sum of the values of the infusions separately (considering that each species contributes 50% in the blend). The lowest values of TPC and TFC were found in MA, PD, and ML (Figs. 3 and 4); however, the last two named infusions showed high values of AE suggesting the presence of non-phenolic antioxidant compounds. It is worth noting that the current study represents the first comprehensive assessment of MA, PD, and ML infusions’ chemical characterization.
Identification of phenolic compounds by HPLC
Phenolic compounds are phytochemicals widely reported in the scientific literature as antioxidant, antimicrobial, and anti-inflammatory agents and exhibit multiple actions in health promotion and disease prevention (Dias et al. 2021; Liu et al. 2023; Reis Nunes et al. 2020). Phytochemical analysis by HPLC revealed the presence of several flavonoids and phenolic acids in the infusions (Table 1). Both infusions of Larrea showed caffeic acid, quercetin, kaempferol, ferulic acid, and epicatechin, as has been reported for other species of the genus (Alonso et al. 2023; Espino et al. 2019; Lorenzo et al. 2019; Marrassini et al. 2021). However, between both infusions, only LC contained p-coumaric and chlorogenic acids (Table 1). This pair of phenolic acids has been well documented for its scavenging and antioxidant properties (Roychoudhury et al. 2021; Wang et al. 2022). The presence of these compounds could account for the higher antioxidant power observed in the LC and GC + LC infusions in the current study.
Like Larrea infusions, MA infusion contained quercetin, ferulic acid, and caffeic acid, though in lower concentrations; however, it was the only infusion in which syringic acid was detected (Table 1). Regarding GC infusion, two phenolic acids were identified, as previously reported by Gastaldi (2018), with a high concentration of chlorogenic acid and a low concentration of caffeic acid in comparison to the other infusions. As expected, the herbal blends, GC + LC and GC + MA, contained all the compounds found in the simple infusions (Table 1).
Early effects on cell viability
To determine whether the infusions used in traditional medicine are toxic or safe, in vitro tests were carried out with the non-tumor VERO cell line. For this, VERO cell cultures at approximately 80% confluence were exposed to different concentrations of the infusions. The concentrations were selected according to their antioxidant capacity determined by the FRAP method (Gallia et al. 2020). In this way, it was possible to compare the toxicity/safety of infusions with similar antioxidant power. To determine if any of these infusions had antitumor activity without affecting non-tumor cells, the concentration at which most of the infusions showed no toxicity in VERO cells (50 μg EAA/mg DW) was tested in MCF-7 cancer cell line. The results presented in Fig. 5 demonstrate that infusions obtained from most of the species studied did not decrease cell viability by more than 20% with respect to the control in both cell types, except for GC infusion, which exhibited significant toxicity (p < 0.05). Certainly, VERO cells exposed to this infusion for two hours at a concentration of 50 μg AAE/mg DW showed a 70% decrease in the density of adherent (viable) cells with respect to the control. MCF-7 cells were more susceptible to this infusion, with a decrease in cell viability greater than 80% at the same concentration. Similarly, GC + MA and GC + LC blends also generated a decrease in cell viability of VERO cells, although not as noticeably (Fig. 5). In these cases, the toxicity was lower than expected at the concentration of GC used, so this result could be related to a protective effect of the infusions of M. aphylla or L. cuneifolia, although it would not be sufficient to prevent the death of VERO cells at the concentrations tested. Unlike what happened in VERO cells, in MCF-7 cells the toxicity of GC was completely inhibited by the other species in the blend, since no significant differences were found with the control; therefore, the protective effect of the infusions of M. aphylla or L. cuneifolia would be more effective in these tumor cells.
Percentage of viability of VERO cells at different concentrations of infusions from medicinal plants. Species: G. chiloensis (GC), L. cuneifolia (LC), L. nitida (LN), M. aphylla (MA), P. dictyocarpa (PD), M. laxa (ML). Blends: G. chiloensis and M. aphylla (GC + MA); G. chiloensis and L. cuneifolia (GC + LC). Values represent the mean ± SD of two independent experiments performed in duplicate
The toxicity of GC infusion was reported for the first time by Gastaldi (2018), who found cytotoxic activity in tumor and non-tumor cells at a concentration of 1.25 mg/mL of extract, while in this work, greater toxicity was found at a concentration of 0.37 mg/mL. To date, there have been no studies reporting which compound or combination of compounds is responsible for the toxicity of G. chiloensis in cells. This species has mainly been investigated due to its high content of diterpenes and other constituents found in the resin of its leaves (Zavala & Ravetta 2001). Hence, these results suggest that G. chiloensis infusion would not be a good candidate for the search for antitumor agents, mainly due to its high toxicity in non-tumor cells.
Regarding the studied species of the genus Larrea, whose infusions are used for their anti-inflammatory, antirheumatic, and balsamic properties, among others, it has been reported that L. cuneifolia and L. divaricata show low toxicity and a protective effect against ROS in non-tumor cell lines and toxicity in tumor cell lines (Bongiovanni et al. 2008; Boris et al. 2015; Lorenzo et al. 2020). In this work, LC infusion did not show toxicity in any cell type at the concentrations studied (Figs. 5 and 6). L. nitida is a species that has not been widely studied. A recent study revealed strong antifungal activity (Moreno et al. 2020). Figure 5 shows that LN infusion significantly increased the viability of VERO cells at 50 μg EAA/mg DW, and this effect was maintained with increasing concentration. In MCF-7 cells, no significant differences were found compared to the control in the concentration used (Figs. 6). These results encourage further research related to the possible applications of the active principles present in the LN infusions in the field of pathologies that induce necrosis or neurodegeneration (Uddin et al. 2020). Regarding M. laxa, its antioxidant activity and cytotoxicity have also not been previously studied. In this study, this infusion did not show significant differences in cell viability compared to the control at the concentrations evaluated. It has been described that extracts made from a species of the same genus, M. velame, did not show toxic effects in vitro and in vivo (Ribeiro et al. 2019). In the case of P. dictyocarpa, neither cytotoxicity nor proliferative effect was found, both in VERO cells and in MCF-7 tumor cells. In available investigations, there are no in vitro studies on its antioxidant or cytotoxic activity; however, its antioxidant activity with a neuroprotective effect against arsenic exposure has been studied in Wistar rats, and it was found that it had no toxic effect and showed chemopreventive activity against damage induced by this metalloid (Cortez et al. 2012).
Percentage of viability of MCF-7 cells incubated with 50 μg AAE/mg DW of infusions from medicinal plants and control. Species: G. chiloensis (GC), L. cuneifolia (LC), L. nitida (LN), M. aphylla (MA), P. dictyocarpa (PD), M. laxa (ML). Blends: G. chiloensis and M. aphylla (GC + MA); G. chiloensis and L. cuneifolia (GC + LC). Values represent the mean ± SD of two independent experiments performed in duplicate. Asterisks indicate significant differences from the control (Tukey's test, p < 0.05)
Lipid peroxidation
TBARS assay is one of the most common methods for measuring the oxidative deterioration of unsaturated fatty acids, which results in rancidity and subsequently, in food deterioration (Antolovich et al. 2002; Shahidi et al 2020). Raw ground beef samples exposed to LC and LN infusions, as well as a control group with water, were evaluated for their TBAR substances during 11 days of storage. In control samples, MDA levels increased significantly from day 4 onwards in comparison to day 1 of storage (Fig. 7). Besides, from day 1, control samples exhibited significantly higher levels of MDA compared to samples treated with Larrea infusions, nearly 34% on day 1, reaching up to 54% on day 11 (Fig. 7). Therefore, the addition of antioxidant-rich infusions from Larrea leaves significantly decreased meat lipoperoxidation during the 11 days of storage, and no significant differences were found between both tested species. In the present study, LC and LN infusions not only have a high antioxidant power but also contain a substantial amount of polyphenols. Given that polyphenols have been described to possess the capability of disrupting the cascade of lipid oxidation chain reactions (Kancheva et al. 2021; Brown et al. 2019), this may account for the protective effect against lipid peroxidation observed in LC and LN infusions. In this context, some in vitro and food studies reported that extracts from species of genus Larrea are capable of inhibiting lipid peroxidation and thereby protecting food from oxidative damage, and this effect has been linked to the high content of phenolic compounds (Micucci et al. 2011; Peralta et al. 2018). It is noteworthy that this report represents the initial exploration of the protective effects of LC and LN infusions against lipid peroxidation in meat.
Effect of LC and LN infusions on lipid peroxidation in raw ground meat incubated for 11 days at 4 °C. Control: meat with water; LC: meat with Larrea cuneifolia infusion; LN: meat with Larrea nitida infusion. Values of TBARS, expressed as μmol malondialdehyde (MDA)/kg meat, represent the mean ± SD of two experiments performed in duplicate. Asterisks indicate significant differences from the control (Tukey's test, p < 0.05)
Conclusions
This work highlights the significance of understanding the antioxidant properties and chemical composition of medicinal plant infusions. The results obtained in this study lend credence to the traditional use of these infusions in treating inflammatory disorders. Notably, the infusions from L. cuneifolia, L. nitida, M. aphylla, M. laxa, G. chiloensis, and P. dictyocarpa emerged as rich sources of phenolic compounds, particularly flavonoids and phenolic acids. Importantly, all these infusions demonstrated robust antioxidant capabilities and exhibited no cytotoxicity in either tumor or non-tumor cells, with one exception: the infusion from G. chiloensis displayed marked cytotoxicity, particularly pronounced in tumor cells. This highlights the safety of the studied infusions, and sheds light on the necessity for comprehensive investigations, particularly regarding G. chiloensis infusion, to ensure the well-being of herbal medicine consumers. Furthermore, the study revealed that L. cuneifolia and L. nitida infusions possess the potential to serve as natural antioxidants for raw ground meat, effectively inhibiting lipid peroxidation. This discovery opens the door to their incorporation in meat products, extending their shelf life while harnessing the benefits of these plant infusions.
Availability of data and materials
The data regarding this study is available and can be produced on proper request to the corresponding author.
Abbreviations
- AAE/mg DW:
-
μg ascorbic acid equivalents per milligram of dry weight
- AE:
-
Antiradical efficiency
- DMEM:
-
Dulbecco's modified essential medium
- DPPH:
-
2,2-diphenyl-1-picryl-hydrazyl radical scavenging assay
- EC40 :
-
Concentration of antioxidants necessary to inhibit the initial amount of the radical DPPH by 40%
- FRAP:
-
Ferric reducing antioxidant power assay
- GAE/mg DW:
-
μg of gallic acid equivalents per milligram of dry weight
- HPLC:
-
High-performance liquid chromatography
- MCF-7:
-
Cells derived from a human breast adenocarcinoma
- TBARS:
-
Thiobarbituric acid reactive substances
- TCA:
-
Trichloroacetic acid
- tEC40 :
-
Time during which a certain concentration of antioxidants inhibits 40% of the DPPH radical
- TFC:
-
Total flavonoid content
- TPC:
-
Total phenolic content
- QE/mg DW:
-
μg of quercetin equivalents per milligram of dry weight
- VERO:
-
African green monkey Cercopithecus aethiops kidney cells
References
Alonso, M. R., Marrassini, C., Saint Martin, M., Cogoi, L., Peralta, I., & Anesini, C. (2023). Larrea divaricata a native Argentine plant with potential activity against SARS-CoV-2? Boletin Latinoamericano y Del Caribe De Plantas Medicinales y Aromaticas, 22(6), 747–769. https://doi.org/10.37360/blacpma.23.22.6.52
Antolovich, M., Prenzler, P. D., Patsalides, E., McDonald, S., & Robards, K. (2002). Methods for testing antioxidant activity. The Analyst, 127(1), 183–198. https://doi.org/10.1039/b009171p
Azar, P. (2008). Utilización de vegetales en las sociedades indígenas norpatagónicas. Contribución a una base de datos. En: Rastros. Arqueología e Historia de La Cuenca Del Río Limay, 2, Buenos Aires.
Barboza, G. E., Cantero, J. J., Núñez, C., Pacciaroni, A., & Ariza Espinar, L. (2009). Medicinal plants: a general review and a phytochemical and ethnopharmacological screening of the native Argentine Flora. Kurtziana, 34(1–2), 7–365.
Benzie, I. F. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry, 239(1), 70–76. https://doi.org/10.1006/abio.1996.0292
Bongiovanni, G. A., Cantero, J., Eynard, A., & Goleniowski, M. (2008). Organic extracts of Larrea divaricata Cav. induced apoptosis on tumoral MCF7 cells with an higher cytotoxicity than nordihydroguaiaretic acid or Paclitaxel. Journal of Experimental Therapeutics and Oncology, 7(1), 1–7.
Bonilla Bonilla, A.M., Gavilánez Buñay, T.C., Bayley, M., Colareda, G.A., Matera, S.I., Flores, M.L., León Córdoba, O., Prieto, J.J., Ruiz, M.E., Consolini, A.E., & Ragone, I.M. (2023). Antispasmodic , cardioprotective and blood-pressure lowering properties of Gomphrena perennis L. and its mechanisms of action. Journal of Traditional and Complementary Medicine. https://doi.org/10.1016/j.jtcme.2023.10.005.
Boris, As., Roca, P., Varas, P., Aguilar, J., Martinez, F., Contigiani, M., & Konigheim, B. (2015). Evaluación de la citotoxicidad de extractos de Larrea cuneifolia Cav. (Zygophyllaceae) como potencial antiviral. Revista de la Facultad de Ciencias Médicas, 2015(1), 146–7.
Borneo, R., León, A. E., Aguirre, A., Ribotta, P., & Cantero, J. J. (2009). Antioxidant capacity of medicinal plants from the province of Córdoba (Argentina) and their in vitro testing in a model food system. Food Chemistry, 112(3), 664–670. https://doi.org/10.1016/j.foodchem.2008.06.027
Brown, N., John, J.A. & Shahidi, F. (2019). Polyphenol composition and antioxidant potential of mint leaves. Food Production, Processing and Nutrition, 1, 1. https://doi.org/10.1186/s43014-019-0001-8.
Burri, S. C. M., Granheimer, K., Rémy, M., Ekholm, A., Håkansson, Å., Rumpunen, K., & Tornberg, E. (2019). Lipid oxidation inhibition capacity of 11 plant materials and extracts evaluated in highly oxidised cooked meatballs. Foods, 8(9), 406. https://doi.org/10.3390/foods8090406
Carabajal, M. P. A., Isla, M. I., & Zampini, I. C. (2017). Evaluation of antioxidant and antimutagenic activity of herbal teas from native plants used in traditional medicine in Argentina. South African Journal of Botany, 110, 258–265. https://doi.org/10.1016/j.sajb.2016.10.006
Carabajal, M. P. A., Piloto-Ferrer, J., Nicollela, H. D., Squarisi, I. S., Prado Guissone, A. P., Esperandim, T. R., Tavares, D. C., Isla, M. I., & Zampini, I. C. (2021). Antigenotoxic, antiproliferative and antimetastatic properties of a combination of native medicinal plants from Argentina. Journal of Ethnopharmacology, 267, 113479. https://doi.org/10.1016/j.jep.2020.113479
Chandrasekara, A., & Shahidi, F. (2018). Herbal beverages: bioactive compounds and their role in disease risk reduction - a review. Journal of Traditional and Complementary Medicine, 8(4), 451–458. https://doi.org/10.1016/j.jtcme.2017.08.006
Chaudhary, P., Mitra, D., Das, P. K., Oana, A., Mon, E., Janmeda, P., Martorell, M., Iriti, M., Ibrayeva, M., Sharifi-rad, J., Santini, A., Romano, R., Calina, D., & Cho, W. C. (2023). Camellia sinensis : insights on its molecular mechanisms of action towards nutraceutical, anticancer potential and other therapeutic applications. Arabian Journal of Chemistry, 16(5), 104680. https://doi.org/10.1016/j.arabjc.2023.104680
Cortez, M. V., Bongiovanni, G. A., Rodrigo Fantón, E. T., Navarra Morero, M. L., & Soria, E. A. (2012). Capacidad antioxidante del consumo de Pteromonnina dictyocarpa frente a neurotoxicidad oxidativa por arsenico. XlIV Reunion Anual de la Sociedad Argentina de Farmacología Experimental- Libro de Resumenes – SAFE 2012, 47.
Dias, M. C., Pinto, D. C. G. A., & Silva, A. M. S. (2021). Plant flavonoids: chemical characteristics and biological activity. In Molecules, 26(17), 5377. https://doi.org/10.3390/molecules26175377
Eguchi, N., Vaziri, N. D., Dafoe, D. C., & Ichii, H. (2021). The role of oxidative stress in pancreatic β cell dysfunction in diabetes. International Journal of Molecular Sciences, 22(4), 1–18. https://doi.org/10.3390/ijms22041509
Espino, M., Boiteux, J., de Fernández, M., & los Á., Pizzuolo, P., & Silva, M.F. (2019). Eco-friendly postharvest protection: Larrea cuneifolia-nades extract against Botrytis cinerea. Revista De La Facultad De Ciencias Agrarias, 51(2), 427–437.
Feng, J., Zheng, Y., Guo, M., Ares, I., Lopez-torres, B., Martı, M., Wang, X., & Anado, A. (2023). Oxidative stress, the blood e brain barrier and neurodegenerative diseases : the critical beneficial role of dietary antioxidants. Acta Pharmaceutica Sinica. B, 13(10), 3988–4024. https://doi.org/10.1016/j.apsb.2023.07.010
Gallia, M. C., Bachmeier, E., Ferrari, A., Queralt, I., Mazzeo, M. A., & Bongiovanni, G. A. (2020). Pehuén (Araucaria araucana) seed residues are a valuable source of natural antioxidants with nutraceutical, chemoprotective and metal corrosion-inhibiting properties. Bioorganic Chemistry, 104, 104175. https://doi.org/10.1016/j.bioorg.2020.104175
Gastaldi, B. (2018). Análisis de los compuestos fenólicos y volátiles de plantas medicinales y aromáticas del noroeste de la Patagonia Argentina, estudio de las actividades antioxidante y citotóxica. Tesis de Doctorado Universidad Nacional de la Patagonia San Juan Bosco. Repositorio Institucional CONICET Digital.
Goleniowski, M. E., Bongiovanni, G. A., Palacio, L., Nuñez, C. O., & Cantero, J. J. (2006). Medicinal plants from the “Sierra de Comechingones”, Argentina. Journal of Ethnopharmacology, 107(3), 324–341. https://doi.org/10.1016/j.jep.2006.07.026
Gramza, A., Marzena, M., Bartosz, B., Anna, K., Świgło, G., Kmiecik, D., Bilska, A., Purłan, M., Wałęsa, L., & Ostrowski, M. (2019). Phenolic compounds and multivariate analysis of antiradical properties of red fruits. Journal of Food Measurement and Characterization, 13(3), 1739–1747. https://doi.org/10.1007/s11694-019-00091-x
Ionescu-Tucker, A., & Cotman, C. W. (2021). Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiology of Aging, 107, 86–95. https://doi.org/10.1016/j.neurobiolaging.2021.07.014
Kancheva, V. D., Dettori, M. A., Fabbri, D., Alov, P., Angelova, S. E., Slavova-Kazakova, A. K., Carta, P., Menshov, V. A., Yablonskaya, O. I., Trofimov, A. V., Tsakovska, I., & Saso, L. (2021). Natural chain-breaking antioxidants and their synthetic analogs as modulators of oxidative stress. Antioxidants, 10(4). https://doi.org/10.3390/antiox10040624.
Krishnan, V., Ahmad, S., & Mahmood, M. (2015). Antioxidant potential in different parts and callus of Gynura procumbens and different parts of Gynura bicolor. BioMed Research International, 2015. https://doi.org/10.1155/2015/147909.
Ladio, A. H., & Lozada, M. (2009). Human ecology, ethnobotany and traditional practices in rural populations inhabiting the Monte region: resilience and ecological knowledge. Journal of Arid Environments, 73(2), 222–227. https://doi.org/10.1016/j.jaridenv.2008.02.006
Lajoie, L., Fabiano-Tixier, A. S., & Chemat, F. (2022). Water as green solvent : methods of solubilisation and extraction of natural products — past. Present and Future Solutions. Pharmaceuticals, 15(12), 1507. https://doi.org/10.3390/ph15121507
Leni, Z., Künzi, L., & Geiser, M. (2020). Air pollution causing oxidative stress. Current Opinion in Toxicology, 20–21, 1–8. https://doi.org/10.1016/j.cotox.2020.02.006
Li, H., Deng, Z., Zhu, H., Hu, C., Liu, R., Young, J. C., & Tsao, R. (2012). Highly pigmented vegetables : anthocyanin compositions and their role in antioxidant activities. Food Research International, 46(1), 250–259. https://doi.org/10.1016/j.foodres.2011.12.014
Liu, W., Cui, X., Zhong, Y., Ma, R., Liu, B., & Xia, Y. (2023). Phenolic metabolites as therapeutic in inflammation and neoplasms : molecular pathways explaining their efficacy. Pharmacological Research, 193, 106812. https://doi.org/10.1016/j.phrs.2023.106812
Lorenzo, M. E., Gomez, P. E., Segovia, A. F., Quiroga, A., Figueroa, L. C., & Baroni, M. V. (2019). Phenolic content and antioxidant activity of ethanolic extracts of Larrea divaricata Cav., Larrea cuneifolia Cav. and Sarcomphalus mistol (Griseb.) hauenschild, coming from different areas of the Catamarca Central Valley. Revista del CIZAS 20 (1–2).
Lorenzo, M. E., Gómez, P. E., Sabatino, E., Segovia, A. F., Figueroa, L. C., & Baroni, M. V. (2020). Phenolic Profile and Antioxidant Activity of Ethanolic Extract of Larrea cuneifolia Cav. Leaves. The 1st International Electronic Conference on Food Science and Functional Foods, 37. https://doi.org/10.3390/foods_2020-07645.
Luo, Q., Luo, L., Zhao, J., Wang, Y., & Luo, H. (2023). Biological potential and mechanisms of tea’s bioactive compounds in tea : an updated review. Journal of Advanced Research. https://doi.org/10.1016/j.jare.2023.12.004
Marrassini, C., Martino, R., Barreiro Arcos, M. L., Saint Martin, E. M., Cogoi, L., Alonso, M. R., & Anesini, C. (2021). Aqueous and polyphenol-rich Larrea divaricata Cav. extracts as potential skin care agents. Medicinal Plant Communications, 4(2), 48–55. https://doi.org/10.37360/mpc.21.4.2.07
Martemucci, G., Portincasa, P., Di Ciaula, A., Mariano, M., Centonze, V., & D’Alessandro, Ä. G. (2022). Oxidative stress, aging, antioxidant supplementation and their impact on human health : an overview. Mechanisms of Ageing and Development, 206, 111707. https://doi.org/10.1016/j.mad.2022.111707
Micucci, P., Alonso, M. R., Turner, S., Davicino, R., & Anesini, C. (2011). Antioxidant and antimicrobial activities of Larrea divaricata Cav. aqueous extract on vitamin C from natural orange juice. Food and Nutrition Sciences, 02(01), 35–46. https://doi.org/10.4236/fns.2011.21005
Moloney, J. N., & Cotter, T. G. (2018). ROS signalling in the biology of cancer. Seminars in Cell and Developmental Biology, 80, 50–64. https://doi.org/10.1016/j.semcdb.2017.05.023
Moreno, M. A., Córdoba, S., Zampini, I. C., Mercado, M. I., Ponessa, G., Sayago, J. E., Ramos, L. L. P., Schmeda-Hirschmann, G., & Isla, M. I. (2018). Argentinean larrea dry extracts with potential use in vaginal candidiasis. Natural Product Communications, 13(2), 171–174. https://doi.org/10.1177/1934578x1801300215
Moreno, M. A., Zampini, I. C., & Isla, M. I. (2020). Antifungal, anti-inflammatory and antioxidant activity of bi-herbal mixtures with medicinal plants from Argentinean highlands. Journal of Ethnopharmacology, 253, 112642. https://doi.org/10.1016/j.jep.2020.112642
Muiño, W. A. (2011). La etnobotánica médica del área de transición pampeano cuyana. Bonplandia, 20(2), 353. https://doi.org/10.30972/bon.2021419
Musaubach, M. G., & Plos, A. (2015). Las plantas de los cazadores-recolectores de la Pampa Occidental Argentina. Base de datos de recursos vegetales potencialmente utilizados. Comechingonia. Revista de Arqueología, 19(2), 257–280. https://doi.org/10.37603/2250.7728.v19.n2.18141
Nwachukwu, I. D., Sarteshnizi, R. A., Udenigwe, C. C., & Aluko, R. E. (2021). A concise review of current in vitro chemical and cell-based antioxidant assay methods. Molecules, 26(16), 4865. https://doi.org/10.3390/molecules26164865
O’Flaherty, C. (2020). Reactive oxygen species and male fertility. Antioxidants, 9(4), 287. https://doi.org/10.3390/antiox9040287
Palchetti, V., Zamudio, F., Zeballos, S., Davies, A., Barboza, G., & Giorgis, M. (2023). Large-scale patterns of useful native plants based on a systematic review of ethnobotanical studies in Argentina. Perspectives in Ecology and Conservation. 21. https://doi.org/10.1016/j.pecon.2023.04.001.
Paz, M., Gúllon, P., Barroso, M. F., Carvalho, A. P., Domingues, V. F., Gomes, A. M., Becker, H., Longhinotti, E., & Delerue-matos, C. (2015). Brazilian fruit pulps as functional foods and additives : evaluation of bioactive compounds. Food Chemistry, 172, 462–468. https://doi.org/10.1016/j.foodchem.2014.09.102
Peralta, I., Marrassini, C., Filip, R., Alonso, M. R., & Anesini, C. (2018). Food preservation by Larrea divaricata extract: participation of polyphenols. Food Science and Nutrition, 6(5), 1269–1275. https://doi.org/10.1002/fsn3.640
Pisoschi, A. M., Pop, A., Iordache, F., Stanca, L., Predoi, G., & Serban, A. I. (2021). Oxidative stress mitigation by antioxidants - an overview on their chemistry and influences on health status. European Journal of Medicinal Chemistry, 209, 112891. https://doi.org/10.1016/j.ejmech.2020.112891
Poswal, F. S., Russell, G., Mackonochie, M., MacLennan, E., Adukwu, E. C., & Rolfe, V. (2019). Herbal teas and their health benefits: a scoping review. In Plant Foods for Human Nutrition, 74(3), 266–276. https://doi.org/10.1007/s11130-019-00750-w
Reis Nunes, C., Barreto Arantes, M., de Faria, M., Pereira, S., Leandro da Cruz, L., De Souza Passos, M., Pereira de Moraes, L., Curcino Vieira, I. J., & Barros de Oliveira, D. (2020). Plants as sources of anti-inflammatory agents. Molecules, 25(3726), 1–22.
Ribeiro, R. V., Mariano, D. B., Arunachalam, K., Soares, I. M., de Aguiar, R. W. S., Ascêncio, S. D., Marlon, R., Colodel, E. M., & de Martins, D. T. O. (2019). Chemical characterization and toxicological assessment of hydroethanolic extract of Mandevilla velame xylopodium. Revista Brasileira de Farmacognosia, 29(5), 605–612. https://doi.org/10.1016/j.bjp.2019.05.002
Roychoudhury, S., Sinha, B., Choudhury, B. P., Jha, N. K., Palit, P., Kundu, S., Mandal, S. C., Kolesarova, A., Yousef, M. I., Ruokolainen, J., Slama, P., & Kesari, K. K. (2021). Scavenging properties of plant-derived natural biomolecule para-coumaric acid in the prevention of oxidative stress-induced diseases. Antioxidants, 10(8). https://doi.org/10.3390/antiox10081205.
Sánchez-Moreno, C., Larrauri, J. A., & Saura-Calixto, F. (1998). A procedure to measure the antiradical efficiency of polyphenols. Journal of the Science of Food and Agriculture., 76(2), 270–276. 10.1002/(sici)1097-0010(199802)76:2<270::aid-jsfa945>3.0.co;2-9.
Shahidi, F., & Zhong, Hy, J. (2020). Methods for measuring lipid oxidation. In: Shahidi F, Eds. Bailey’s industrial oil and fat products. Wiley. pp. 1–27. https://doi.org/10.1002/047167849x.bio050.pub2.
Soria, E. A., Goleniowski, M. E., Cantero, J. J., & Bongiovanni, G. A. (2008). Antioxidant activity of different extracts of Argentinian medicinal plants against arsenic-induced toxicity in renal cells. Human and Experimental Toxicology, 27(4), 341–346. https://doi.org/10.1177/0960327108092192
Tuli, H. S., Garg, V. K., Bhushan, S., Uttam, V., Sharma, U., Jain, A., Sak, K., Yadav, V., Lorenzo, J. M., Dhama, K., Behl, T., & Sethi, G. (2023). Natural flavonoids exhibit potent anticancer activity by targeting microRNAs in cancer: a signature step hinting towards clinical perfection. Translational Oncology, 27, 101596. https://doi.org/10.1016/j.tranon.2022.101596
Uddin, M. S., Hossain, M. F., Mamun, A. A., Shah, M. A., Hasana, S., Bulbul, I. J., Sarwar, M. S., Mansouri, R. A., Ashraf, G. M., Rauf, A., Abdel-Daim, M. M., & Bin-Jumah, M. N. (2020). Exploring the multimodal role of phytochemicals in the modulation of cellular signaling pathways to combat age-related neurodegeneration. Science of the Total Environment, 725, 138313. https://doi.org/10.1016/j.scitotenv.2020.138313
Vatner, S. F., Zhang, J., Oydanich, M., Berkman, T., Naftalovich, R., & Vatner, D. E. (2020). Healthful aging mediated by inhibition of oxidative stress. Ageing Research Reviews, 101194. https://doi.org/10.1016/j.arr.2020.101194.
Wang, L., Pan, X., Jiang, L., Chu, Y., Gao, S., Jiang, X., Zhang, Y., Chen, Y., Luo, S., & Peng, C. (2022). The biological activity mechanism of chlorogenic acid and its applications in food industry: a review. Frontiers in Nutrition, 9(June), 1–22. https://doi.org/10.3389/fnut.2022.943911
Zavala, J. A., & Ravetta, D. A. (2001). Allocation of photoassimilates to biomass, resin and carbohydrates in Grindelia chiloensis as affected by light intensity. Field Crops Research, 69(2), 143–149. https://doi.org/10.1016/S0378-4290(00)00136-2
Zheng, F., Gonçalves, F. M., Abiko, Y., Li, H., Kumagai, Y., & Aschner, M. (2020). Redox toxicology of environmental chemicals causing oxidative stress. Redox Biology, 34, 101475. https://doi.org/10.1016/j.redox.2020.101475
Zuloaga, F. O., Belgrano, M. J., & Zanotti, C. A. (2019). An update of the catalogue of the vascular plants of the southern cone. Darwiniana, 7(2), 208–278. https://doi.org/10.14522/darwiniana.2019.72.861
Acknowledgements
The authors thank Chem. Marcela Amaro for her assistance with the HPLC determinations, and English Professor Paola Lardone for her help in reviewing the manuscript's English.
Funding
This research was supported by the following grants: Universidad Nacional del Comahue (UNCo) (PIN 04/N037), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP 2020–1243), and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (PICT 2020–3049).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. M. C. G.: investigation, data collection, writing—review and editing, visualization. L. B.: HPLC analysis, data collection, and analysis. A. F. and G. A. B.: science directors, supervision, review and editing. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gallia, M.C., Ferrari, A., Bajda, L. et al. Antioxidant activity and phenolic content of herbal infusions from medicinal plants used in Argentina. Food Prod Process and Nutr 6, 45 (2024). https://doi.org/10.1186/s43014-024-00224-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43014-024-00224-w