Abstract
Diagnosing chronic periprosthetic joint infection (PJI) requires clinical suspicion in combination with both serological and synovial fluid tests, the results of which are generally applied to validated scoring systems or consensus definitions for PJI. As no single “gold standard” test exists, the diagnosis becomes challenging, especially in the setting of negative cultures or equivocal test results. This review aims to address the workup of chronic PJI and considerations for clinical evaluation to guide treatment. Following aspiration of the joint in question, a multitude of tests has been developed in an attempt to assist with diagnosis, including cell synovial white blood cell count, gram stain, cultures, leukocyte esterase, alpha-defensin, synovial C-reactive protein, multiplex polymerase chain reaction, next-generation sequencing, and interleukins. Each test has advantages and disadvantages and should be used in conjunction with the overall clinical picture to guide further clinical evaluation and treatment in this complex patient population.
Similar content being viewed by others
Background
The diagnosis of periprosthetic joint infection (PJI) is rarely as clear-cut as a draining sinus tract and no single, perfect diagnostic test exists. A solitary, “gold standard” test has yet to be established. Thus, multiple consensus groups have developed clinical definitions for PJI with associated scoring systems to assist in diagnosis and guide clinical treatment [1,2,3]. A variety of serological and synovial fluid tests exist to aid in the diagnosis of periprosthetic joint infection, with some being more beneficial in a chronic infection setting compared to others. The aim of this article is to review the considerations for the workup of chronic PJI and to discuss the advantages and disadvantages of the current tests available to assist in PJI diagnosis in conjunction with clinical findings.
Overview
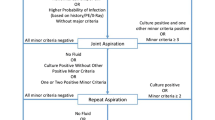
Serological testing (i.e., C-reactive protein CRP and erythrocyte sedimentation rate [ESR]) is a ubiquitous test that has been shown to be cost-effective and highly sensitive as a preliminary screening tool in patients with a painful total joint arthroplasty [4,5,6]. The 2018 ICM minor diagnostic criteria thresholds for chronic PJI are 30 mm/h for ESR and 10 mg/L for CRP [1]. We do not send for serum white blood cell (WBC) and differential analysis since this does little to improve the diagnostic accuracy of PJI [7]. When either the ESR or CRP is elevated, aspiration of the joint is recommended. Additionally, if there is a high index of suspicion for infection in the setting of normal serum laboratory values, performing an arthrocentesis and synovial fluid analysis should be done to rule out PJI [8]. Currently, there are no guidelines for the ideal “timing” of an aspiration to rule out chronic PJI. It is the authors’ opinion that any elevation in serological testing and/or a high clinical index of suspicion warrants an aspiration. Furthermore, many of the tests discussed below may assist when “standard” tests are equivocal.
Aspiration of the knee is performed employing the standard sterile technique via a superolateral approach. In patients with excess soft tissue around the knee, we recommend the use of a spinal needle to ensure that adequate depth is achieved to obtain the synovial fluid sample. In rare circumstances, ultrasound or fluoroscopic guidance may be necessary for the aspiration to achieve a sufficiently-sized sample of fluid. It is recommended that hip aspirations are performed under image guidance (i.e., ultrasound or fluoroscopy).
The manufacturing guidelines should be referenced for each planned test prior to aspiration to ensure ample fluid is acquired. For commercially available tests, the amount of synovial fluid required ranges from 20 µL to 2 mL depending on the desired test. The minimum amount for alpha-defensin and cell count at most laboratories is 0.5 mL. Next-generation sequencing often requires 2 mL of fluid. Rockov et al. found that higher aspiration volumes are more likely to correlate with intraoperative cultures and that an even higher volume was required for slow-growing microorganisms. This study reports that at least 1 mL is required to send for culture, though 5 mL allows for gram stain, fungal stain and acid-fast bacillus stain to all be run, and above 10 mL the sensitivity of each of these cultures is maximized. The optimal volume cutoff for concordant intraoperative cultures was 3.5 mL for typical organisms and 12.5 mL for slow-growing organisms [9]. If aspiration is performed such that blood is included in the sample, a collection tube containing ethylenediaminetetraacetic acid (EDTA), commonly used for complete blood counts (CBC), may be useful to limit clot formation or aberrant test results. Finally, special considerations should be observed in the setting of metallosis as metal debris may alter the results of certain laboratory values. If minimal or no synovial fluid is obtained, we recommend against the addition of saline since this may reduce the sensitivity of traditional laboratory values and contemporary synovial fluid biomarkers [10].
When synovial fluid is obtained, it should be sent for synovial fluid WBC count with differential and conventional culture, including both aerobic and anaerobic analyses. The synovial WBC count and differential analysis have been shown to perform well in the diagnosis of PJI [11]. In the setting of chronic infection, the threshold for minor diagnostic criteria, as defined by the 2018 International Consensus Meeting (ICM) to indicate a high likelihood of infection, is ≥ 3,000 cells/µL for synovial WBC count and ≥ 80% for the percentage of polymorphonuclear neutrophil (PMN) cells in the synovial cell differential [1]. The 2018 ICM thresholds for the minor diagnostic criteria have been included in Table 1 [1]. While false elevation in automated synovial WBC count has been detected in the setting of hip corrosion, there appears to be a substantial risk with all patients with THA or TKA. It has been suggested that clinicians should exercise caution when interpreting elevated automated synovial WBC count and consideration should be given to requesting a manual synovial WBC count to verify the accuracy of the automated cell count [12]. We typically do not rely on gram stain since the sensitivity as a screening tool has been shown to be poor [13,14,15]. All cultures should be kept in the laboratory for at least 14 days to allow for sufficient time for indolent infection (i.e., Cutibacterium acnes) growth. Fungal and acid-fast bacillus (AFB) cultures may also be sent as clinically indicated, particularly in immunocompromised patients or those with persistent infections. Studies have found that diabetes, prolonged use of antibiotics, prior PJI, and immunosuppression are among the risk factors for fungal PJI specifically [16]. When multiple risk factors exist and in patients with poor host immunity, evaluation of synovial fluid for fungal infection is recommended, including the use of fungal-specific culture mediums and a longer incubation time of at least 14 days [16]. The detection of fungal PJI with systemic serum markers and synovial WBC count remains a diagnostic dilemma [17].
In addition to standard synovial fluid tests, several currently available synovial biomarkers are also available, which may be beneficial in the diagnosis of PJI, and are described in more detail below and summarized in Table 2.
Leukocyte esterase
Leukocyte esterase (LE) is an enzyme produced by activated neutrophils in response to inflammation or infection. The test results are available instantaneously by reading a colorimetric reagent test strip that has been exposed to synovial fluid. Studies have demonstrated a sensitivity of 49%–95%, a specificity of 82%–100%, and a positive predictive value ranging from 72%–100% [18, 19]. The test is readily available, technically easy to perform and inexpensive, making it an ideal point-of-care test for PJI. A “clean” synovial fluid sample (i.e., without blood or metallic debris) is unlikely to yield false positive results [20]. It appears that the LE strip test can be utilized as a reliable diagnostic tool for the diagnosis of PJI even when prior antibiotics have been administered [21, 22]. Additionally, it may serve as a screening tool to rule out PJI in patients with failure of THA secondary to metal particle release [23]. However, the major disadvantage of this test is that results may be influenced by the presence of blood or metal debris within the synovial fluid sample. When blood is present, the centrifuge may be utilized to help maintain the reliability and accuracy of the test [54]. Its overall performance has made it a valuable part of the 2018 ICM criteria [24].
Alpha-defensin
Alpha-defensin (AD) is an antimicrobial peptide produced naturally by neutrophils in the presence of bacterial pathogens. Ranges for sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) have been cited at 65%–95%, 87%–99.6%, 75%–98% and 84%–98%, respectively [18]. While AD lateral flow immunoassay techniques are faster, some studies suggested that sensitivity, specificity and PPV are improved when the test is performed in the laboratory by enzyme-linked immunosorbent assay (ELISA) [18, 25], while others demonstrated no difference in accuracy when comparing the two techniques [26]. One potential disadvantage of AD is that the diagnosis accuracy is decreased in the presence of metallosis with adverse local tissue reaction (ALTR) [27, 28] and in crystalline arthropathies [29].
The diagnostic accuracy of AD for PJI diagnosis is comparable but not superior to that of synovial WBC count and percent PMN combined [55]. Additionally, AD has not been shown to change the clinical management of patients being worked up for PJI or in the setting of reimplantation of chronic PJI when compared with traditional laboratory values [56,57,58]. These studies questioned the routine use of this synovial biomarker. Lastly, premature antibiotic administration in the setting of PJI may compromise the sensitivity of traditional diagnostic laboratory results. When antibiotics have been administered, AD has been shown to have a higher sensitivity and provide better screening for chronic PJI than ESR, CRP, synovial fluid neutrophil percentage and synovial fluid culture [30]. However, no statistically significant improvement was noted over synovial WBC count. As such, its greatest utility may be for equivocal cases as an adjunct for the diagnosis of PJI.
Synovial CRP
Parvizi et al. [31] originally demonstrated that the synovial biomarker was more accurate in the diagnosis of PJI than its serum counterpart. Some studies have indicated that synovial CRP may have higher sensitivity and specificity for PJI compared to serum CRP [32]. However, others have shown that it has similar sensitivity, specificity and positive predictive value, providing no additional diagnostic advantage compared with serum CRP [33]. Recent studies suggested that the combination of both synovial and serum CRP might improve the diagnostic value in the prediction of PJI, especially in the setting of chronic infection [59, 60]. Lastly, Deirmengian et al. demonstrated increased sensitivity and specificity (97% and 100%, respectively) with the combination of synovial CRP and alpha-defensin, even in complex patient populations with systemic inflammatory disease or recent antibiotic use [34]. While synovial CRP alone may not be sufficient to diagnose PJI, it is included in the validated workup by Parvizi et al. [2] and its inclusion in the workup as an adjuvant should be considered.
Multiplex polymerase chain reaction
Multiplex polymerase chain reaction (mPCR) is a test that provides the genotypic evaluation of bacteria of a sample specimen. Lausmann et al., by using mPCR, have demonstrated a sensitivity of 85%, specificity of 98%, PPV of 97.6% and NPV of 87.5%, with an overall accuracy of 91.8% [25]. Other studies have also shown improved sensitivity and specificity compared to conventional cultures alone, yet mPCR in combination with traditional cultures provides the greatest diagnostic accuracy [35, 36]. The major advantage of this test is that it provides information regarding the presence of infection as well as the causative organism for the infection and potential antibiotic resistance markers regardless of concurrent antibiotic use [25]. By using this test, the simultaneous detection of an entire panel of potentially causative organisms is possible, even with limited synovial fluid obtainable via aspiration [35]. Additionally, the test results are typically available within approximately 5 h compared to several days with traditional culture results. Some studies have suggested that it may be superior to traditional culture in the detection of low-virulence organisms and have the added advantage of requiring only 180 µl of fluid for evaluation [25, 36]. A limitation of this test is that if the proper primer for the causative organism is not present on the cassette, the test result will be negative and a diagnosis of PJI may be missed [25, 36]. The test is also not readily available at all institutions.
Next-generation sequencing
Next-generation sequencing (NGS) is a technique by which all of the DNA in a sample specimen is detected and sequenced to determine the presence of specific microorganisms [61]. This technique is still being investigated but offers the advantages of being able to detect multiple causative organisms and determine the presence of possible antibiotic resistance markers [37,38,39]. Additionally, the data are accurate regardless of antibiotic use by the patient and the presence of systemic inflammatory disease. In particular, NGS may be most useful in patients with culture-negative aspiration results [38, 39]. A recent review demonstrated that the sensitivity of NGS at identifying organisms may be greater than conventional culture, with rates up to 89% in culture-negative PJI [40]. However, Kildow et al. reported improved sensitivity and specificity for PJI detection using traditional culture compared to NGS and mPCR [41]. More recent reductions in the cost of this test, as well as improvements in techniques and the hardware required to run the test have made NGS more accessible for clinical application [40]. Torchia et al. reported that the cost-effectiveness of NGS for PJI diagnosis is dependent upon the pretest probability of PJI and the performance of the technology used for the test, suggesting that it should ideally be reserved for patients with a high pretest probability of infection [42]. Certain NGS methods are also available and the detection of multiple microbe types (bacteria, fungi, parasites, and viruses) by using the methods is possible [40]. While this test offers multiple advantages, a major limitation is the high rate of false positives due to low specificity and high risk of contamination [40]. Additionally, the run time of 2–5 days should also be considered, though this time may be less if facilities have in-house NGS capabilities.
Interleukins
Interleukins (IL) are inflammatory cytokines that are currently under investigation for use as a diagnostic tool for PJI. IL-6 is the most cited at this time and has the advantage of maintaining accuracy even in patients actively taking antibiotics. There are equivocal results for its use in those with systemic inflammatory disease. Synovial cutoff values are still being studied but values > 9,000 pg/mL have shown a specificity of nearly 100% [43]. Some studies have demonstrated increased positive predictive value when IL-6 is used in combination with serum CRP [43]. The accuracy of the results also depends on the immunoassay technique used, with lateral flow being less accurate than radioimmunoassay techniques [18]. Interleukins as a diagnostic test can be considered for inclusion in the workup of PJI, though further studies are likely needed to evaluate the utility and appropriate settings for the use of these particular biomarkers.
Calprotectin
Calprotectin is a pro-inflammatory protein released from activated neutrophils and macrophages during inflammation. It has been studied as a potential synovial fluid biomarker for the diagnosis of chronic PJI. Using a lateral flow assay, expeditious analysis can be used intraoperatively for calprotectin, affording the ability to make immediate intraoperative decisions during revision surgery [44]. The lateral flow assay is made available by a company in Norway and photometrically evaluates 20 µL sample after a 15-min waiting period via a smartphone application provided by the manufacturer. The application provides a quantitative value of calprotectin and proposes 3 different risk stratifications for the assessment of the risk of PJI [44]. An additional advantage of this biomarker is its consistent accuracy in the setting of other etiologies for inflammation, including inflammatory arthritis, fracture, dislocation, or recent surgery that may mimic infection [45, 46]. The sensitivity of the test has been reported to be at 71%–94% and the specificity at 81%–88%, with a PPV of 71%–77% and an NPV of 76%–98% [44, 45, 47]. These test kits are not yet widely available in the USA and a specific threshold has not been determined to definitively rule in or out infection. Future studies are warranted to further evaluate this biomarker, but current research suggests it should be considered moving forward, especially in the setting of other etiologies of inflammation.
Neutrophil gelatinase-associated lipocalin
Neutrophil gelatinase-associated lipocalin (NGAL) is an antibacterial peptide that affects the iron ion metabolism of pathogens and is secreted by neutrophils during an inflammatory response [48]. It is measured using ELISA techniques and only 0.5 mL of synovial fluid is required. A specific threshold that defines infection has not yet been determined and the levels of NGAL may be highly dependent on the number of neutrophils present in the sample [48]. Studies have reported a sensitivity of 86%–100% and a specificity of 77%–100% [48,49,50,51]. One advantage of this biomarker is its efficacy in the setting of recent antibiotic, use according to Huang et al. [48]. Further research is necessary to determine appropriate cutoff values to define PJI and clinical settings in which NGAL is most appropriate for use.
Lactoferrin
Lactoferrin is a glycoprotein involved in the iron metabolism in pathogens, is secreted by neutrophils in the early inflammatory response and plays a complex role in the immune cascade [53]. The biomarker is typically analyzed using ELISA or multiplex PCR techniques, though specific cut-off values have not been determined to definitively rule in or out PJI. Studies have reported a sensitivity of 97%–100% and a specificity of 90%–100% [51, 53]. Wang et al. also found correlations of synovial fluid WBC/PMN percentage with lactoferrin values, which may suggest it is dependent on the number of neutrophils within the sample collected [53]. Studies have found it is a reliable test for the detection of PJI even in patients with inflammatory arthropathies [46], but it has not been widely tested in other clinical scenarios, including in the setting of recent antibiotic use. Clinical studies to further evaluate the efficacy of this biomarker are needed, as well as studies to determine appropriate clinical scenarios for its use. Lastly, there are many other synovial fluid biomarkers, including, tumor necrosis factor-alpha, interferon-gamma, lactate and others, that show promise that is not readily available for use at all institutions and may prove to be advantageous but future studies are needed before these are widely endorsed [62,63,64,65].
Conclusions
The diagnosis of periprosthetic joint infection in the setting of equivocal test results, negative cultures or chronic, indolent infection can raise significant dilemmas, especially without a single, “gold standard” diagnostic test available. The presence of metallosis, inflammatory arthropathies, other concurrent inflammatory processes, recent antibiotic use and other clinically-related factors should be considered when deciding which synovial test is most appropriate. Each test provides valuable information to help guide the surgeon in their evaluation of patients with possible chronic PJI, but the accuracy of such tests is variable and no single test can be relied upon for definitive clinical decisions. These tests should be utilized as adjunct data and, as such, a combination of appropriate tests based on the clinical scenario may be most valuable. However, an exact combination of tests has yet to be determined to most accurately diagnose chronic PJI. Validated scoring systems and consensus definitions of periprosthetic infection [1,2,3] in conjunction with the overall clinical picture should guide the clinical evaluation and treatment of patients presenting with clinical findings concerning infection.
Availability of data and materials
Not applicable.
Abbreviations
- PJI:
-
Periprosthetic joint infection
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- WBC:
-
White blood cell
- EDTA:
-
Ethylenediaminetetraacetic acid
- CBC:
-
Complete blood count
- ICM:
-
International Consensus Meeting
- PMN:
-
Polymorphonuclear neutrophil
- AFB:
-
Acid-fast bacillus
- LE:
-
Leukocyte esterase
- AD:
-
Alpha-defensin
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- ELISA:
-
Enzyme-linked immunosorbent assay
- ALTR:
-
Adverse local tissue reaction
- mPCR:
-
Multiplex polymerase chain reaction
- NGS:
-
Next-generation sequencing
- IL:
-
Interleukins
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
References
Parvizi J, Gehrke T, International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29(7):1331.
Parvizi J, Tan TL, Goswami K, Higuera C, della Valle C, Chen AF, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33(5):1309-1314.e2.
Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, della Valle CJ, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469(11):2992–4.
Berbari E, Mabry T, Tsaras G, Spangehl M, Erwin PJ, Murad MH, et al. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2010;92(11):2102–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20810860. Cited 2022 Aug 27.
Austin MS, Ghanem E, Joshi A, Lindsay A, Parvizi J. A simple, cost-effective screening protocol to rule out periprosthetic infection. J Arthroplasty. 2008;23(1):65–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18165031. Cited 2022 Aug 27.
Ting NT, della Valle CJ. Diagnosis of periprosthetic joint infection—an algorithm-based approach. J Arthroplasty. 2017;32(7):2047–50.
Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis. A complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589–93.
Goud A, Nützinger D, van der Bij A, Jenniskens K, Groenewold J, de Gast A, et al. Synovial-based tests outperform serum markers to rule out infection in total knee arthroplasty and total hip arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2022;37(4):802-808.e5.
Rockov ZA, Clarke HD, Grys TE, Chang YHH, Schwartz AJ. Is there an optimal cutoff for aspiration fluid volume in the diagnosis of periprosthetic joint infection? J Arthroplasty. 2020;35(8):2217–22.
Deirmengian C, Feeley S, Kazarian GS, Kardos K. Synovial Fluid Aspirates Diluted with Saline or Blood Reduce the Sensitivity of Traditional and Contemporary Synovial Fluid Biomarkers. Clin Orthop Relat Res. 2020;478(8):1805–13.
Levent A, Neufeld ME, Piakong P, Lausmann C, Gehrke T, Citak M. Which international consensus meeting preoperative minor criteria is the most accurate marker for the diagnosis of periprosthetic joint infection in hip and knee arthroplasty? J Arthroplasty. 2021;36(11):3728–33. Elsevier B.V.
Deirmengian CA, Kazarian GS, Feeley SP, Sizer SC. False-positive automated synovial fluid white blood cell counting is a concern for both hip and knee arthroplasty aspirates. J Arthroplasty. 2020;35(6):S304–7.
Morgan PM, Sharkey P, Ghanem E, Parvizi J, Clohisy JC, Burnett RSJ, et al. The value of intraoperative Gram stain in revision total knee arthroplasty. J Bone Joint Surg Am. 2009;91(9):2124–9. Available from: http://journals.lww.com/00004623-200909000-00008. Cited 2022 Sep 4.
Johnson AJ, Zywiel MG, Stroh DA, Marker DR, Mont MA. Should gram stains have a role in diagnosing hip arthroplasty infections? Clin Orthop Relat Res. 2010;468(9):2387–91. Available from: https://journals.lww.com/00003086-201009000-00014. Cited 2022 Sep 4.
Zywiel MG, Stroh DA, Johnson AJ, Marker DR, Mont MA. Gram stains have limited application in the diagnosis of infected total knee arthroplasty. Int J Infect Dis. 2011;15(10):e702-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21764346. Cited 2022 Sep 4.
Sambri A, Zunarelli R, Fiore M, Bortoli M, Paolucci A, Filippini M, et al. Epidemiology of fungal periprosthetic joint infection: a systematic review of the literature. Microorganisms. 2023;11(1):84. MDPI.
Bracken CD, Berbari EF, Hanssen AD, Mabry TM, Osmon DR, Sierra RJ. Systemic inflammatory markers and aspiration cell count may not differentiate bacterial from fungal prosthetic infections. Clin Orthop Relat Res. 2014;472(11):3291–4.
Aalirezaie A, Bauer TW, Fayaz H, Griffin W, Higuera CA, Krenn V, et al. Hip and knee section, diagnosis, reimplantation: proceedings of international consensus on orthopedic infections. J Arthroplasty. 2019;34:S369–79. Churchill Livingstone Inc.
Wyatt MC, Beswick AD, Kunutsor SK, Wilson MJ, Whitehouse MR, Blom AW. The alpha-defensin immunoassay and leukocyte esterase colorimetric strip test for the diagnosis of periprosthetic infection a systematic review and meta-analysis. J Bone Joint Surg Am. 2016;98(12):992–1000.
McNabb DC, Dennis DA, Kim RH, Miner TM, Yang CC, Jennings JM. Determining false positive rates of leukocyte esterase reagent strip when used as a detection tool for joint infection. J Arthroplasty. 2016;3–5. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0883540316302443.
Shahi A, Alvand A, Ghanem E, Restrepo C, Parvizi J. The leukocyte esterase test for periprosthetic joint infection is not affected by prior antibiotic administration. J Bone Joint Surg Am. 2019;101(8):739–44.
Shahi A, Tan TL, Kheir MM, Tan DD, Parvizi J. Diagnosing periprosthetic joint infection: and the winner is? J Arthroplasty. 2017;32(9):S232–5.
Tischler EH, Plummer DR, Chen AF, della Valle CJ, Parvizi J. Leukocyte esterase: metal-on-metal failure and periprosthetic joint infection. J Arthroplasty. 2016;31(10):2260–3.
Chisari E, Yacovelli S, Goswami K, Shohat N, Woloszyn P, Parvizi J. Leukocyte esterase versus ICM 2018 criteria in the diagnosis of periprosthetic joint infection. J Arthroplasty. 2021;36(8):2942-2945.e1.
Lausmann C, Kolle KN, Citak M, Abdelaziz H, Schulmeyer J, Delgado GD, et al. How reliable is the next generation of multiplex-PCR for diagnosing prosthetic joint infection compared to the MSIS criteria? Still missing the ideal test. HIP Int. 2020;30(1_suppl):72–7.
Kuiper JWP, Verberne SJ, Vos SJ, van Egmond PW. Does the alpha defensin ELISA test perform better than the alpha defensin lateral flow test for PJI diagnosis? A systematic review and meta-analysis of prospective studies. Clin Orthop Relat Res. 2020;478:1333–44. Lippincott Williams and Wilkins.
Kasparek MF, Kasparek M, Boettner F, Faschingbauer M, Hahne J, Dominkus M. Intraoperative diagnosis of periprosthetic joint infection using a novel alpha-defensin lateral flow assay. J Arthroplasty. 2016;31(12):2871–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27329580. Cited 2022 Sep 4.
Okroj KT, Calkins TE, Kayupov E, Kheir MM, Bingham JS, Beauchamp CP, et al. The alpha-defensin test for diagnosing periprosthetic joint infection in the setting of an adverse local tissue reaction secondary to a failed metal-on-metal bearing or corrosion at the head-neck junction. J Arthroplasty. 2018;33(6):1896–8.
Partridge DG, Gordon A, Townsend R. False-positive synovial fluid alpha-defensin test in a patient with acute gout affecting a prosthetic knee. Eur J Orthop Surg Traumatol. 2017;27(4):549–51.
Shahi A, Parvizi J, Kazarian GS, Higuera C, Frangiamore S, Bingham J, et al. The alpha-defensin test for periprosthetic joint infections is not affected by prior antibiotic administration. Clin Orthop Relat Res. 2016;474(7):1610–5.
Parvizi J, Jacovides C, Adeli B, Jung KA, Hozack WJ, Mark B. Coventry Award: synovial C-reactive protein: a prospective evaluation of a molecular marker for periprosthetic knee joint infection. Clin Orthop Relat Res. 2012;470(1):54–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21786056. Cited 2022 Sep 4.
Omar M, Ettinger M, Reichling M, Petri M, Guenther D, Gehrke T, et al. Synovial C-reactive protein as a marker for chronic periprosthetic infection in total hip arthroplasty. Bone Joint J. 2015;97-B(2):173–6.
Tetreault MW, Wetters NG, Moric M, Gross CE, della Valle CJ. Is synovial C-reactive protein a useful marker for periprosthetic joint infection? Clin Orthop Relat Res. 2014;472(12):3997–4003.
Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg. 2014;96(17):1439–45.
Suren C, Feihl S, Cabric S, Banke IJ, Haller B, Trampuz A, et al. Improved pre-operative diagnostic accuracy for low-grade prosthetic joint infections using second-generation multiplex Polymerase chain reaction on joint fluid aspirate. https://doi.org/10.1007/s00264-020-04552-7.
Sigmund IK, Windhager R, Sevelda F, Staats K, Puchner SE, Stenicka S, et al. Multiplex PCR Unyvero i60 ITI application improves detection of low-virulent microorganisms in periprosthetic joint infections. Int Orthop. 2019;43(8):1891–8.
Tang Y, Zhao D, Wang S, Yi Q, Xia Y, Geng B. Diagnostic value of next-generation sequencing in periprosthetic joint infection: a systematic review. Orthop Surg. 2022;14:190–8. Sociedade Brasileira de Matematica Aplicada e Computacional.
Tarabichi M, Shohat N, Goswami K, Parvizi J. Can next generation sequencing play a role in detecting pathogens in synovial fluid? Bone Joint J. 2018;100-B(2):127–33.
Tarabichi M, Shohat N, Goswami K, Alvand A, Silibovsky R, Belden K, et al. Diagnosis of periprosthetic joint infection: the potential of next-generation sequencing. J Bone Joint Surg. 2018;100(2):147–54.
Indelli PF, Ghirardelli S, Violante B, Amanatullah DF. Next generation sequencing for pathogen detection in periprosthetic joint infections. EFORT Open Rev. 2021;6(4):236–44.
Kildow BJ, Ryan SP, Danilkowicz R, Lazarides AL, Penrose C, Bolognesi MP, et al. Next-generation sequencing not superior to culture in periprosthetic joint infection diagnosis. Bone Joint J. 2021;103-B(1):26–31.
Torchia MT, Austin DC, Kunkel ST, Dwyer KW, Moschetti WE. Next-generation sequencing vs culture-based methods for diagnosing periprosthetic joint infection after total knee arthroplasty: a cost-effectiveness analysis. J Arthroplasty. 2019;34(7):1333–41.
Patel R, Alijanipour P, Parvizi J. Advancements in diagnosing periprosthetic joint infections after total hip and knee arthroplasty. Open Orthop J. 2016;10(1):654–61.
Lazic I, Burdach A, Pohlig F, von Eisenhart-Rothe R, Suren C. Utility of synovial calprotectin lateral flow test to exclude chronic prosthetic joint infection in periprosthetic fractures: a prospective cohort study. Sci Rep. 2022;12(1):18385.
Lazic I, Prodinger P, Stephan M, Haug AT, Pohlig F, Langer S, et al. Synovial calprotectin is a reliable biomarker for periprosthetic joint infections in acute-phase inflammation — a prospective cohort study. Int Orthop. 2022;46(7):1473–9.
Tahta M, Simsek ME, Isik C, Akkaya M, Gursoy S, Bozkurt M. Does inflammatory joint diseases affect the accuracy of infection biomarkers in patients with periprosthetic joint infections? A prospective comparative reliability study. J Orthop Sci. 2019;24(2):286–9.
Suren C, Lazic I, Haller B, Pohlig F, von Eisenhart-Rothe R, Prodinger P. The synovial fluid calprotectin lateral flow test for the diagnosis of chronic prosthetic joint infection in failed primary and revision total hip and knee arthroplasty. Int Orthop. 2023;47(4):929–44.
Huang Z, Zhang Z, Li M, Li W, Fang X, Zhang W. Synovial fluid neutrophil gelatinase-associated lipocalin can be used to accurately diagnose prosthetic joint infection. Int J Infect Dis. 2022;1(123):170–5.
Vergara A, Fernández-Pittol MJ, Muñoz-Mahamud E, Morata L, Bosch J, Vila J, et al. Evaluation of lipocalin-2 as a biomarker of periprosthetic joint infection. J Arthroplasty. 2019;34(1):123–5.
Dijkman C, Thomas AR, Koenraadt KLM, Ermens AAM, van Geenen RCI. Synovial neutrophilic gelatinase-associated lipocalin in the diagnosis of periprosthetic joint infection after total knee arthroplasty. Arch Orthop Trauma Surg. 2020;140(7):941–7.
Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254–62.
Zhang Z, Huang Y, Li M, Li W, Fang X, Huang Z, Zhang W. Rapid and quantitative detection of neutrophil gelatinase-associated lipocalin in synovial fluid using fluorescence immunochromatographic test strips for diagnosing prosthetic joint infections. Diagn Microbiol Infect Dis. 2023;106(4):115929. https://doi.org/10.1016/j.diagmicrobio.2023.115929. Epub ahead of print. PMID: 37244008.
Wang C, Wang Q, Li R, Qin J, Song L, Zhang Q, et al. LTF, PRTN3, and MNDA in synovial fluid as promising biomarkers for periprosthetic joint infection: identification by quadrupole orbital-trap mass spectrometry. J Bone Joint Surg Am. 2019;101(24):2226–34.
Li X, Li R, Ni M, Chai W, Hao L, Zhou Y, et al. Leukocyte esterase strip test: a rapid and reliable method for the diagnosis of infections in arthroplasty. Orthopedics. 2018;41(2):e189–93.
Ivy MI, Sharma K, Greenwood-Quaintance KE, Tande AJ, Osmon DR, Berbari EF, et al. Cite this article. Bone Joint J. 2021;103(6):1119–26.
Kleeman-Forsthuber LT, Dennis DA, Brady AC, Pollet AK, Johnson RM, Jennings J. Alpha-defensin is not superior to traditional diagnostic methods for detection of PJI in THA and TKA patients. J Arthroplasty. 2021;4–9. https://doi.org/10.1016/j.arth.2021.01.036.
Kleeman-Forsthuber LT, Johnson RM, Brady AC, Pollet AK, Dennis DA, Jennings JM. Alpha-defensin offers limited utility in routine workup of periprosthetic joint infection. J Arthroplasty. 2020;(2020). https://doi.org/10.1016/j.arth.2020.12.018.
Owens JM, Dennis DA, Abila PM, Johnson RM, Jennings JM. Alpha-defensin offers limited utility in work-up prior to reimplantation in chronic periprosthetic joint infection in total joint arthroplasty patients. J Arthroplasty. 2022. Available from: http://www.ncbi.nlm.nih.gov/pubmed/35803520. Cited 2022 Sep 4.
Baker CM, Goh GS, Tarabichi S, Shohat N, Parvizi J. Synovial C-reactive protein is a useful adjunct for diagnosis of periprosthetic joint infection. J Arthroplasty. 2022;37(12):2437-2443.e1.
Wang H, Qin L, Wang J, Hu N, Huang W. Combined serum and synovial C-reactive protein tests: a valuable adjunct to the diagnosis of chronic prosthetic joint infection. BMC Musculoskelet Disord. 2021;22(1):670.
Koboldt DC, Steinberg KM, Larson DE, Wilson RK, Mardis ER. The next-generation sequencing revolution and its impact on genomics. Cell. 2013;155(1):27–38.
Grassi M, Salari P, Farinelli L, D’Anzeo M, Onori N, Gigante A. Synovial biomarkers to detect chronic periprosthetic joint infection: a pilot study to compare calprotectin rapid test, calprotectin ELISA immunoassay and leukocyte esterase test. J Arthroplasty. 2022;37(4):781–6.
Lee YS, Koo KH, Kim HJ, Tian S, Kim TY, Maltenfort MG, et al. Synovial fluid biomarkers for the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2017;99(24):2077–84.
Warren J, Anis HK, Bowers K, Pannu T, Villa J, Klika AK, et al. Diagnostic utility of a novel point-of-care test of calprotectin for periprosthetic joint infection after total knee arthroplasty: a prospective cohort study. J Bone Joint Surg Am. 2021;103(11):1009–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34038396. Cited 2022 Sep 4.
Sharma K, Ivy M, Block DR, Abdel MP, Hanssen AD, Beauchamp C, et al. Comparative analysis of 23 synovial fluid biomarkers for hip and knee periprosthetic joint infection detection. J Orthop Res. 2020;38(12):2664–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32485031. Cited 2022 Sep 4.
Acknowledgements
Not applicable.
Funding
No Funding was required for this manuscript.
Author information
Authors and Affiliations
Contributions
N.Q. was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Quinlan, N.D., Jennings, J.M. Joint aspiration for diagnosis of chronic periprosthetic joint infection: when, how, and what tests?. Arthroplasty 5, 43 (2023). https://doi.org/10.1186/s42836-023-00199-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42836-023-00199-y