Abstract
Herbicides in wastewater are considered as a serious issue to environmental pollution. Different impregnated metal zinc oxide (Cu/ZnO and Ni/ZnO) as catalysts were prepared through wet impregnation method for the degradation of herbicides Isoproturon and triasulfuron. The prepared impregnated catalysts were characterized using scanning electron microscopy (SEM) and x-ray diffraction (XRD), energy dispersive x-ray (EDX) analysis, Fourier-transform infrared spectroscopyand surface area. The degradation of selected herbicides were investigated using combined effect of photocatalysis and sonication. The experimental parameters such as pH, irradiation time, photocatalyst dose, effect of oxidants, diverse ion effect, herbicide concentration and catalyst reusability have been optimized. The percent removal of isoproturon was found to be 99 and 98% at pH 7 and triasulfuron was 98% at pH 6 using Cu/ZnO and 99% at pH 7 using Ni/ZnO photocatalysts respectively.
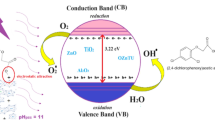
Graphical Abstract
Graphical abstract of Ultrasound Assisted Photocatalytic Degradation of Isoproturon and Triasulfuron Herbicides

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.1 Introduction
While increased use of pesticide results in more crops grown, it has a negative impact on organisms and causes environmental contamination. Pesticides have a negative impact on the environment, using them in large quantities leads to a deadly situation. Accumulation in soil can make it unsuitable for cultivation. Living organisms and food can be affected by the persistence of pesticides in them. Mixing with runoff and groundwater can contaminate water resulting in pollution [1, 2]. Water resources are continuously contaminated by pesticides and other chemicals used in fields, farms and our environment. These chemicals not only affect the quality of drinking water, but are also harmful to aquatic life. All pesticides are toxic, but their degree of toxicity to living things varies. Some pesticides are very toxic, while others are less harmful [3]. Environmental pollution caused by herbicides is a growing threat to humans. Herbicides released from various sources not only affect humans, but also cause contamination of food, soil and water [4]. Photocatalysis is an important method for treating wastewaters due to its ability to remove contaminants in the ppb range, absence of formation of aromatic compounds, greater speed and lower cost [1, 5]. Traditional methods of wastewater treatment like filtration, air stripping, ozone oxidation, photo Fenton, electrochemical degradation, co precipitation, ion exchange, biodegradation [6] and adsorption [7,8,9] were used for the removal of herbicides from the water sample but these methods suffer from the limitations such as incomplete removal, complex sludge formation and slow degradation [10]. Alternatively, photocatalysis is an economical and widely used technique for water purification. The photocatalysis procedure not only aims to decompose the organic compound, but also to mineralize the intermediates into water, salt and mineral acids [11]. Impregnated photocatalysts are the catalyst of which surface pore has been filled by active substances. It is superior to simple catalyst due to increase surface area, high catalytic activity and selectivity [12]. Different nanoparticles are used as catalysts in a wide range of applications due to their large surface area and strong catalytic property [13]. Semiconductor metal oxides including titania, zinc oxide, silver oxide, copper sulphide, tin oxide, nickel oxide, cupreous oxide, magnetic and cadmium sulfide have been utilized as photocatalysts. Impregnated catalysts have also been used for the degradation of organic contaminants due to ease of use, low cost, faster degradation and reusability [14]. Surface modified semiconductor decreases the band gap between the conduction band and valence band, thus shifting absorption to longer wavelength. Among the modified surfaces, the impregnated zinc oxide presents great potential for the removal of several types of organic compounds, mainly for emerging organic contaminates [15].
For the present work, two herbicides are selected. Isoproturon (3-(4-isopropylphenyl)-1, 1-dimethylurea) is substituted urea herbicide. The chemical formula is C12H18N2O. It is absorbed by the roots and stem, inhibiting the photosynthesis of unwanted plants [16]. It is used in cereal crops to control the growth of weeds and annual grasses. Isoproturon is one of the most detected herbicides in soil and drinking water [17]. Triasulfuron (2-(6-methoxy-4-methyl, 1, 3, 5-triazine-2-yl)-1-(2-(2-cholrethoxy) phenylsulfonyl urea) is a selective sulfonyl urea pre- and post-emergence herbicide [18]. It suppresses broadleaf and grassy weeds in wheat, barley, pastures and arid pastures. It controls weeds by inhibiting valine and isoleucine synthesis which impede cell growth and therefore plant growth. It is toxic to the aquatic ecosystems.
2 Experimental
2.1 Material and Methods
Reagents like sodium chloride, sodium perchlorate, hydrochloric acid, zinc oxide were of analytical grade purity manufactured by BDH, England. Sodium hydroxide from Merck, Darmstadt, Germany. Potassium persulfate K2S2O8 from Sigma, Aldrich (Burlington, MA, USA). Hydrogen peroxide (H2O2, 30% w/w), Zinc oxide ZnO powder (99% purity), copper nitrate Cu(NO3)2·5H2O, nickel nitrate Ni(NO3)2·7H2O were used for the preparation of impregnated catalyst. Standard references of drugs were provided by Cirin Pharmaceutical (Pvt) Ltd., Hatter, Pakistan. Commercial formulations of the isoproturon having molecular formula: C12H18N2O, molecular weight, 206 g mol−1, solubility in water, 0.07 g L−1, percent purity 98.3% was used and phenyl sulfonylurea having molecular formula: C14H16ClN5O5S, molecular weight, 401 g mol−1, solubility in water, 0.815 g L−1, 97% percent purity was used in this research. As an ultrasonic radiation source, a Kum Sung Ultrasonic bath with 40 kHz frequencies was used. As a visible source for photocatalytic degradation of emerging pollutants, a 200 W tungsten filament lamp was used. The TOC-VCPH analyzer (Shimadzu, Japan) was used to measure the contents of TOC.
The surface morphology and particle size of the support ZnO impregnation were evaluated before and after by 30 kV Scanning Electron Microscope (SEM) (JSM5910, JEOL, Japan). The samples were prepared using conventional methods; the powdered samples were mounted on standard specimen stubs with double adhesive carbon tape. For elemental analysis, the Energy Dispersive X-ray (EDX) mappings of the catalyst were obtained using EDX-INCA 200, UK Oxford Instrument. X-ray diffraction (XRD) patterns were taken using a JDX-3532 JEOL (Japan) diffractometer with monochromatic Cu-Kα radiation (λ = 1.5418 Å) at 40 kV and 30 mA in the 2θ range of 10–80° with 1.03° min−1. The surface area of Cu and Ni impregnated ZnO catalysts was determined through Brunauer Emmett-Teller method. Before the analysis, the samples were dried for 2 h at 50 °C in oven to remove all adsorbed moisture and then it was grinded well before analysis.
2.2 Preparation of Photocatalysts
Nickel and copper impregnated zinc oxide (Ni/ZnO, Cu/ZnO) catalysts were prepared using the wet impregnation method as reported in our previous work [19,20,21]. Nitrate salts of the metals Cu and Ni were impregnated over zinc oxide as a supporting material. The amount of copper and nickel in the form of their nitrate salts for 3% impregnation was calculated and weighted for a known weight (97%) of the support. The precursor metal salts were dissolved in appropriate amount of water and were added to the slurry of zinc oxide supporting material kept on magnetic stirrer. The mixture was stirred at 50 °C for 30 min, followed by drying in oven at 120 °C for 4 h. The sample was calcined at 300 °C for 4 h and then passed through mesh of size < 445 µm.
2.3 Sonication assisted photocatalytic study
Degradation experiments were carried out in small beakers, containing known amount of herbicides (isoproturon and triasulfuron) and photocatalysts (Cu/ZnO or Ni/ZnO). The solutions were allowed for 30 min in dark to attain equilibrium and then under the visible light lamp in the sonicator. Aliquots of 5 mL were taken periodically after every 10 min from the mixture and centrifuged to remove particles, the absorbance was measured at maximum absorption wavelength of isoproturon (230 nm) and triasulfuron (340 nm). The concentration of the remaining herbicides in the solution was calculated from the linear equation of the calibration curve of standards. The degradation is noted in %, which is the percent ratio of the concentration of herbicide after and before the degradation (Eq. 1). Optimization studies were performed using standard solution containing isoproturon and triasulfuron. Parameters like pH of solution, catalyst dose, substrate concentration, and irradiation time and scavenger effect were investigated.
where initial concentration of herbicides is ACo and ACf is the final concentration of herbicide solutions after irradiation at time t. All the experimental work was performed at room temperature (25 °C) and in triplicate.
3 Results and discussion
3.1 Characterization of photocatalysts
The prepared impregnated catalysts (Cu/ZnO and Ni/ZnO) were characterized using SEM, EDX, XRD and surface area analysis. SEM was used to investigate the physical nature and surface morphology of the ZnO and ZnO support impregnated with copper and nickel to confirm that copper and nickel after impregnation were significantly dispersed throughout the ZnO surface. The morphology of Cu/ZnO and Ni/ZnO and ZnO is shown in Fig. 1a, b and c. The dispersed and mosaic nature of impregnated Cu/ZnO and Ni/ZnO compared to naked ZnO shows that the active metals Cu and Ni are successfully impregnated onto the zinc oxide surface. The same results were also obtained by other researchers [22, 23].
The EDX spectra for Cu/ZnO, Ni/ZnO and ZnO are given in Fig. 2a, b and c, respectively. The spectra show that the concentration of Cu and Ni shows that copper and nickel are successfully impregnated in ZnO. The same results were also obtained by other researchers [24].
The XRD patterns of ZnO, Cu/ZnO and Ni/ZnO are given in Fig. 2d, XRD of pure ZnO with ICCD number 11136, 30,888, 361,451 and 11,244 and shows peak at 28, 31, 32, 34, 36, 47, 50, 56, 62, 66, 67 and 69°. Figure 2d (b) is XRD of Cu/ZnO and its pattern according to ICDD number 11136, 30,879, 30,981, 50,661 and 50,664 whereas Cu shows peak at 43.6°, 50.7° and 74.4. The XRD diffraction pattern of Ni/ZnO are given in Fig. 2d (c). The XRD micrograph of Ni/ZnO showing pattern ICCD number 30888, 30,891, 50,664, 211,486, 471,019 and 11,025 (software Cmpr and Logic). In these figures, the corresponding peaks at angle greater than 30° show residue peaks that might have come from any residue present in water. It is observed that 2θ values for the major reflections of Cu/ZnO is in range of 28° to 74.4° while for ZnO ranges from 28° to 69°. Based on Scherer's equation, the thicknesses of the crystal lattice were found in the range of 10.8 to 24.8 nm for ZnO, 11.2 to 25.5 nm for Cu/ZnO and 15.5 to 30.2 nm for Ni/ZnO. For Ni impregnation on ZnO, XRD patterns attain similar spectra to ZnO, except decreased peak intensities which occur due to impregnation of Ni ion into ZnO. As atomic radii of Ni2+ is slightly less than Zn2+ so no additional peaks have been obtained [25] however in case of Cu/ZnO additional peaks of Cu were obtained 43.6°, 50.7° and 74.4°. From the XRD data the shape of the photocatalysts was also found using a XRD software LOGIC and CMPR and it was found hexagonal for ZnO and octahedral for Cu/ZnO and Ni/ZnO. Surface area of ZnO, Cu/ZnO and Ni/ZnO was found to be 181, 190 and 186 m2 g–1. When the strength of radiations for 200 W was measured using a digital lux meter, the average light intensity was 3205 Lux. Band gap and FTIR of ZnO, Cu/ZnO and Ni/ZnO are reported in our previous work [20].
3.2 Effect of pH
The solution pH is important in the process of ultrasound assisted photocatalytic degradation of herbicides. The influence of pH on ultrasound assisted photocatalytic degradation of isoproturon was studied by varying the solution pH from 2 to 10 using 0.1 g of photocatalysts (Cu/ZnO and Ni/ZnO), irradiation time (30 min) and herbicide solution (50 µg mL−1) in the presence of sonication and visible light. pH was adjusted using Britton-Robinson buffer. The effect of pH on % degradation of isoproturon by Ni/ZnO and Cu/ZnO was investigated (Fig. 3a). The results indicate that % degradation of isoproturon increases with increase in pH and reach to its maximum value of 80% at pH 7 using Ni/ZnO and 76% using Cu/ZnO. The influence of pH on % degradation of traisulfuron by Cu/ZnO and Ni/ZnO was also investigated from pH 2 to pH 10 (Fig. 3b). The results showed that % degradation of triasulfuron increases with increase in pH reaching to its maximum value (75 and 73%) at pH 7 and pH 6 using Cu/ZnO and Ni/ZnO, respectively. It can be explained that at lower pH, catalyst has positive surface charge and the concentration of H+ is high that competes with herbicides for active sites leading to reduction in percentage degradation. The ultrasound assisted photocatalytic degradation decreases when pH increases from 8 to 12, decrease in % degradation at higher pH is due to negatively charged hydroxyl anion which inhibit free radicals in solution and active site of catalyst. As well as non-ionic nature of isoproturon are also responsible for maximum degradation of isoproturon at neutral pH [26]. The increase in degradation on basic side as compared to acidic side is mainly due to attack of free radicals on methyl group of herbicides [27].
a pH effect on ultrasound assisted photocatalytic degradation of isoproturon and b triasulfuron (Conditions: pH from 2 to 10, 0.1 g of photocatalysts (Cu/ZnO and Ni/ZnO), irradiation time (30 min) and herbicides concentration (50 µg mL−1) in the presence of sonication and visible light) c Effect of sonication and irradiation time on isoproturon ultrasound assisted photocatalytic degradation and d triasulfuron (Conditions: neutral pH, 0.1 g of photocatalysts (Cu/ZnO and Ni/ZnO), irradiation time (5–60 min), temperature 25 °C and herbicides concentration (50 µg mL.−1) in the presence of sonication and visible light)
3.3 Effect of irradiation time
The time necessary for the ultrasound assisted photocatalytic treatment of herbicides was studied. The irradiation time was gradually changed from 5 to 60 min under the visible light in the sonicator by loading 0.1 g of Cu/ZnO and Ni/ZnO impregnated photocatalysts into 50 µg mL−1 sample solutions of isoproturon and triasulfuron. The sonication passes ultrasound waves from the solution, increases the flow of solution to photocatalysts surface and homogenously distribute it within the solution. The effect of time on photocatalytic degradation efficiency of isoproturon under visible light is given in Fig. 3c. The considerable increase in photocatalytic degradation of isoproturon with increase in sonication and illumination time may be explained as the sonication helps in mixing of substrate with photocatalysts and brings the herbicides into contact with catalyst resulting in increase in degradation efficiency. Maximum degradation of 83 and 78% was observed at optimum time of 25 and 30 min using Cu/ZnO and Ni/ZnO respectively. The degradation efficiency was seen to decrease beyond the optimum time. Due to prolong irradiation and sonication the impregnated metals get discharged from the surface of ZnO catalyst and as a result its efficiency decreases. Results of the degradation of triasulfuron containing 0.1 g catalyst, at neutral pH and varying irradiation time, are given in Fig. 3d. The results suggest greater degradation with increasing irradiation time and reaches to maximum value (85 and 79%) at 25 min for Cu/ZnO and Ni/ZnO, respectively. Further increases in time above the optimum value has no significant effect on degradation process. But it decreases with more sonication and illumination time [28].
3.4 Effect of photocatalysts dose
For economic removal of herbicides from the wastewater the effect of photocatalysts dosage on photocatalytic degradation of herbicides was studied using impregnated Cu/ZnO and Ni/ZnO photocatalysts. Experiments were performed by varying the amount of photocatalysts form 0.02 to 0.3 g keeping all other experimental conditions constant. Initially the photocatalytic activity increases with the amount of catalyst reaches to maximum degradation 86 and 80% using 0.1 g of Cu/ZnO and Ni/ZnO for isoproturon photocatalytic degradation respectively (Fig. 4a). The increase in catalyst amount beyond the optimum values does not significantly increase the efficiency of photocatalytic degradation. The photocatalytic degradation was slightly reduced beyond the 0.2 to 0.3 g of photocatalysts, this is due to large amount of photocatalyst amount in solution leads to light scattering by the particles, less light penetration through the herbicides solution and reduction in transparency of aqueous medium. The degradation of triasulfuron with varying amount of Cu/ZnO and Ni/ZnO catalyst was also investigated and results are given in Fig. 4b. There is increase in degradation of triasulfuron with catalyst dosage until the maximum photocatalytic decomposition 89% and 86% is obtained for Cu/ZnO (0.08 g) and Ni/ZnO (0.1 g). The degradation slightly decreases beyond 0.15 to 0.30 g of catalyst, explained as greater amount of catalyst in solution causes light scattering, reduction in solution transparency and less light penetration [26].
a Effect of catalysts dose on ultrasound assisted photocatalytic degradation of isoproturon and b triasulfuron (Conditions: pH 7 for isoproturon and pH 7 and pH 6 using Cu/ZnO and Ni/ZnO for triasulfuron, irradiation time (30 min), temperature 25 °C and herbicides concentration (50 µg mL.−1) in the presence of sonication and visible light)
3.5 Effect of oxidants
Different oxidants such as potassium persulfate, sodium perchlorate and hydrogen peroxide were applied in concentration range of 1 to 6 mM and its effect on ultrasound assisted photocatalytic degradation was studied. Experimental work was performed by varying the concentration of enhancers keeping optimized pH, catalyst dosage and concentration of herbicides for 30 min. The sample was placed in dark to attain equilibrium and then placed in sonicator under tungsten filament lamp and after each known amount of the solution was taken, absorbance was noted and percent degradation was calculated. The effect of oxidizing agents on the degradation of isoproturon was determined and 81% degradation was observed using Cu/ZnO for 4 mM of K2S2O8, 89% degradation for 4 mM of NaClO4 and 93% for 3 mM of H2O2. Using Ni/ZnO photocatalyst 83% degradation was observed with 5 mM of K2S2O8, 87% degradation with 3 mM of NaClO4 and 91% degradation with 4 mM of H2O2 was observed (Fig. 5a, b and c). The influence of addition of oxidants on ultrasound assisted photocatalytic degradation of triasulfuron was also studied and the results are presented in (Fig. 5e, f and g). It is shown that degradation increases with oxidant due to more radical’s formation [22]. As these free radicals are responsible for the degradation so by increasing the concentration of oxidizing agents the generation of free radicals increases but after optimum value the degradation efficiency decreases due to inhibition of radicals due to interaction of radicals with each other. 5 mM of potassium persulfate concentration was found to be optimum for triasulfuron and 88 and 82% degradation was found using Cu/ZnO and Ni/ZnO visible light induced photocatalysts respectively (Fig. 5d). Effect of sodium perchlorate as an enhancer was also studied and with 5 mM of NaClO4 93% degradation was found using Cu/ZnO as photocatalyst and with 4 mM of NaClO4 86% degradation was observed using Ni/ZnO for triasulfuron (Fig. 5e). Hydrogen peroxide effect as an enhancer was also investigated and 93 and 91% degradation was observed with 5 mM of H2O2 using Cu/ZnO and Ni/ZnO (Fig. 5f).
Effect of oxidizing agents on ultra-sonic assisted degradation of herbicides a Potassium sulfate effect as an oxidizing agent on isoproturon degradation b Effect of sodium perchlorate effect on isoproturon degradation c Effect of hydrogen peroxide effect on isoproturon degradation d Effect of potassium persulfate effect on triasulfuron degradation e Effect of sodium chlorate effect on triasulfuron degradation f Effect of Hydrogen peroxide effect on triasulfuron degradation (Conditions: pH 7 for isoproturon and pH 7 and pH 6 using Cu/ZnO and Ni/ZnO for triasulfuron, 0.1 g of photocatalysts (Cu/ZnO and Ni/ZnO), irradiation time (30 min), temperature 25 °C and herbicides concentration (50 µg mL.−1) in the presence of sonication and visible light)
3.6 Effect of herbicide concentration
The effect of herbicide concentration on ultrasound assisted photocatalytic degradation of isoproturon and triasulfuron was studied in the range of 5 to 50 µg mL−1 using Cu/ZnO and Ni/ZnO (Fig. 6). Experimental work was performed using pH 7 for isoproturon and pH 7 and pH 6 using Cu/ZnO and Ni/ZnO for triasulfuron. 3 mM of H2O2 using Cu/ZnO and 4 mM of H2O2 using Ni/ZnO for isoproturon and 5 mM of H2O2 using Cu/ZnO and Ni/ZnO for triasulfuron, 0.1 g of photocatalysts (Cu/ZnO and Ni/ZnO) for irradiation time of 30 min and varying herbicides concentration in the presence of sonication and visible light. The results showed that 99% degradation of 5 µg mL−1 isoproturon was achieved in the presence of Cu/ZnO and 98% degradation with Ni/ZnO. While 98 and 99% ultrasonic assisted photocatalytic degradation of 5 µg mL−1 triasulfuron was achieved in the presence of Cu/ZnO and Ni/ZnO photocatalysts respectively. The degradation decreases with increase in concentration. With 50 µg mL−1 the degradation of isoproturon decreases with increase in concentration and it was found to be 91 and 88% with Cu/ZnO and Ni/ZnO while in case of 50 µg mL−1 concentration of triasulfuron the degradation efficiency decreases to 91 and 87% with Cu/ZnO and Ni/ZnO photocatalysts, respectively. The decrease is explained by the enhanced light scattering with increase in herbicide concentration and decrease in the concentration of free radicals, as these •OH are responsible for the degradation of herbicides [29]. At the optimized conditions (pH 7 for isoproturon and pH 7 and pH 6 using Cu/ZnO and Ni/ZnO for triasulfuron, 0.1 g of photocatalysts (Cu/ZnO and Ni/ZnO), irradiation time (30 min), temperature 25 °C and 3 mM of H2O2 using Cu/ZnO and 4 mM of H2O2 using Ni/ZnO for isoproturon and 5 mM of H2O2 using Cu/ZnO and Ni/ZnO for triasulfuron in the presence of sonication and visible light) the TOC removal of isoproturon was found to be 87 and 89% and triasulfuron was 89% using Cu/ZnO and 86% using Ni/ZnO photocatalysts respectively.
a Effect of isoproturon concentration on ultrasound assisted photocatalytic degradation b Effect of triasulfuron concentration on ultrasound assisted photocatalytic degradation (Conditions: pH 7 for isoproturon and pH 7 and pH 6 using Cu/ZnO and Ni/ZnO for triasulfuron, 0.1 g of photocatalysts (Cu/ZnO and Ni/ZnO), irradiation time (30 min), temperature 25 °C and 3 mM of H2O2 using Cu/ZnO and 4 mM of H2O2 using Ni/ZnO for isoproturon and 5 mM of H2O2 using Cu/ZnO and Ni/ZnO for triasulfuron in the presence of sonication and visible light)
3.7 Effects of interferences
The influence of the presence of other chemical ions on degradation efficiency of isoproturon and triasulfuron is necessary to study its interaction with the degradation mechanism. The effect of commonly present ions such as sulfates, carbonates and chlorides on the photocatalytic activity was studied by adding the variable amounts of ions in concentration range of (1 to 5 mM) to reaction mixture, while keeping all other experimental conditions that is pH 7 for isoproturon and pH 7 and pH 6 using Cu/ZnO and Ni/ZnO for triasulfuron, 3 mM of H2O2 using Cu/ZnO and 4 mM of H2O2 using Ni/ZnO for isoproturon and 5 mM of H2O2 using Cu/ZnO and Ni/ZnO for triasulfuron, Catalyst amount (0.1 g Cu/ZnO and Ni/ZnO), irradiation time (30 min), herbicides concentration (50 µg mL−1) as constant. The results showed that isoproturon degradation decreases to 73% with 6 mM of SO42− using Cu/ZnO and Ni/ZnO photocatalysts (Fig. 7a), 75% using 6 mM of CO32− in the presence of Cu/ZnO and 74% using Ni/ZnO photocatalysts (Fig. 7b) and 82% using 6 mM of Cl− in the presence of Cu/ZnO and 81% using Ni/ZnO photocatalysts (Fig. 7c). While in case of triasulfuron the ultrasound assisted photocatalytic degradation was also affected both by concentration and size of the added diverse ions. It is explained that diverse ions diminish the free radicals formed in the photocatalytic process due to its larger size and greater charge, therefore slows down the photocatalytic degradation process [12]. The degradation of triasulfuron decreases to 75% with 6 mM of SO42− using Cu/ZnO and 71% using Ni/ZnO photocatalysts (Fig. 7d), 76% using 6 mM of CO32− in the presence of Cu/ZnO and 78% using Ni/ZnO photocatalysts (Fig. 7e) and 86% using 6 mM of Cl− in the presence of Cu/ZnO and 82% using Ni/ZnO photocatalysts (Fig. 7f).
( Effect of a sulfate ions b carbonate ions c chloride ions on isoproturon ultrasound assisted photocatalytic degradation d Effect of sulfate ions e carbonate ions f and chloride on triasulfuron photocatalytic degradation (Conditions: pH 7 for isoproturon and pH 7 and pH 6 using Cu/ZnO and Ni/ZnO for triasulfuron, 0.1 g of photocatalysts (Cu/ZnO and Ni/ZnO), irradiation time (30 min), temperature 25 °C and 3 mM of H2O2 using Cu/ZnO and 4 mM of H2O2 using Ni/ZnO for isoproturon and 5 mM of H2O2 using Cu/ZnO and Ni/ZnO for triasulfuron in the presence of sonication and visible light)
3.8 Recovery and reusability
After the photocatalytic treatment, the recovery and reuse of the photocatalysts was verified. The ease of reuse and good recovery after photocatalytic activity is of great economic advantage, with used photocatalysts being recovered by filtration (Whatman filter paper, pore size (2.5 µm), followed by washing and drying in an oven. The photocatalysts were washed with distilled water and then with organic solvents such as ethanol and acetone. After grinding, the recovered impregnated zinc oxide photocatalysts were ready for reuse. The efficiency of ultrasound-assisted photocatalytic degradation of the first, second and third photocatalysts recycled using isoproturon is given in Fig. 8a. The degradation efficiencies of Cu/ZnO are 93, 92 and 90% and those of Ni/ZnO are 92, 91 and 89% for first, second and third run of isoproturon degradation. It was observed that the photocatalytic activity of the recycled photocatalysts is very close to the photocatalytic activity of the fresh photocatalyst. Thus Cu/ZnO and Ni/ZnO can be recycled without compromising the photocatalytic activity. Sonication-assisted photodegradation of triasulfuron for reusability is shown in Fig. 8b. The degradation efficiencies of Cu/ZnO are 97, 95 and 93% and of Ni/ZnO are 95, 93 and 91% for triasulfuron photocatalytic degradation after first, second and third run of the catalyst. The results shows no significant decrease in herbicide degradation. The proposed method is better for commercial applications due to its reusability of the photocatalyst. The prepared photocatalysts were compared with other catalysts and degradation methods. Comparison of the results of the proposed method of ultrasound-assisted photocatalytic degradation of isoproturon and triasulfuron with other methods are given in Tables 1, 2 and 3. The possible degradation mechanisms of isoproturon are given in Fig. 9. For the ultrasound-assisted photocatalytic degradation of isoproturon, light and ultrasonic radiation are responsible. When light falls on the surface of photocatalysts (Cu/ZnO and Ni/ZnO), they accelerate electrons towards the conduction band, leaving holes in the valence band. These electron–hole pairs are responsible for the generation of free radicals. The generated free radicals reacts with isoproturon and transform them into highly unstable intermediates, leading to further degradation and resulting in products such as CO2, H2O and mineral acids. The degradation of isoproturon is accelerated due to the synergistic effect of ultrasonic radiation also. Ultrasonic radiation causes sonocatalytic degradation of isoproturon due to sonoluminescence, hot spots and leakage of oxygen atoms [30]. These three phenomena caused by sonication accelerate the degradation. Intermediates formed during the degradation are highly unstable and attacked by free radicals [26]. The electron hole pair generated due to sonoluminescence in a range of 200–700 nm causes the generation of free radicals. The cavitation bubbles in the ultrasonic radiation and hot spot further cause the generation of free radicals. All the free radicals, electrons and holes cause the degradation of herbicides resulting in the formation of CO2, H2O and mineral acids.
a Re-usability of ultrasound assisted photocatalytic degradation of isoproturon b. Re-usability of ultrasound assisted photocatalyst for photocatalytic degradation of triasulfuron (Conditions: pH 7 for isoproturon and pH 7 and pH 6 using Cu/ZnO and Ni/ZnO for triasulfuron, 0.1 g of photocatalysts (Cu/ZnO and Ni/ZnO), irradiation time (30 min), temperature 25 °C and 3 mM of H2O2 using Cu/ZnO and 4 mM of H2O2 using Ni/ZnO for isoproturon and 5 mM of H2O2 using Cu/ZnO and Ni/ZnO for triasulfuron in the presence of sonication and visible light)
4 Conclusions
Copper and nickel impregnated zinc oxide photocatalysts were used for photocatalytic degradation of isoproturon and triasulfuron herbicides. Cu/ZnO and Ni/ZnO was prepared using wet impregnation method, different characterization techniques such as SEM, EDX, XRD, FTIR, and surface area confirms the preparation of Cu/ZnO and Ni/ZnO. Sonication assisted photocatalytic degradation of herbicides in the wastewater was studied under different experimental parameters. The optimum experimental conditions have been proposed for the significant sonophotocatalytic degradation of isoproturon and triasulfuron. Copper and nickel impregnated photocatalyst showed higher degradation efficiency in visible light region. The good recovery and reuse of impregnated zinc oxide photocatalyst many times are the major advantages along with the higher degradation efficiency in visible light region.
Availability of data and materials
The data and materials will be provided on demand.
References
Winayu BNR, Mao WH, Chu H. Combination of rGO/S, N/TiO2 for the enhancement of visible light-driven toluene photocatalytic degradation. Sustain Environ Res. 2022;32:34.
Vaya D, Surolia PK. Semiconductor based photocatalytic degradation of pesticides: An overview. Environ Technol Inno. 2020;20:101128.
Ahmed S, Rasul MG, Brown R, Hashib MA. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J Environ Manage. 2011;92:311–30.
de Oliveira R, da Silva Martini W, Sant'Ana AC. Combined effect involving semiconductors and plasmonic nanoparticles in photocatalytic degradation of pesticides. Environ Nanotechnol, Monit Manage. 2022;17:100657.
Nasir MS, Yang GR, Ayub I, Nasir A, Yan W. Higher hydrogen production by photocatalytic water splitting using a hollow tubular graphitic carbon nitride-zinc telluride composite. Environ Chem Lett. 2022;20:19–26.
El Bouraie M, El Din WS. Biodegradation of Reactive Black 5 by Aeromonas hydrophila strain isolated from dye-contaminated textile wastewater. Sustain Environ Res. 2016;26:209–16.
Ullah T, Gul H, Khitab F, Khattak R, Ali Y, Rasool S, et al. Adsorption of Remazol Brilliant Violet-5R from aqueous solution using sugarcane bagasse as biosorbent: kinetic and thermodynamic studies. Water-Sui. 2022;14:3014.
Ahmed SF, Mofijur M, Parisa TA, Islam N, Kusumo F, Inayat A, et al. Progress and challenges of contaminate removal from wastewater using microalgae biomass. Chemosphere. 2022;286:131656.
Lim S, Shi JL, von Gunten U, McCurry DL. Ozonation of organic compounds in water and wastewater: a critical review. Water Res. 2022;213:118053.
Abuhasel K, Kchaou M, Alquraish M, Munusamy Y, Jeng YT. Oily wastewater treatment: overview of conventional and modern methods, challenges, and future opportunities. Water-Sui. 2021;13:980.
Irfan M, Zahid M, Tahir N, Yaseen M, Qazi UY, Javaid R, et al. Enhanced photo-Fenton degradation of Rhodamine B using iodine-doped iron tungstate nanocomposite under sunlight. Int J Environ Sci Te. 2023;20:3645–60.
Fan GD, Yang SW, Du BH, Luo J, Lin X, Li X. Sono-photo hybrid process for the synergistic degradation of levofloxacin by FeVO4/BiVO4: Mechanisms and kinetics. Environ Res. 2022;204:112032.
An XF, Chen Y, Ao MH, Jin YH, Zhan LW, Yu B, et al. Sequential photocatalytic degradation of organophosphorus pesticides and recovery of orthophosphate by biochar/α-Fe2O3/MgO composite: A new enhanced strategy for reducing the impacts of organophosphorus from wastewater. Chem Eng J. 2022;435:135087.
Da Costa-Serra JF, Guil-Lopez R, Chica A. Co/ZnO and Ni/ZnO catalysts for hydrogen production by bioethanol steam reforming. Influence of ZnO support morphology on the catalytic properties of Co and Ni active phases. Int J Hydrogen Energ. 2010;35:6709–16.
Makofane A, Motaung DE, Hintsho-Mbita NC. Photocatalytic degradation of methylene blue and sulfisoxazole from water using biosynthesized zinc ferrite nanoparticles. Ceram Int. 2021;47:22615–26.
Kaur M, Verma A, Rajput H. Potential use of foundry sand as heterogeneous catalyst in solar photo-Fenton degradation of herbicide isoproturon. Int J Environ Res. 2015;9:85–92.
Sharma MVP, Durgakumari V, Subrahmanyam M. Solar photocatalytic degradation of isoproturon over TiO2/H-MOR composite systems. J Hazard Mater. 2008;160:568–75.
Vulliet E, Emmelin C, Grenier-Loustallot MF, Paisse O, Chovelon JM. Simulated sunlight-induced photodegradations of triasulfuron and cinosulfuron in aqueous solutions. J Agr Food Chem. 2002;50:1081–8.
Khitab F, Jan MR, Shah J. Removal of hazardous textile dyes from water using Ni impregnated ZnO through sonophotocatalytic degradation. Desalin Water Treat. 2020;205:357–72.
Khitab F, Shah J, Jan MR. Systematic assessment of visible light driven photocatalysts for the removal of cefixime in aqueous solution sonophotocatalytically. Int J Environ An Ch. 2022; doi: https://doi.org/10.1080/03067319.2022.2025790.
Shah J, Jan MR, Khitab F. Sonophotocatalytic degradation of textile dyes over Cu impregnated ZnO catalyst in aqueous solution. Process Saf Environ. 2018;116:149–58.
Karthik KV, Raghu AV, Reddy KR, Ravishankar R, Sangeeta M, Shetti NP, et al. Green synthesis of Cu-doped ZnO nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants. Chemosphere. 2022;287:132081.
Khalid A, Ahmad P, Khan A, Muhammad S, Khandaker MU, Alam MM, et al. Effect of Cu doping on ZnO nanoparticles as a photocatalyst for the removal of organic wastewater. Bioinorg Chem Appl. 2022;2022:9459886.
Ponnambalam P, Kamalakkannan J, Jayaseelan R, Selvi G. Novel synthesis of Cu–ZnO heterostructure for photoelectric, medicinal, and sun-light dye degradative applications. Inorg Nano-Met Chem. 2022;52:1214–25.
Mustafa SM, Barzinjy AA, Hamad AH, Hamad SM. Green synthesis of Ni doped ZnO nanoparticles using dandelion leaf extract and its solar cell applications. Ceram Int. 2022;48:29257–66.
Reddy PAK, Reddy PVL, Sharma VM, Srinivas B, Kumari VD, Subrahmanyam M. Photocatalytic degradation of isoproturon pesticide on C, N and S doped TiO2. J. Water Resource and Protection. 2010;2:235–44.
Garcia-Munoz P, Carbajo J, Faraldos M, Bahamonde A. Photocatalytic degradation of phenol and isoproturon: Effect of adding an activated carbon to titania catalyst. J Photoch Photobio A. 2014;287:8–18.
Al-Musawi TJ, McKay G, Rajiv P, Mengelizadeh N, Balarak D. Efficient sonophotocatalytic degradation of acid blue 113 dye using a hybrid nanocomposite of CoFe2O4 nanoparticles loaded on multi-walled carbon nanotubes. J Photoch Photobio A. 2022;424:113617.
Phanikrishna Sharma MV, Durga Kumari V, Subrahmanyam M. Photocatalytic degradation of isoproturon herbicide over TiO2/Al-MCM-41 composite systems using solar light. Chemosphere. 2008;72:644–51.
Bembibre A, Benamara M, Hjiri M, Gomez E, Alamri HR, Dhahri R, et al. Visible-light driven sonophotocatalytic removal of tetracycline using Ca-doped ZnO nanoparticles. Chem Eng J. 2022;427:132006.
Schieppati D, Galli F, Peyot ML, Yargeau V, Bianchi CL, Boffito DC. An ultrasound-assisted photocatalytic treatment to remove an herbicidal pollutant from wastewaters. Ultrason Sonochem. 2019;54:302–10.
Verma A, Prakash NT, Toor AP. An efficient TiO2 coated immobilized system for the degradation studies of herbicide isoproturon: Durability studies. Chemosphere. 2014;109:7–13.
Haque MM, Muneer M. Heterogeneous photocatalysed degradation of a herbicide derivative, isoproturon in aqueous suspension of titanium dioxide. J Environ Manage. 2003;69:169–76.
Fenoll J, Hellin P, Flores P, Martinez CM, Navarro S. Photocatalytic degradation of five sulfonylurea herbicides in aqueous semiconductor suspensions under natural sunlight. Chemosphere. 2012;87:954–61.
Vulliet E, Emmelin C, Chovelon JM, Guillard C, Herrmann JM. Photocatalytic degradation of sulfonylurea herbicides in aqueous TiO2. Appl Catal B-Environ. 2002;38:127–37.
Pathania D, Sharma A, Kumar S, Srivastava AK, Kumar A, Singh L. Bio-synthesized Cu-ZnO hetro-nanostructure for catalytic degradation of organophosphate chlorpyrifos under solar illumination. Chemosphere. 2021;277:130315.
Gholami M, Jonidi-Jafari A, Farzadkia M, Esrafili A, Godini K, Shirzad-Siboni M. Photocatalytic removal of bentazon by copper doped zinc oxide nanorods: Reaction pathways and toxicity studies. J Environ Manage. 2021;294:112962.
Javed M, Qamar MA, Shahid S, Alsaab HO, Asif S. Highly efficient visible light active Cu-ZnO/S-g-C3N4 nanocomposites for efficient photocatalytic degradation of organic pollutants. RSC Adv. 2021;11:37254–67.
Jung HJ, Koutavarapu R, Lee S, Kim JH, Choi HC, Choi MY. Enhanced photocatalytic degradation of lindane using metal–semiconductor Zn@ZnO and ZnO/Ag nanostructures. J Environ Sci-China. 2018;74:107–15.
Jonidi-Jafari A, Gholami M, Farzadkia M, Esrafili A, Shirzad-Siboni M. Application of Ni-doped ZnO nanorods for degradation of diazinon: Kinetics and by-products. Sep Sci Technol. 2017;52:2395–406.
Adabavazeh H, Saljooqi A, Shamspur T, Mostafavi A. Synthesis of polyaniline decorated with ZnO and CoMoO4 nanoparticles for enhanced photocatalytic degradation of imidacloprid pesticide under visible light. Polyhedron. 2021;198:115058.
Lemessa G, Negussa D, Bedi PS. Synthesis and Characterization of Co-doped nickel-ZnO/polypyrrole nano-composites, and their effect on photo-catalytic degradation of p-nitrophenol under solar irradiation. International Research Journal of Pure and Applied Chemistry. 2018;17:1–12.
Hosseini M, Esrafili A, Farzadkia M, Kermani M, Gholami M. Degradation of ciprofloxacin antibiotic using photo-electrocatalyst process of Ni-doped ZnO deposited by RF sputtering on FTO as an anode electrode from aquatic environments: Synthesis, kinetics, and ecotoxicity study. Microchem J. 2020;154:104663.
Gulipalli P, Punugoti T, Nikhil P, Poiba VR, Vangalapati M. Synthesis and characterization of Ni/Zn dually doped on multiwalled carbon nanotubes and its application for the degradation of dicofol. Mater Today-Proc. 2021;44:2760–6.
Acknowledgements
Not applicable.
Funding
The authors have no funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, R. J. and J. S.; methodology, J. S.; software, K. K.; validation, R. J., J. S. and K.K.; formal analysis, F.K.; investigation, J.S.; resources, R.J.; data curation, K.K.; writing—original draft preparation, K.K.; writing—review and editing, J.S.; visualization, R.J.; supervision, J.S.; project administration, R.J.; funding acquisition, R.J. All authors have read and agreed to the published version of the manuscript.” Please turn to the Credit taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, K., Khitab, F., Shah, J. et al. Ultrasound assisted photocatalytic degradation of isoproturon and triasulfuron herbicides using visible light driven impregnated zinc oxide catalysts. Sustain Environ Res 33, 24 (2023). https://doi.org/10.1186/s42834-023-00184-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42834-023-00184-9