Abstract
The bioactive compounds in a food matrix can exist either in their free form or bound to other endogenous compounds, which may affect their bioactivities. This study investigated the impact of endogenous proteins and lipids on the phenolics profile, anti-nephrolithiasis, and antioxidant activities of Monodora myristica (African nutmeg) seed. Endogenous proteins and lipids in M. myristica seed flour were removed by deproteinization and defatting, to obtain the deproteinized, defatted, and deproteinized-defatted flours. The native flour served as a control. Phenolics compounds in the flours were quantified using HPLC-DAD, while nephrolithiasis-associated enzymes (xanthine oxidase and urease) inhibitory and antioxidant activities were determined using a spectrophotometer. Nine phenolic compounds (gallic, chlorogenic, caffeic, p-coumaric, ellagic acids, catechin, rutin, quercetin and luteolin) were detected in the native flour, with quercetin as the most abundant. The concentrations of the phenolic compounds, nephrolithiasis-associated enzymes inhibitory, and antioxidant activities of the flour decreased significantly due to deproteinization and defatting. Overall, the native flour had the highest concentrations of phenolics and the most potent enzymes inhibitory and antioxidant activities, followed by the defatted, deproteinized, and deproteinized-defatted flours. Hence, endogenous proteins and lipids may enhance the phenolics profile, anti-nephrolithiasis and antioxidant activities of M. myristica seed. This could have application in the development of functional food products and nutraceuticals targeting nephrolithiasis.
Graphical Abstract

Similar content being viewed by others
Introduction
Matrix interaction is an important factor affecting the bioaccessibility, bioavailability, and bioactivity of bioactive compounds in foods, including polyphenolic compounds (Pinarli et al. 2020). Plant-derived bioactive compounds usually exist either in their free form or bound with the macronutrients (proteins, carbohydrates, and lipids) in the food matrix (Jakobek 2015; Irondi et al. 2022). Phenolic compounds, for example, occur in their free, soluble conjugated, and insoluble bound forms (Jimenez-Pulido et al. 2022). They exist mainly as glycosides bonded to different sugar moieties or as other complexes bonded to lipids, amines, carbohydrates, other phenols, and organic acids (Liu et al. 2007). Further, some recent studies have demonstrated that food matrix affects its functionalities, including the functional properties, bioactive constituents, and biological activities. For instance, Pessato et al. (2018) reported that the interaction of whey proteins with phenolic compounds from tea and coffee reduced the whey protein’s allergenicity. Similarly, Sęczyk et al. (2021) demonstrated that phenolic–food matrix components interactions negatively impacted the antioxidant activity, in vitro bioaccessibility of phenolic compounds, and nutrient digestibility.
Phenolic compounds have attracted the most attention among the bioactive compounds in plant foods, due to their diverse biological activities and health benefits. They are notable for their antioxidant, antidiabetic, digestive enzymes inhibitory, and anti-hypertensive activities (Servili et al. 2013; Irondi et al. 2019). They also impact some important functional qualities of food products, such as flavour, colour, and oxidative stability (Sęczyk et al. 2021), thereby maintaining their nutritional quality (Avila-Roman et al. 2021; Irondi et al. 2022). In addition, phenolic compounds were reported to inhibit xanthine oxidase and urease (Nile et al. 2017), and possess anti-nephrolithiasis (Irondi 2020) activity.
Nephrolithiasis (also known as urolithiasis or kidney stone) is a heterogeneous disease that results from an imbalance between the rate of solubility and precipitation of salts in the urinary tract and kidneys, leading to the presence of renal calculi (Han et al. 2015; Mukasa & Sung 2020; Toole et al. 2021). Among the urinary diseases, it ranks as the third most common disorder, with 10 to 12% of people in industrialized countries having it during their lifetime (Nirumand et al. 2018). Depending on the aetiological factor, different types of nephrolithiasis, including uric acid, struvite, and calcium nephrolithiasis, are known. Uric acid nephrolithiasis accounts for about 10% of all nephrolithiasis cases (Sakhaee 2014), and its development has been linked to the activities of xanthine oxidase (Irondi 2020). Xanthine oxidase catalyzes the last two reactions of purine nucleotides catabolism, leading to the formation of xanthine and, subsequently, uric acid (Berry & Hare 2004). The accumulation of the uric acid so-produced, precipitates the pathogenesis of uric acid nephrolithiasis (Leonardo et al. 2018). On the other hand, struvite nephrolithiasis accounts for 1–5% of all nephrolithiasis cases. It develops as a result of repeated infection with urease-producing gram-negative bacteria (Sorokin & Pearle 2018). The urease, produced by the gram-negative bacteria in the urinary system of susceptible humans, is a nickel-containing metalloenzyme that catalyzes the hydrolysis of urea, forming ammonium and carbon (IV) oxide (Khan et al. 2014).
The involvement of xanthine oxidase and urease in the development of uric acid and struvite nephrolithiasis underpins the clinical use of the inhibitors of these two enzymes for their treatment. Hence, allopurinol (xanthine oxidase inhibitor) and acetohydroxamic acid (urease inhibitor), are used for the treatment of uric acid and struvite nephrolithiasis, respectively (Sorokin & Pearle 2018). However, the clinical use of these two drugs is associated with some adverse effects that undermine their therapeutic effectiveness. Some of the side effects of allopurinol include hypertransaminasemia and Stevense-Johnson syndrome, while those of acetohydroxamic acid include anaemia, thrombophlebitis, gastrointestinal discomfort, rash and headache (Sorokin & Pearle 2018). Against the backdrop of these side effects, recent studies have shown that bioactive constituents of plant origin, especially phenolic compounds, are potent inhibitors of these enzymes, and may be a safer alternative for the treatment of nephrolithiasis (Irondi 2020).
Some plants reported to be beneficial for preventing and managing kidney stones include green tea (Camellia sinensis), pomegranate (Punica granatum), black-cumin (Nigella sativa), roselle (Hibiscus sabdariffa) (Nirumand et al. 2018). Albeit, the concentration and biological activities of phenolic compounds in plant foods, such as Monodora myristica (African nutmeg), are affected by different factors. Some of those factors include food matrix interaction (Irondi et al. 2022) and processing method (Domínguez-Fernandez et al. 2021). Pertaining to food matrix interaction, the removal of endogenous lipids and proteins from sorghum flour led to a decrease in the bioactive constituents’ levels and antioxidant activity of the flour (Irondi et al., 2022; Ye et al., 2018) also demonstrated that endogenous proteins and lipids removal in rice flour resulted in an increased in vitro starch digestibility of rice flour.
M. myristica, a member of the Anonaceae family, is an under-utilized spice in Africa and Asia that is rich in bioactive constituents (Afolabi et al. 2021; Ekeanyanwu et al. 2021). It is indigenous to West, East and Central Africa, and thrives in the West African evergreen forest. The seed of M. myristica is aromatic and its powder serves as a condiment that adds flavour to soups, stews, desserts, and cakes (Moukette et al. 2015). The seed’s powder also serves as a stimulant in pepper soup for alleviating post-parturition passive uterine hemorrhage in women and relieving constipation (Ekeanyanwu 2013). Some bioactivities that have been reported for M. myristica include anti-depressant (Ekeanyanwu et al. 2021), anti-inflammatory, and anti-nociceptive (Ishola et al. 2016). With these reported bioactivities, the influence of matrix on the bioactive constituents and bioactivities of M. myristica may have important implications on its health benefits, as earlier suggested in other staples (Kaur et al. 2016). Also, understanding food matrix interactions can provide vital information on their functionalities in real food systems (Zhang & Hamaker 2003). Therefore, this study investigated the impact of endogenous proteins and lipids on the phenolics profile, anti-nephrolithiasis, and antioxidant activities of M. myristica seed, in vitro.
Methodology
Chemicals and reagents
Jack bean urease, thiourea, urea, allopurinol, xanthine oxidase, xanthine, Trolox, 2,2´-azino-bis-3-ethylbenzthiazoline-6-sulphonic (ABTS), L-ascorbic acid, and 2,2-diphenylpicrylhydrazyl (DPPH) were Sigma (St. Louis, USA) products. HPLC grade methanol, formic acid, acetic acid, gallic acid, chlorogenic acid, p-coumaric acid, ellagic acid, caffeic acid, catechin, rutin, luteolin and quercetin were products of Merck (Darmstadt, Germany). All other reagents, chemicals and solvents used in the experiments were of analytical grade.
Sample collection and preparation
A sample (500 g) of M. myristica seeds (Fig. 1) was bought from Ipata market in Ilorin, Kwara State, Nigeria. Subsequently, the sample was sorted, pulverized into fine flour with a kitchen grinder, and kept air-tight in a refrigerator for further analysis.
Deproteinization and defatting of sample
Endogenous proteins and lipids were removed from three different portions (100 g each) of the M. myristica flour by deproteinization, defatting, and deproteinization followed by defatting (deproteinization-defatting) as per the procedure reported by Annor et al. (2013), with a slight modification. The flour was deproteinized by alkaline protease hydrolysis. This process was carried out by mixing 100 g of the flour with 800 mL of alkaline protease solution (120 U/mL), prepared in a carbonate buffer (0.02 M, pH 9.0) in a beaker. Next, the suspension was kept in a water bath (at 45oC) for 4 h, stirring continuously to achieve hydrolysis. Afterwards, the suspension was subjected to centrifugation for 10 min at 4000 rpm, and the solid residue was collected. The alkaline protease hydrolysis was repeated on the residue, after it was washed with distilled water to a neutral pH (7.0) to yield the deproteinized flour.
The flour was defatted by blending 100 g with petroleum ether (500 mL), followed by continuous stirring for 4 h at room temperature. The suspension was filtered through a Whatman filter paper (No. 2) and the resulting residue was collected. The defatting process was repeated twice on the residue, after which the defatted flour was obtained. Deproteinization-defatting process was performed by deproteinizing and defatting 100 g of the flour sequentially, according to the procedures described above. Finally, the deproteinized, defatted, and deproteinized-defatted samples were oven-dried to a constant weight at 45oC, and stored in air-tight sample containers for further analysis.
Preparation of flour extracts
The procedure outlined by Engida et al. (2013) was adopted to prepare the flours extracts for analyses. This was done by soaking 2 g of each of the flours (native, deproteinized, defatted, and deproteinized-defatted) with 20 mL of methanol for 24 h. Subsequently, the suspension was spun in a centrifuge at 4000 rpm for a period of 5 min. The supernatant was collected, concentrated at 45 °C in a rotary evaporator, and later reconstituted with methanol (6 mL).
Quantification of phenolics compounds by high-performance liquid chromatography (HPLC)
A reverse-phase HPLC system (Shimadzu, Kyoto, Japan) was used for the quantification of phenolic compounds (Irondi et al. 2018). The HPLC instrumentation consisted of an auto-sampler, a reciprocating pumps attached to a degasser with a CBM 20 A integrator, diode-array detector (DAD), and LC solution 1.22 SP1 software. Flour extract (15 mg/mL) was injected into a reverse-phase Phenomenex C18 column (4.6 mm x 250 mm) packed with 5 μm diameter particles. The flow rate and injection volume were of 0.7 mL/min and 40 µL, respectively. The mobile phase consisted of 0.5% (v/v) aqueous formic acid (solvent A) and 1% (v/v) acetic acid in methanol (solvent B). The extract and mobile phase were filtered through a 0.45 μm membrane filter (Millipore) and then degassed by ultrasonic bath prior to injection. Stock solution (0.030–0.250 mg/mL) of each phenolic compound’s standard reference was prepared in the HPLC mobile phase. The binary elution system used was as follows: 2% B at an initial 5 min to wash the column, a linear gradient of 8% B (15 min), 12% B (30 min), 24% B (45 min). After 50 min, the organic phase concentration was brought back to 2% (B) and lasted 10 min for column equilibration. The wavelengths for detections were 254 nm (gallic and ellagic acids), 280 nm (catechin), 327 nm (caffeic, chlorogenic, and p-coumaric acids), and 366 nm (rutin, quercetin and luteolin). Confirmation of chromatography peaks and quantification of their corresponding phenolic compounds were achieved by comparing their retention time with those of reference standards and by DAD spectra (200 to 500 nm). All chromatography operations were carried out at ambient temperature.
Enzymes inhibition assays
Assay for xanthine oxidase inhibition
The procedure outlined by Osada et al. (1993) was adopted to assay for the xanthine oxidase inhibitory activity of the flour extract. In this assay, xanthine and allopurinol was used as substrate and reference inhibitor, respectively. Solution of 15 mM xanthine and 0.1 mU/µL xanthine oxidase were prepared fresh with 50 mM Tris–HCl buffer (pH 7.4). Thereafter, xanthine oxidase (10 µL) was mixed with 1950 µL of varied concentrations (10, 20, 30, 40 µg/mL) of the extract and xanthine solutions (40 µL), and incubated for 10 min at 37oC. Afterwards, the hydrolytic reaction was stalled by adding 50 µL of 3.2% (v/v) perchloric acid solution in Tris–HCl buffer (50 mM, pH 7.4). The absorbance reading of the uric acid produced was taken at 292 nm. Subsequently, the inhibition of xanthine oxidase (%) and IC50 (extract concentration that inhibited 50% of xanthine oxidase activity) were calculated.
Assay for urease inhibition
The protocol of Jaffary et al. (2016) was followed to assay for the urease inhibitory activity of the flour extracts, with urea and thiourea as the substrate and reference inhibitor, respectively. In this assay, jack bean urease (500 µL) and 100 µL of different concentrations of the extract (10, 20, 30, 40 µg/mL) was incubated for 30 min at 37 °C. Thereafter, urea (1100 µL) was added and the mixture was further incubated for 30 min at 37 °C. Next, phenol reagent (1% phenol and 0.005% sodium nitroprusside, w/v) and alkali reagent (0.5% NaOH and 0.1% sodium hypochlorite, w/v), were added to the test mixture. Afterwards, the test mixture was subjected to incubation for 2 h at 37 °C, and the absorbance reading of the ammonia resulting from urease-catalyzed hydrolytic reaction was taken at 635 nm. The inhibition of urease (%) and IC50 (extract concentration that inhibited 50% of urease activity) were calculated.
Antioxidant activity assays
Assay for ABTS*+ scavenging capacity
The procedure outlined by Re et al. (1999) was adopted to assay for the ABTS*+ scavenging capacity of the extracts. ABTS•+ reagent was prepared by mixing aqueous solutions of ABTS•+ (7 millimole/L) and K2S2O8 (2.45 millimole/L) in an equal volume and incubating the mixture in the dark at room temperature for 16 h. After that, the reagent’s absorbance reading at 734 nm was adjusted to 0.70 ± 0.02 with ethanol (95%). Next, 2.0 mL of the ABTS•+ reagent and 0.2 mL of the four extract were dispensed in a test tube, vortexed and incubated for 15 min in the dark at room temperature. The absorbance was read at 734 nm and the ABTS•+ scavenging capacity of the extract was calculated from a trolox (1–20 mM) calibration curve and expressed as trolox equivalent antioxidant capacity (TEAC) in millimole per gram.
Assay for DPPH• scavenging capacity
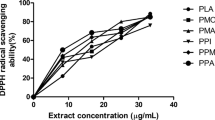
Assay for DPPH• scavenging capacity was performed as per the protocol of Cervato et al. (2000). A mixture consisting of 1.0 mL of different dilutions of the flour extract (or ascorbic acid, a reference antioxidant) and 3.0 mL of DPPH• solution (60 micromole/L) was incubated for 30 min at room temperature. Afterward, the absorbance reading was taken at 517 nm and the DPPH• scavenging capacity of the extract was expressed as SC50 (extract concentration that scavenged 50% of DPPH•).
Assay for reducing power
Assay for ferric reducing power of the extract was performed as per the procedure of Oyaizu (1986). Flour extract (2.5 mL) was mixed with 200 mM sodium phosphate buffer, pH 6.6, (2.5 mL) and 1% potassium ferricyanide (2.5 mL). The mixture was incubated at 50oC for 20 min, after which 10% trichloroacetic acid (2.5 mL) was added to it. Next, 2.5 mL of the mixture was transferred into two different test tubes, followed by the addition of distilled H2O (2.5 mL) and 0.1% ferric chloride (1 mL) to each tube. Thereafter, the absorbance reading was taken at 700 nm and the reducing power of the extract was calculated from a gallic acid (10–100 mg gallic acid/mL) calibration curve, and expressed as GAE mg/g (gallic acid equivalent in mg per g).
Assay for Iron (II) chelation
Assay for iron (II) was performed by adopting the protocol outlined by Puntel et al. (2005). A mixture of 0.1 M Tris-HCl, pH 7.4, (168 µL), normal saline (218 µL) and different dilutions of the extract (or ascorbic acid, a reference antioxidant) and freshly prepared 500 micromole/L FeSO4 (150 µL) was incubated for 5 min at room temperature. Subsequently, 0.25% 1,10-phenanthroline (13 µL) was added and the absorbance reading was taken at 510 nm. Iron (II) chelation by the extract was later calculated and expressed as SC50 (extract concentration that chelated 50% of Fe2+) in µg/mL.
Data analysis
Results of triplicate determinations of the different treatments were subjected to one-way analysis of variance (ANOVA). This was followed by Duncan’s multiple range test for mean values comparison at p < 0.05. The Statistical Package for Social Science (SPSS) software, 17th version, was used for the data analysis.
Results and discussion
Phenolic compounds in native and treated M. myristica flour
The phenolic compounds, quantified using HPLC-DAD, in the native and treated (deproteinized, defatted, and deproteinized-defatted) M. myristica flours are presented in Table 1. Figure 2 also depicts a representative chromatogram of the phenolic compounds’ profile. The phenolic compounds quantified in the flours belong to three major classes of polyphenols, including phenolic acids (gallic, chlorogenic, caffeic, p-coumaric and ellgic acids), flavonoids (rutin, quercetin and luteolin) and tannins (catechin). Quantitatively, the flavonoids, quercetin followed by rutin, were the most abundant. These were followed by chlorogenic acid, a phenolic acid. The levels of phenolics compounds, including gallic acid, catechin, chlorogenic acid, caffeic acid, p-coumaric acid, ellagic acid, rutin, quercetin and luteolin quantified in the M. myristica native flour in this study differed from those reported by Feyisoyo and Oluokun (2014). The differences may be due to variations in biotic and abiotic factors, sample collection period and extraction method, which are known to affect the contents of bioactive compounds in plants, as well as their biological activities (Mpofu et al. 2006). In addition, variations in analytical techniques may have contributed to the differences: we used a reverse-phase HPLC-DAD for the phenolics quantification in this study, while Feyisoyo and Oluokun (2014) used gas chromatography coupled to flame ionization detector (GC-FID) in their study.
Since phenolic compounds exist in their free, soluble conjugated, and insoluble bound forms (Jimenez-Pulido et al. 2022), we investigated the effect of endogenous proteins and lipids on the phenolic compounds’ levels of the M. myristica flour. Deproteinization, defatting, and deproteinization-defatting of the M. myristica generally led to significant losses (p < 0.05) in the levels of the individual phenolic compounds, with deproteinization-defatting causing the highest loss, followed by deproteinization and then defatting. It was also observed that deproteinization-defatting led to absolute loss of catechin and p-coumaric acid, as these two compounds were not detected in the deproteinized-defatted flour. The observed losses of the phenolic compounds might be attributed to the leaching of the phenolic compounds during the treatments. For the deproteinization process, the alkaline protease-catalyzed hydrolysis, subsequent decantation of the hydrolyzed protein, and washing the resulting residue may have facilitated the leaching of the water-soluble free and protein-bound phenolic compounds, leading to the observed losses. Pertaining to the losses due to the defatting process, the fat-soluble free and lipid-bound phenolic compounds may have also been lost through leaching into the organic solvent (petroleum ether) used for the fats removal. The susceptibility of lipophilic phenolic compounds to degradation (Zhou et al. 2021) may have also contributed to the losses. Zhu (2015) had earlier revealed that the de-compartmentalization and disintegration of plant cells occurred during food processing under mechanical shearing forces, leading to the release of different chemical constituents from their respective cell compartments. These processes may have led to the loss of the phenolic compounds in the M. myristica flour.
Nephrolithiasis-associated enzymes (xanthine oxidase and urease) inhibitory activity of native and treated M. myristica flour
The nephrolithiasis-associated enzymes (xanthine oxidase and urease) inhibitory activity of the M. myristica flour extract, expressed in terms of IC50 (extract concentration that inhibited the activity of the enzyme by 50%), is presented in Table 2. The IC50 values for both enzymes were in a consistent order of native < defatted < deproteinized < deproteinized-defatted. Since a lower IC50 value is indicative of a more potent inhibitory activity (Irondi et al. 2018), the native flour with the least IC50 values against xanthine oxidase and urease (20.56 ± 1.72 and 17.86 ± 1.13 µg/mL, respectively), had the most potent inhibitory activity, followed by the defatted, deproteinized, and deproteinized-defatted flour. However, allopurinol and thiourea, the reference inhibitors of xanthine oxidase and urease, respectively, displayed stronger inhibitory activities against their respective enzymes than the M. myristica flour.
The decreasing order in the xanthine oxidase and urease inhibitory activities of the extracts due to deproteinization, defatting, and deproteinization-defatting corresponded with that of their phenolics compounds contents (Table 1). This trend is instructive as it accentuates the prominent role of phenolic compounds as antioxidant and anti-nephrolithiasis compounds in plants. Plant-derived polyphenolic compounds have an affinity for proteins, including enzymes, via hydrogen and hydrophobic bonding, and this enhances polyphenolics-rich plant extract’s capacity to inhibit enzymes by denaturing them (Villiger et al. 2015). Mechanistically, the OH− groups at the fifth (C-5) and seventh (C-7) carbons of flavones and flavonols are capable of replacing the OH− groups at the second (C-2) and sixth (C-6) carbons of xanthine in the active site of xanthine oxidase (Cos et al. 1998; Irondi et al. 2018), leading to xanthine oxidase inhibition. Pertaining to urease inhibition, it is possible that the dihydroxyl group at the C-ring of quercetin structure may have interacted with the amino acid residues around the nickel atoms in urease active to inhibit it, as does thiourea (urease standard inhibitor) (Nile et al. 2017).
Further, although the phenolic compounds in M. myristica extract may have acted synergistically in the observed inhibition of the nephrolithiasis-associated enzymes tested in this study, it is possible that quercetin, the most abundant of them, contributed majorly. This is supported by the findings of some previous studies that demonstrated the anti-nephrolithiasis activity of quercetin. Structurally, quercetin (C15H10O7) is a pentahydroxyflavone (with five hydroxyl groups), a property that confers on it some unique bioactivities such as antioxidant and enzymes inhibitory activities. It is reputed as an effective phyto-therapeutics for managing kidney stones due to its inhibitory effect on calcium oxalate crystals deposition, anti-apoptotic effects and antioxidant activity against the injury of renal tubular cell (Park et al. 2008; Nirumand et al. 2018). Quercetin was also reported to be a very strong urease inhibitor (Nile et al. 2017). As observed in Table 1, quantitatively quercetin was followed by rutin (3, 3ʹ, 4ʹ, 5, 7-pentahydroxyflavone-3-Rhamnoglucoside), a derivative of quercetin with both antioxidant and anti-nephrolithiasis activities (El oumari et al. 2021). The co-administration of rutin and curcumin was reported to inhibit calcium oxalate formation, restore the normal levels of urinary calcium and oxalate, and inhibit calcium oxalate monohydrate crystals aggregation and growth (Ghodasara et al. 2011).
Antioxidant activity of native and treated M. myristica flour
Previous studies suggested that increasing the body’s antioxidant capacity could be beneficial for preventing stone development and/or recurrence (Irondi 2020; Guzel et al. 2021). Hence, the antioxidant activity of the native and treated flours was tested and the results are presented in Table 3. The native flour had the highest reducing power and ABTS•+ scavenging ability and the lowest DPPH• and Fe2+ chelation IC50 values (9.39 ± 0.39 and 14.91 ± 0.82 µg/mL, respectively). As with the xanthine oxidase and urease inhibitory activities, the flour’s antioxidant activity decreased as the phenolic compound’s levels decreased due to deproteinization, defatting, and deproteinization-defatting. Thus, the antioxidant activity was also in the order of native > defatted > deproteinized > deproteinized-defatted. This also buttresses the role of phenolics compounds, which exist either in their free form or bound with other endogenous chemicals, as the major determinants of the antioxidant activity (Beta & Hwang 2018) in the M. myristica flour. These phenolic compounds display their antioxidant activity by various mechanisms, including the inhibition of lipid radicals formation, suppression of singlet oxygen, disruption of chain auto-oxidation reactions the propagation, chelation of transition metal ions, reduction of hydrogen peroxides to form stable compounds, activation of endogenous antioxidant enzymes, and inhibition of endogenous prooxidative enzymes (Sęczyk et al. 2019). Hence, the loss in phenolic compounds due to deproteinization and/or defatting (Table 1) was accompanied with a reduction in the antioxidant activity of the flour. Moreover, the deproteinization of the M. myristica flour may have also contributed to the loss of antioxidant activity, since plant-derived bioactive peptides exhibit antioxidant activity (Sánchez & Vázquez 2017).
Taken together, the reducing power, free radicals (ABTS•+ and DPPH•) scavenging ability, and Fe2+ chelation of the M. myristica flour suggest its potential to mitigate or prevent oxidative stress. It is well-known that oxidative stress results when the level of oxidants (free radicals and reactive species – reactive oxygen species and reactive nitrogen species) overwhelms the body’s antioxidant level. Further, since the activities of xanthine oxidase and urease result in the generation of reactive oxygen species, the antioxidant activity of M. myristica may complement its anti-nephrolithiasis activity. The Fe2+ chelating capacity of the M. myristica flour also suggests that it may mitigate transition metals availability, thereby decelerating and/or inhibiting free radicals-mediated oxidative chain reactions in food and biological systems. As earlier reported by (Smeriglio et al. 2016), this can improve food quality, stability and safety, and human health.
Conclusions
Deproteinization, defatting, and deproteinization-defatting resulted in a reduction in the levels of all the phenolic compounds in M. myristica seed. The nephrolithiasis-associated enzymes (xanthine oxidase and urease) inhibitory and antioxidant activities of the seed also decreased due to deproteinization, defatting, and deproteinization-defatting. Overall, the native flour (containing proteins and lipids) had the highest concentrations of the phenolics compounds and the most potent nephrolithiasis-associated enzymes inhibitory and antioxidant activities, followed by the defatted, deproteinized, and deproteinized-defatted flours. These findings suggest that endogenous proteins and lipids may enhance the phenolics profile, anti-nephrolithiasis, and antioxidant activities of M. myristica seed.
Availability of data and materials
The data supporting the findings in this article are included in the manuscript. More details about the data and materials are available on request from the corresponding author.
References
Afolabi, O. B., Oloyede, O. I., Aluko, B. T., & Johnson, J. A. (2021). Biosynthesis of magnesium hydroxide nanomaterials using Monodora myristica, antioxidative activities and effect on disrupted glucose metabolism in streptozotocin-induced diabetic rat. Food Bioscience, 41, 101023. https://doi.org/10.1016/j.fbio.2021.101023
Annor, G. A., Marcone, M., Bertoft, E., & Seetharaman, K. (2013). In vitro starch digestibility and expected glycemic index of Kodo millet (Paspalum scrobiculatum) as affected by starch–protein–lipid interactions. Cereal Chemistry, 90, 211–217. https://doi.org/10.1094/CCHEM-06-12-0074-R
Avila-Roman, J., Soliz-Rueda, J. R., Bravo, F. I., Aragones, G., Suarez, M., Arola-Arnal, A., Mulero, M., Salvado, M., Torres-Fuentes, L. C., & Mugueza, B. (2021). Phenolic compounds and biological rhythms: Who takes the lead? Trends in Food Science and Technology, 113, 77–85. https://doi.org/10.1016/j.tifs.2021.04.050
Berry, C. E., & Hare, J. M. (2004). Xanthine oxidoreductase and cardiovascular disease: Molecular mechanisms and pathophysiological implications. Journal of Physiology, 555, 589–606.
Beta, T., & Hwang, T. (2018). Influence of heat and moisture treatment on carotenoids, phenolic content, and antioxidant capacity of orange maize flour. Food Chemistry, 246, 58–64. https://doi.org/10.1016/j.foodchem.2017.10.150
Cervato, G., Carabelli, M., Gervasio, S., Cittera, A., Cazzola, R., & Cestaro, B. (2000). Antioxidant properties of oregano (Origanum vulgare) leaf extracts. Journal of Food Biochemistry, 24, 453–465.
Cos, P., Ying, L., Calomme, M., Hu, J. P., Cimanga, K., Poel, B. V., Pieters, L., Vlietinck, A. J., & Berghe, D. V. (1998). Structure–activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. Journal of Natural Products, 61, 71–76.
Domínguez-Fernandez, M., Irigoyen, A., Vargas-Alvarez, M. A., Ludwig, I. A., De Pena, M. P., & Cid, C. (2021). Influence of culinary process on free and bound (poly)phenolic compounds and antioxidant capacity of artichokes. International Journal of Gastronomy and Food Science, 25, 100389. https://doi.org/10.1016/j.ijgfs.2021.100389
Ekeanyanwu, R. C. (2013). Evaluation of the crude protein and amino acid composition of nigerian Monodora myristica (Ehuru). Pakistan Journal of Nutrition, 12(3), 219–223.
Ekeanyanwu, R. C., Nkwocha, C. C., & Ekeanyanwu, C. L. (2021). Behavioural and biochemical indications of the antidepressant activities of essential oils from Monodora myristica (Gaertn) seed and Xylopia aethiopica (Dunal) fruit in rats. IBRO Neuroscience Report, 10, 66–74.
El oumari, F. E., Bousta, D., Grafov, A., & Houssaini, T. S. (2021). Phytomolecules investigated for the prevention and treatment of urinary stones. Mediterranean Journal of Chemistry, 11, 126–132. https://doi.org/10.13171/mjc02102271568fee
Engida, A. M., Kasim, N. S., Tsigie, Y. A., Ismadji, S., Huynh, L. H., & Ju, Y. (2013). Extraction, identification and quantitative HPLC analysis of flavonoids from sarang semut (Myrmecodia pendan). Industrial Crops and Products, 41, 392–396.
Feyisoyo, A., & Oluokun, O. O. (2014). Comparative analysis of phenolic profile of Monodora myristica and Monodora tenuifolia. African Journal of Agricultural Research, 9, 1296–1302.
Ghodasara, J., Pawar, A., & Deshmukh, C. (2011). Inhibitory effect of rutin and curcumin on experimentally-induced calcium oxalate urolithiasis in rats. Pharmacognosy Research, 2, 388–392.
Guzel, A., Yunusoglu, S., Calapoglu, M., Candan, I. A., Onaran, I., Oncu, M., Ergun, O., & Oksay, T. (2021). Protective Effects of Quercetin on oxidative Stress-Induced tubular epithelial damage in the experimental rat Hyperoxaluria Model. Medicina, 57, 566. https://doi.org/10.3390/medicina57060566
Han, H., Segal, A. M., Seifter, J. L., & Dwyer, J. T. (2015). Nutritional management of kidney stones (nephrolithiasis). Clinical Nutrition Research, 4, 37–52.
Irondi, E. A. (2020). Phenolics-rich extract of guava stem bark inhibits enzymes associated with nephrolithiasis and obesity in vitro. Journal of Complementary Medicine Research, 11, 34–42.
Irondi, E. A., Agboola, S. O., & Boligon, A. A. (2018). Inhibitory effects of tropical almond leaf extract on xanthine oxidase, pancreatic lipase, and angiotensin 1-converting enzyme, in vitro. Journal of Food Biochemistry, 42, e12481. https://doi.org/10.1111/jfbc.12481
Irondi, E. A., Adegoke, B. M., Effion, E. S., Oyewo, S. O., Alamu, E. O., & Boligon, A. A. (2019). Enzymes inhibitory property, antioxidant activity and phenolics profile of raw and roasted red sorghum grains in vitro. Food Science and Human Wellness, 8, 142–148. https://doi.org/10.1016/j.fshw.2019.03.012
Irondi, E. A., Adewuyi, A. E., & Aroyehun, T. M. (2022). Effect of endogenous lipids and proteins on the antioxidant, in vitro starch digestibility, and pasting properties of sorghum flour. Frontiers in Nutrition, 8, 809330. https://doi.org/10.3389/fnut.2021.809330
Ishola, I. O., Ikumawoyi, V. O., Afolayan, G. O., & Olorife, O. J. (2016). Antinociceptive and anti-inflammatory properties of hydroethanolic seed extract of Monodora myristica (Annonaceae) in rodents. West African Journal of Pharmacy, 27, 22–32.
Jaffary, S. R. A., Ahmed, S. W., Shakeel, S., Asif, H. M., & Usmanghani, K. (2016). Evaluation of in vitro urease and lipoxygenase inhibition activity of weight reducing tablets. Pakistan Journal of Pharmaceutical Science, 29, 1397–1400.
Jakobek, L. (2015). Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chemistry, 175, 556–567. https://doi.org/10.1016/j.foodchem.2014.12.013
Jimenez-Pulido, I. J., Daniel, R., Perez, J., Martínez-Villaluenga, C., De Luis, D., & Martín Diana, A. B. (2022). Impact of protein content on the antioxidants, anti-inflammatory properties and glycemic index of wheat and wheat bran. Foods, 11, 2049. https://doi.org/10.3390/foods11142049
Kaur, B., Ranawana, V., & Henry, J. (2016). The Glycemic Index of Rice and Rice Products: A review, and table of GI values. Critical Reviews in Food Science and Nutrition, 56, 215–236.
Khan, S. S., Khan, A., Wadood, A., Farooq, U., Ahmed, A., Zahoor, A., Ahmad, V. U., Sener, B., & Erdemoglu, N. (2014). Urease inhibitory activity of ursane type sulfated saponins from the aerial parts of Zygophyllum fabago Linn. Phytomedicine, 21, 379–382.
Leonardo, S., Pietro, F. M., Giovanni, G., Salvatore, B., & Marco, D. (2018). Metabolic syndrome and uric acid nephrolithiasis: Insulin resistance in focus. Metabolism, 83, 225–233.
Liu, R. H. (2007). Whole grain phytochemicals and health. Journal of Cereal Science, 46, 207–219. https://doi.org/10.1016/j.jcs.2007.06.010
Moukette, B. M., Pieme, C. A., Njimou, J. R., Biapa, C. P. N., Marco, B., & Ngogang, J. Y. (2015). In vitro antioxidant properties, free radicals scavenging activities of extracts and polyphenol composition of a non-timber forest product used as spice: Monodora myristica. Biological Research, 48, 15. https://doi.org/10.1186/s40659-015-0003-1
Mpofu, A., Sapirstein, H. D., & Beta, T. (2006). Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. Journal of Agricultural and Food Chemistry, 54, 1265–1270. https://doi.org/10.1021/jf052683d
Mukasa, D., & Sung, J. (2020). A prediction model of Nephrolithiasis Risk: A population-based cohort study in Korea. Investigative Clinical Urology, 61, 188–199. https://doi.org/10.4111/icu.2020.61.2.188
Nile, S. H., Nile, A. S., Keum, Y. S., & Sharma, K. (2017). Utilization of quercetin and quercetin glycosides from onion (Allium cepa L.) solid waste as an antioxidant, urease and xanthine oxidase inhibitors. Food Chemistry, 235, 119–126.
Nirumand, M. C., Hajialyani, M., Rahimi, R., Farzaei, M. H., Zingue, S., Nabavi, S. M., & Bishayee, A. (2018). Dietary plants for the Prevention and management of kidney Stones: Preclinical and clinical evidence and molecular mechanisms. International Journal of Molecular Sciences, 19, 765. https://doi.org/10.3390/ijms19030765
Osada, Y., Tsuchimoto, M., Fukushima, H., Takahashi, K., Kondo, S., Hasegawa, M., & Komoriya, K. (1993). Hypouricemic effect of the novel xanthine oxidase inhibitor, TEI-6720, in rodents. European Journal of Pharmacology, 241, 183–188.
Oyaizu, M. (1986). Studies on products of browning reaction: Antioxidative activity of products of browning reaction prepared from glucosamine. Japan Journal of Nutrition, 44, 307–315. https://doi.org/10.5264/eiyogakuzashi.44.307
Park, H. K., Jeong, B. C., Sung, M. K., Park, M. Y., Choi, E. Y., Kim, B. S., Kim, H. H., & Kim, J. I. (2008). Reduction of oxidative stress in cultured renal tubular cells and preventive effects on renal stone formation by the bioflavonoid quercetin. Journal of Urology, 179, 1620–1626.
Pessato, T. B., de Morais, F. P. R., de Carvalho, N. C., Figueira, A. C. M., Fernandes, L. G. R., de Zollner, R. L., & Netto, F. M. (2018). Protein structure modification and allergenic properties of whey proteins upon interaction with tea and coffee phenolic compounds. Journal of Functional Foods, 51, 121–129. https://doi.org/10.1016/j.jff.2018.10.019
Pinarli, B., Simge Karliga, E., Ozkan, G., & Capanoglu, E. (2020). Interaction of phenolics with food matrix: In vitro and in vivo approaches. Mediterranean Journal of Nutrition and Metabolism, 13, 63–74. https://doi.org/10.3233/mnm-190362
Puntel, R. L., Nogueira, C. W., & Rocha, J. B. T. (2005). Krebs cycle intermediates modulate thiobarbituric reactive species (TBARS) production in rat brain. vitro Neurochemical Research, 30, 225–235. https://doi.org/10.1007/s11064-004-2445-7
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radicals in Biology and Medicine, 26, 1231–1237.
Sakhaee, K. (2014). Epidemiology and clinical pathophysiology of uric acid kidney stones. Journal of Nephrology, 27, 241–245.
Sánchez, A., & Vázquez, A. (2017). Bioactive peptides: A review. Food Quality and Safety, 1, 29–46. https://doi.org/10.1093/fqs/fyx006
Sęczyk, Ł., Świeca, M., Kapusta, I., & Gawlik-Dziki, U. (2019). Protein-phenolic interactions as a factor affecting the physicochemical properties of white bean proteins. Molecules, 24, 408. https://doi.org/10.3390/molecules24030408
Sęczyk, Ł., Gawlik-Dziki, U., & Świeca, M. (2021). Influence of phenolic-food matrix interactions on in vitro bioaccessibility of selected phenolic compounds and nutrients digestibility in fortified white bean paste. Antioxidants, 10, 1825. https://doi.org/10.3390/antiox10111825
Servili, M., Sordini, B., Esposto, S., Urbani, S., Veneziani, G., Maio, I. D., Selvaggini, R., & Taticchi, A. (2013). Biological Activities of Phenolic Compounds of Extra Virgin Olive Oil. Antioxidants, 3, 1–23. https://doi.org/10.3390/antiox3010001
Smeriglio, A., Galati, E. M., Monforte, M. T., Lanuzza, F., D’Angelo, V., & Circosta, C. (2016). Polyphenolic compounds and antioxidant activity of Cold-Pressed seed oil from Finola Cultivar of Cannabis sativa L. Phytotherapy Research, 30, 1298–1307. https://doi.org/10.1002/ptr.5623
Sorokin, I., & Pearle, M. S. (2018). Medical therapy for nephrolithiasis: State of the art. Asian Journal of Urology, 5, 243–255. https://doi.org/10.1016/j.ajur.2018.08.005
Toole, K. P., Frank, C., Jarvis, M. K., Pluckebaum, S., & Wiles, B. (2021). Ureterolithiasis in Adolescents: A Case Report. Journal of Pediatric Health Care, 35, 327–331. https://doi.org/10.1016/j.pedhc.2021.01.004
Villiger, A., Sala, F., Suter, A., & Butterweck, V. (2015). In vitro inhibitory potential of Cynara scolymus, Silybum marianum, Taraxacum officinale, and Peumus boldus on key enzymes relevant to metabolic syndrome. Phytomedicine, 22, 138–144. https://doi.org/10.1016/j.phymed.2014.11.015
Ye, J., Hu, X., Luo, S., McClements, D. J., Liang, L., & Liu, C. (2018). Effect of endogenous proteins and lipids on starch digestibility in rice flour. Food Research International, 106, 404–409. https://doi.org/10.1016/j.foodres.2018.01.008
Zhang, G., & Hamaker, B. R. (2003). A three component interaction among starch, protein, and free fatty acids revealed by pasting profiles. Journal of Agricultural and Food Chemistry, 51, 2797–2800.
Zhou, H., Zheng, B., & McClements, D. J. (2021). Encapsulation of lipophilic polyphenols in plant-based nanoemulsions: Impact of carrier oil on lipid digestion and curcumin, resveratrol and quercetin bioaccessibility. Food and Function, 12, 3420–3432. https://doi.org/10.1039/d1fo00275a
Zhu, F. (2015). Interactions between starch and phenolic compound. Trends in Food Science and Technology, 43, 129–143.
Acknowledgements
The Department of Biochemistry, Kwara State University, Malete, Nigeria, provided the facilities for the wet analyses reported in this article. The support from the laboratory staff is acknowledged.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Emmanuel A. Irondi: Conceptualization, Resources, Supervision, Writing – original draft & editing. Tolulope M. Aroyehun: Resources, Investigation, Formal analysis. Arinze F. Anyiam: Resources, Writing – reviewing, Formal analysis. Milan K. Lal: Resources, Writing -review & editing, Validation. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors do not have any competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Irondi, E.A., Aroyehun, T.M., Anyiam, A.F. et al. Phenolics profile, anti-nephrolithiasis, and antioxidant activities of Monodora myristica seed: impact of endogenous proteins and lipids. Food Prod Process and Nutr 5, 52 (2023). https://doi.org/10.1186/s43014-023-00167-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43014-023-00167-8