Abstract
The increasing global aging population has led to a continual rise in the prevalence of bone and joint diseases, posing challenges to both the quality of life for patients and healthcare resources. Type II collagen, a pivotal protein for sustaining joint function, has gained substantial attention in recent years. The oral administration of undenatured type II collagen (UC-II) has demonstrated noteworthy advancements in tackling bone and joint diseases. This article presents a comprehensive review of the structure and extraction methods of UC-II, discusses the relationship between UC-II and arthritis, and thoroughly examines its therapeutic role and potential mechanisms in the treatment process. In addition, future perspectives for clinical application of UC-II are discussed. It was found that the oral administration of UC-II, through induction of oral tolerance mechanisms, exhibits promise in alleviating joint inflammation and pain in patients with osteoarthritis (OA) and rheumatoid arthritis (RA). This method can significantly ameliorate joint inflammation and pain, with high patient acceptance and minimal side effects, demonstrating its potential as a well-tolerated treatment option for joint diseases.
Graphical Abstract

Similar content being viewed by others
1 Introduction
Bone and joint health are crucial for preserving quality of life and vitality of individuals. The incidence of bone and joint diseases has exhibited a significant upward trend due to the accelerating aging of the world population. According to the statistical data in 2020, the global number of arthritis sufferers reached 595 million, marking a 132% increase from the 1990 figure of 256 million. By 2050, this number is projected to approach 1 billion [1]. This trend not only subjects patients to pains, limited mobility and dysfunction, but also places substantial pressure on healthcare resources in society [2]. Hence, there is an urgent need for in-depth research to explore innovative solutions that can effectively enhance osteoarthritic conditions in the elderly, aiming to enhance the life quality of patients and alleviate the strain on healthcare resources.
In this context, type II collagen has emerged as a compelling area of research. Type II collagen, a structural protein predominantly found in cartilage tissue, contributes to joint stability and elasticity, facilitating the absorption of shock and stress while sustaining normal joint function [3]. It has been shown that a close association between the deficiency or damage to type II collagen and the development of various bone and joint diseases. As cartilage quality and elasticity diminish, patients may experience pain, joint swelling, restricted movement, and dysfunction. Therefore, preserving the stability and function of type II collagen is essential for preventing and treating osteoarthritic diseases.
In recent years, significant progress has been made in osteoarthritis research, particularly regarding the role of undenatured type II collagen (UC-II). Advanced therapeutic approaches, including the oral administration of UC-II, have been extensively studied [4]. Recent studies have delved into the effectiveness of UC-II in alleviating osteoarthritis, providing in-depth insights into its structure, function, and therapeutic potential at both cellular and animal levels [5]. The expanded understanding encompasses novel therapeutic strategies, significant clinical trials, patient outcomes, and detailed mechanistic analyses [6], thereby paving the way for innovative treatments in osteoarthritis management. Nonetheless, the action mechanism of UC-II and its long-term safety and efficacy warrant further comprehensive investigation to fully comprehend the therapeutic potential and implications. This work aims to delineate the structure and extraction process of UC-II, while delving further into the association between UC-II and osteoarthritic diseases. Its role in the treatment of osteoarthritic diseases will be carefully scrutinized, and the potential mechanisms underlying its therapeutic effects will be thoroughly explored. Addressing current research gaps, the future prospects of UC-II in clinical treatments are also discussed. This encompasses novel therapeutic strategies and therapies, aiming to pave the way for the application of type II collagen in the treatment of osteoarthritic diseases.
2 Overview of type II collagen
Collagen, the most abundant protein in the animal body, is widely prevalent. Abundant in glycine, proline, hydroxyproline, and other essential amino acids for human body, collagen is predominantly present in various animal tissues, such as skin, bones, cartilage, teeth, tendons, ligaments, blood vessels, and the intramuscular space ligaments [7]. Constituting 25–30% of total protein in mammals, or 6% of body weight, collagen serves as a vital structural protein in connective tissue, accounting for 20–30% of composition [8]. Due to the high collagen content, connective tissue exhibits distinct structural and mechanical properties, including tensile strength, tear strength, and viscoelasticity, contributing to the support and protection of organs [9]. Identified by different gene sequences, 29 collagen types have been reported, with prevalent types, including type I, II, III, V, and XI [10]. Type II collagen, generated by chondrocytes, is evenly distributed across all layers of cartilage, constituting more than 80% of chondrocytes [11]. As a principal component of cartilage matrix, type II collagen plays a crucial role in maintaining the integrity of cartilage tissue, stimulating chondrocyte growth and redifferentiation, and promoting overall bone health [12]. Type II collagen constitutes the primary macromolecule within the collagen fiber network, featuring three α1 peptide chains denoted as [α1(II)]3. Each peptide chain contains a sequence of tripeptide repeats, typically Gly-X-Y (G-X-Y), represented as (G-X-Y)n [13]. Notably, the X-position commonly hosts a Pro residue (20–30%), with tripeptides like Gly-Pro-Y constituting approximately one-third of the total tripeptide count. The secondary structure of type II collagen manifests as a triple-stranded helix, formed by the intertwining of three left-handed helices, collectively creating a right-handed helical structure, known as the superhelix [14]. This triple helix is maintained through various interactions, including hydrogen bonding, dipole-dipole bonding, ionic bonding, and van der Waals forces. During maturation, enzymes hydrolyze the N- and C-terminal propeptides of mature type II collagen, leaving behind the triple helix structure and telopeptide as procollagen. This procollagen undergoes cross-linking, forming a network of collagen fibers.
Notably, the triple helix structure of type II collagen is susceptible to damage. The elevated ambient temperatures, for instance, can weaken the hydrogen bonds between the three-stranded peptide chains. This weakening can lead to a reduction in the helix of peptide chain, advancing towards de-helixing and an overall increase in protein disorder. Such changes can result in the denaturation of type II collagen, affecting both its structure and activity. The effectiveness and viability of type II collagen as a crucial element in treating arthritic conditions are intimately related to its undenatured structure. In its undenatured state, type II collagen preserves its original structure and bioactivity. Denaturation, on the other hand, disrupts its molecular arrangement, weakening interactions with pertinent cells and molecules. This compromises its ability to absorb joint loads and maintain normal joint function. Moreover, the immune tolerance induced by UC-II relies on its immunodominant T-cell epitopes, and denaturation may alter immune recognition dynamics [15]. Therefore, the undenatured extraction of type II collagen holds paramount importance for its therapeutic potential in the treatment of arthritic conditions.
3 Extraction methods for UC-II
The structure of UC-II is intricate, featuring filamentous collagen fibers interwoven with elastin and glycoproteins, forming a reticular framework. Preserving its triple helix structure during the extraction process poses a challenge. Presently, the predominant extraction methods for UC-II employ the acid-enzyme combination approach. Specific proteases are chosen to cleave peptide bonds at phenylalanine, leucine, and glutamic acid residues [12], thereby removing the telopeptide situated in the non-helical region at the ends of type II collagen (Fig. 1). Subsequently, collagen within the helical region is dissolved using an acidic solution, culminating in the attainment of high-purity UC-II [10]. Other methods, such as ultrasound-assisted extraction [16], ion-exchange chromatography [17], and salt precipitation followed by dialysis [12], also employ enzymes to remove terminal peptides. Subsequently, these methods utilize various treatments to efficiently isolate and purify type II collagen from enzymatic digest.
Several recent studies have explored the extraction of UC-II from animal cartilage. Trentham et al. [18] utilized pepsin to extract UC-II from chicken cartilage powder. The process involved washing the powder with 0.05 mol/L Tris/ 2 mol/L MgCl2, followed by pepsin-induced extraction at 0.5 mol/L acetic acid, pH 2.5, and 4 °C, with gentle shaking for 72 h. The obtained UC-II underwent dialysis, purification, and lyophilization. In the study conducted by Cheng et al. [19], a comparison was made between NaCl salting and guanidine hydrochloride dissolution for the removal of non-collagenous components from chicken cartilage powder. The extraction efficiency of type II collagen following guanidine hydrochloride solubilization was 56.95%, significantly surpassing 28.62% yield achieved through NaCl extraction. Maity et al. [20] proposed an expeditious method utilizing pepsin (0.04%, w/w) for the digestion of goat ear cartilage tissue, resulting in a 55% extraction rate while preserving the natural intermolecular cross-linking and triple-helix structure.
Beyond terrestrial animals, marine organisms can also serve as a source for UC-II extraction. Che et al. [21] extracted UC-II from Chinese sturgeon cartilage using enzymatic dissociation in 0.5 mol/L glacial acetic acid containing pepsin (0.1%f) for 48 h. By summarizing various extraction studies, a consistent extraction rate of around 55% was observed. Despite different cartilage sources, the common extraction methods involved pre-treatment, impurity removal, enzymatic hydrolysis, and purification. The commonly used methods, such as pepsin combined with acetic acid digestion, guanidine hydrochloride for heteroprotein removal, and DEAE52 ion exchange column chromatography, indicated the effectiveness of the acid-enzyme method for type II collagen extraction. However, these methods present challenges, including limited processing capacity, intricate production processes, time consumption, high reagent costs, and impracticality for large-scale production. Therefore, there is an urgent need for a more economical, rapid, and effective UC-II extraction method [2].
In recent years, deep eutectic solvents (DES), recognized as an emerging green solvent, have exhibited numerous advantages, including wide availability, low cost, straightforward preparation, high extraction efficiency, non-toxicity, environmental friendliness, and the ability to maintain the structure of the extracted material. They have practical application in various industries, particularly in the extraction of natural active substances, and have gained popularity in the extraction of food proteins. This includes plant proteins [22,23,24,25], animal proteins [26,27,28], amino acids [29,30,31], and enzymes [32,33,34]. Bai et al. [26] utilized choline chloride-oxalic acid DES to extract collagen peptides from cod skin. They investigated the impact of extraction conditions on the efficiency and purity of collagen peptides. Following optimization, the optimal conditions for high molecular weight collagen peptides were determined as choline chloride: oxalic acid = 1:1, a solid-liquid ratio of 1:80 g/ml, and extraction for 2 h at 65 °C, resulting in yields and purities of 91.57% and 92.85%, respectively. For low molecular weight collagen peptides, the optimal conditions were choline chloride: oxalic acid = 1:1, a solid-liquid ratio of 1:120 g/mL, and extraction at 65 °C for 6 h, yielding 96.36% with 100% purity. Furthermore, in addition to achieving a high extraction rate, the cluster effect extraction mechanism of DES has demonstrated the capability to preserve the structure of the extracted material. This presents a promising approach for the non-denaturing and efficient extraction of type II collagen.
4 Association of type II collagen with joint disease
Type II collagen is a vital component of articular cartilage and plays a crucial role in the context of joint diseases. Articular cartilage, a specialized type of non-vascular, non-nervous connective tissue, covers the surfaces of articular bones, offering support, shock absorption, and resistance to shear forces [35]. Comprising an extracellular matrix and a sparse population of chondrocytes, the matrix of articular cartilage consists of a network of collagen fibers, proteoglycans, and water. Its principal role is to preserve the smoothness of the cartilage structure and cushion against external forces [36].
The collagen fiber network within the extracellular matrix imparts elasticity and toughness to articular cartilage, enabling joints to withstand stress and absorb shocks. Chondrocytes, the principal cells in cartilage, secrete the extracellular matrix, which regulates the dynamic stability and balance of cartilage during synthesis and degradation [37, 38]. The stability of the collagenous network relies on the equilibrium between collagen synthesis and degradation, especially the balance in the synthesis and degradation of type II collagen. The imbalance in this process can lead to cartilage tissue damage, gradual wear, and degradation, ultimately resulting in direct bone contact between joints, leading to pain, inflammation, and impaired joint function.
Moreover, an amplified inflammatory response mechanism can expedite cartilage degeneration. Inflammatory cytokines, including interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6), trigger the cellular immune system, stimulating the production of matrix metalloproteinases (MMPs) and polyprotein polyglucomutases (A Disintegrin and Metalloproteinase with Thrombospondin Motifs-4, ADAMTS-4 and A Disintegrin and Metalloproteinase with Thrombospondin Motifs-5, ADAMTS-5) [39, 40]. These enzymes possess the ability to degrade various collagens, including type II collagen, fibronectin, laminin, elastin, and diverse proteoglycans [41]. The production of these enzymes accelerates the rate of cartilage matrix degradation, while inflammatory cytokines weaken the ability of chondrocytes to repair the cartilage matrix, induce chondrocyte apoptosis, exacerbate joint disease, and cause irreversible damage [42].
Joint diseases encompass a group of disorders involving inflammation and lesions in the joints and surrounding tissues, with degenerative and inflammatory joint diseases being the most common and extensively studied. Degenerative joint diseases, resulting from the degeneration and wear of articular cartilage, are commonly encountered [43]. The most prevalent of these is osteoarthritis (OA), characterized by the degeneration and deterioration of articular cartilage. OA primarily impacts weight-bearing joints, such as knees, hips, spine, and wrists, particularly among the elderly population [44]. As pathological progression intensifies, it can result in joint swelling and restricted range of motion, significantly impacting daily activities and diminishing the patient’s overall quality of life. Histologically, OA is marked by cartilage degeneration, the formation of redundant bone, subchondral bone resorption, and chronic synovial inflammation [44].
The pathogenesis of OA can be divided into three main phases. It begins with a decrease in cartilage strength due to collagen network degradation and an increase in water content, followed by a compensatory rise in chondrocyte anabolism for repair. Eventually, the reparative process becomes unsustainable, leading to the destruction of cartilage tissue. The etiology of OA is complex, involving genetic factors, joint injury, obesity, age, and inflammation [45].
Inflammatory joint diseases stem from an inflammatory process, normally involving an abnormal immune system response, leading to inflammation, swelling, and pain in the joints, with rheumatoid arthritis (RA) being a prominent instance [46]. RA is a chronic, inflammatory autoimmune disease primarily affecting the joints, wherein the immune system erroneously attacks the body tissues, resulting in inflammation of joint membranes and synovitis. The typical presentation of RA involves symmetrical arthritis affecting multiple joints, particularly smaller joints such as fingers, wrists, ankles, and knees. Systemic symptoms may also manifest, including fatigue, fever, loss of appetite, and weight loss [47]. Moreover, RA can impact other organs and tissues, such as the skin, eyes, lungs, and cardiovascular system.
The primary pathological change in RA involves the destruction of collagen in the articular cartilage matrix [48]. Pro-inflammatory cytokines stimulate chondrocytes and synoviocytes to secrete collagenases, including cartilage matrix metalloproteinase (MMP), which leads to an imbalance between the synthesis and degradation of cartilage extracellular matrix, disrupting the peripheral environment of chondrocytes and promoting chondrocyte apoptosis [49,50,51]. The complex pathogenesis of RA is influenced by various factors such as genetic factors, environmental factors, and immune system abnormalities. It has been suggested that RA may be triggered by an autoimmune response within joint tissues to specific occult antigens, resulting in an imbalance in the body immune regulation and an excessive inflammatory response, ultimately destroying joint structures [39,40,41, 52].
Remarkably, type II collagen is identified as one of the autoantigens in RA, initiating autoimmunity in synovium and cartilage in animal models of the disease. It is widely utilized in rodent models of collagen-induced arthritis (CIA). The glycosylated peptide 256–270 of type II collagen is recognized as the major immunodominant T cell epitope involved in CIA. This peptide is internalized by antigen-presenting cells, degraded into smaller peptides, and contributes to the formation of the collagen type II-major histocompatibility complex II (CII-MHC-II) complex. This complex is further transported to T cells, initiating their activation and the release of cytokines and triggering an immune response [15].
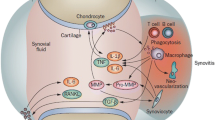
Figure 2 illustrates a comparison among OA, normal joints, and RA joints. OA is characterized by severe joint degeneration, resulting in significant destruction of the cartilage matrix (in blue). While RA also exhibits damage to the cartilage matrix (the blue area), it is generally less extensive than in OA. Additionally, RA is distinguished by synovitis and opacification, indicating cloudiness or increased opacity in the joint cavity due to exudate and inflammatory cell accumulation as a result of the inflammatory response. Despite disparities in etiology, pathogenesis, and pathological manifestations between osteoarthritis (OA) and rheumatoid arthritis (RA), both conditions involve inflammation that leads to the degradation of the cartilage matrix. Specifically, this process includes the breakdown of the fibrous scaffold of type II collagen and the destruction of chondrocyte cells. Therefore, the primary treatment objective should focus on mitigating inflammation and facilitating the repair and rebuilding of type II collagen fiber scaffold.
5 Application of UC-II for potential treatment of joint disease
The identification of therapies capable of efficaciously retarding joint degeneration, enhancing joint mobility, and mitigating joint pain has emerged as a formidable challenge. It has been shown that UC-II, undenatured form of collagen II, can impact joint inflammation by modulating local immunity. The oral administration of modest quantities of UC-II has exhibited promising results in offering relief from joint diseases [53].
Several recent studies have reported the therapeutic effects of UC-II on OA in different animal models, including rats, mice, horses, and dogs (Table 1) [42, 54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70]. Orhan et al. [55] investigated the therapeutic effects and mechanisms of UC-II on OA using a rat model induced by monosodium iodoacetate (MIA). The MIA was injected into the rats to induce the OA model, and the rats were fed with chicken-derived UC-II at a dose of 4 mg/kg/day for 30 days. Kellgren-Lawrence arthritis scores were assessed, and serum levels of inflammatory cytokines were measured. The study revealed that UC-II markedly decreased MIA-induced Kellgren-Lawrence scores by 53.3%, indicating a significant reduction in joint degradation. Additionally, UC-II supplementation led to notable decreases in key serum inflammatory markers, with reductions of 7.8% in IL-1β, 18.0% in IL-6, 25.9% in TNF-α, 16.4% in COMP, and 32.4% in CRP, showcasing its potent anti-inflammatory effects (P < 0.0001). The authors also observed that UC-II could inhibit the levels of NF-κB, TNF-α, IL-1β, IL-6, COX-2, and IKK-γ in OA rats. UC-II may alleviate inflammation and pain in OA joints by inhibiting the activation of the NF-κB pathway in arthritic rats. In a study using the same rat model [56], significant effects were observed after orally administering 8 mg/kg of chicken UC-II for 5 weeks. There was an 18.72% increase in the knee joint pressure pain threshold, along with a 26.41% decrease in serum TNF-α level, a 9.12% decrease in MMP-13 level, and a 35.18% increase in TGF-β level. Pathologically, there was a marked reduction in synovial hyperplasia, characterized by decreased fibrous tissue proliferation and inflammatory cell infiltration, leading to an effective improvement in the degenerative changes and injuries of articular cartilage tissues (P < 0.05). In contrast, in the glucosamine + chondroitin sulfate treatment group, while there was a reduction in the joint pressure pain threshold and MMP-13 level, along with alleviated synovial tissue inflammation, no significant changes were observed in TNF-α and TGF-β levels or joint cartilage tissue inflammation. This indicated that the action mechanisms of UC-II differ from those of glucosamine and chondroitin sulfate. Similarly, Fan et al. [54] observed a reduction in inflammatory factors in the aging db/db mouse model. Following the oral administration of 6 mg/kg/day chicken-derived UC-II for 3 months, a significant decrease was noted in serum levels of inflammatory factors. This included a 50% reduction in MMP-3 and MMP-13 levels. Additionally, there was a noteworthy increase in anti-inflammatory factors: a 74% rise in IL-4 levels, a 123% increase in IL-10 levels, a 67% increase in CTX-II levels, and an 84% increase in TGF-β levels. Marzia et al. [71] conducted a study on the therapeutic effects of UC-II in dogs with osteoarthritis (OA). The study involved dogs diagnosed with OA, which were treated with oral chicken-derived UC-II at the dosage of 40 mg/day for 30 days. The evaluation included the assessment of LOAD (load score), MOBILITY (activity score), CLINICAL (clinical score), and COAST (Canine Osteoarthritis Staging Tool) scores in the dogs. The results indicated the efficacy of UC-II in the treatment of dogs with OA, as evidenced by improvements in associated clinical signs. There were varying degrees of decrease in all scores: a 31.4% reduction in the LOAD score, a 25% reduction in the MOBILITY score, an 18.8% reduction in the CLINICAL score, and a 15.2% reduction in the COAST score. Orie et al. [57] conducted a study on the long-term (150 days) effects of oral UC-II treatment in dogs with osteoarthritis (OA). The assessment included parameters such as body weight, body temperature, heart and respiratory rates, liver function, renal function, cardiovascular function in serum samples, overall pain, pain from limb manipulation, and pain after physical exertion. The results revealed a substantial 54.3% reduction in overall pain, a notable 65.2% reduction in pain from limb manipulation, and a significant 62.6% reduction in pain from physical exertion. Importantly, there were no significant changes observed in body weight, body temperature, heart or respiratory rate, blood chemistry, liver function, or renal cardiovascular function before or after the treatment period. Gupta et al. [59] investigated the ameliorative effects of daily administration of UC-II to horses with osteoarthritis (OA). The researchers administered chicken-derived UC-II at a daily dosage of 160 mg for 150 days. The evaluation encompassed overall pain, pain after limb manipulation, physical examination, as well as liver and renal function. The findings revealed a significant reduction in joint pain in horses following the administration of UC-II, with a remarkable 91% decrease in overall pain and an 80% decrease in pain after limb manipulation. In comparison to horses in the glucosamine (5.4 g) and chondroitin (1.8 g) groups, where overall pain was 68% and pain after limb manipulation was 69%, the UC-II group exhibited superior results. In this study, clinical parameters were assessed, including liver function indicators, such as bilirubin, gamma-glutamyl transferase (GGT), and alkaline phosphatase (ALP), as well as renal function markers (e.g. blood urea nitrogen and creatinine), all of which remained unchanged, indicating good tolerability. In a recent study by Oentarini et al. [62], a total of 102 individuals with osteoarthritis participated in a 90-day clinical trial where they received a daily dose of 40 mg of UC-II. A control group was given microcrystalline cellulose as a placebo. The results demonstrated considerable improvement in the UC-II group, with a substantial 81.6% reduction in WOMAC scores, a 75.8% decrease in the Lequesne Functional Index, and a 67.9% decrease in VAS scores. Moreover, the study revealed no significant deviations in vital signs and clinical lab tests, when compared to the placebo group, indicating the safety and efficacy of UC-II in osteoarthritis treatment. Overall, the studies collectively suggest the potential therapeutic benefits of UC-II in mitigating OA symptoms in various animal models.
In addition to animal studies, UC-II has also demonstrated effectiveness in clinical trials. Cheng et al. [74] conducted a 12-week trial involving 101 patients aged 40–65 with OA of the knee. Participants were supplemented with 40 mg of chicken UC-II per day, and joint health improvement was assessed using the Western Ontario and McMaster Universities OA Index (WOMAC). The results revealed significant efficacy, with a 37.73% decrease in the total WOMAC score, a 39.6% decrease in the pain score, a 37.1% decrease in the stiffness score, and a 37.4% decrease in the dysfunction score. Vital signs and biomarkers remained within normal range. Compared to the glucosamine + chondroitin sulfate (G + C) group, the UC-II group exhibited more significant improvements in the five domains assessing quality of life, including mobility, self-care, activities of daily living, pain and discomfort, anxiety and depression, as well as VAS scores, suggesting that UC-II was more effective in enhancing overall quality of life.
Similarly, in a 90-day study, subjects supplemented with 40 mg of UC-II per day showed improvements in pain, joint stiffness, and quality of life, with a 54.2% increase in SF-12 physical domain scores, a 53.5% decrease in VAS scores, and a 17.6% decrease in WOMAC scores [73]. Fan et al. [54] assessed various OA indices and demonstrated a 35.3% decrease in the total WOMAC score, a 50% increase in gait speed, and improvements in other parameters, including knee flexibility. Serum markers of inflammatory and oxidative stress also showed positive changes, with increased superoxide dismutase (SOD) levels and decreased high-sensitivity C-reactive protein (hs-CRP) levels. A previous study on the effect of UC-II on knee flexibility in humans showed significant improvement after 24-week supplementation with 40 mg/d of chicken UC-II, indicating an increased range of motion in flexion and extension, compared to control group [72]. Numerous studies collectively support the efficacy of UC-II in treating OA, highlighting its superior effectiveness and good tolerability, compared to various dietary supplements, including chondroitin and glucosamine.
Studies on the therapeutic effects of UC-II in RA (Table 2) can be categorized based on the experimental animal model employed. The most frequently utilized models include adjuvant-induced arthritis (AIA) and collagen-induced arthritis (CIA). AIA is also recognized as Freund’s adjuvant arthritis, with complete Freund’s adjuvant (CFA) and incomplete Freund’s adjuvant (IFA) [76]. CFA was formulated by combining 1 mL of a mixture of liquid paraffin and anhydrous lanolin in a 2:1 or 6:4 ratio(v/v), autoclaved, and subsequently blended with inactivated, attenuated BCG or Mycobacterium tuberculosis. Those lacking inactivated BCG or M. tuberculosis are termed IFA. AIA models can be induced by dorsal subcutaneous injection of CFA or IFA mixtures [77, 78].
The AIA model is a classic and straightforward modeling method with a high success rate and cost-effectiveness [79]. It is widely employed for routine RA drug efficacy testing, particularly in the preclinical evaluation of non-steroidal anti-inflammatory drugs. For instance, Zhao et al. [68] investigated the therapeutic effects of orally administered chicken type II collagen on AIA arthritis in rats by administering 25 mg/kg of chicken UC-II through gavage over 2 weeks. The study assessed the incidence and severity of arthropathies, changes in peripheral blood T-cell subpopulations, and alterations in TNF-α levels in rats. The results indicated a significant improvement in arthropathy in the UC-II group compared to the arthritis model control group: the peripheral blood CD3+ T-cell level increased by 20.6%, the CD8+ T-cell level rose by 31.7%, and the CD4+ T-cell level decreased by 16.9%. Furthermore, the peripheral blood TNF-α level decreased to 1485.0 ng/L, significantly lower than that of the arthritis model group (1785.4 ng/L).
Similarly, Chen et al. [63] explored the efficacy of shark type II collagen (SC-II) in RA. Rats were orally administered SC-II (3 mg/mL) daily for 6 weeks. Evaluation parameters included delayed-type hypersensitivity reaction, circulating immune complexes, interleukin-10 serum levels, ankle joint histomorphology, and cartilage surface repair status. The findings revealed that SC-II could regulate the organism’s cellular immune function, restore delayed-type hypersensitivity reaction to normal levels, increase the number of rats with negative circulating immune complexes in the SC-II group, and bring the proportion of rats with negative circulating immune complexes close to that of the blank group. Additionally, the level of IL-10 significantly increased by 110.6% compared to the model control group. Microscopic observation of pathological sections of rat ankle joints showed a smooth surface of the ankle joint, and the synovial surface of the articular cartilage exhibited signs of repair.
The CIA model is currently regarded as the most suitable experimental animal model for studying RA [80, 81]. Despite its more classical preparation method, CIA remains the most widely used model in both clinical and basic research on RA due to its highly reproducible and manipulable advantages. Orie et al. [57] investigated the therapeutic effects of orally administered UC-II on CIA rats. They induced a rat CIA model by orally administering water-soluble chicken undenatured collagen at 1 mg/kg for 9 days. The therapeutic effects were assessed using arthritis index, hind limb histopathology, serum immune factor levels, and spleen tissue lymphocyte composition analysis. Results showed a significant reduction in the arthritis index in the experimental group from day 4 (P = 0.0091), surpassing the reduction in the Glu (glucosamine hydrochloride, 300 mg/kg) group (P = 0.0030). Histopathological examination revealed limited synovial infiltration, clear articular cartilage, reduced swelling, inflammation, and improved joint degeneration in the experimental group. Serum immune factor levels indicated a significant decrease in anti-CII antibody levels (P = 0.03) and IL-6 levels (P = 0.04), along with a significant increase in IL-2 levels (P = 0.04) in the experimental group. Peripheral blood CD4 + IL-10+ T-cell and CD4 + CD25+ T-cell levels were enhanced and proportionally increased. Reduced frequencies of CD4 + IL-10+ T cells in the inflamed synovium and peripheral blood of RA patients have been reported to contribute to the loss of tolerance. CD4 + CD25+ T cells, phenotypically similar to Treg cells, have been implicated in actively suppressing self-reactive T lymphocytes and maintaining self-tolerance in the control of chronic arthritis [57].
Lu et al. [67] investigated the immunotherapeutic effects of soluble chicken type II collagen (SCC-II) in CIA rats. They established a rat CIA model and administered 3 mg/kg of SCC-II for a total of 9 days. The study evaluated serum anti-type II collagen antibodies, IL-1 and TNF-α levels produced by peritoneal macrophages (PMΦ) and synoviocytes, secondary inflammatory responses, and immune organ coefficients in CIA rats. Results indicated that SCC-II significantly inhibited the delayed-type hypersensitivity reaction to SCC-II in the skin and reduced inflammation on the non-inflammatory side of the joints in CIA rats after 7 consecutive days of SCC-II administration. Compared to the positive control group of dexamethasone, which lost efficacy soon after discontinuation of treatment, the SCC-II group exhibited continued efficacy even 2 weeks after treatment cessation. Additionally, SCC-II suppressed the hypersecretion of IL-1 and TNF-α by PMΦ and synoviocytes, and restored body weight and immune organ indices.
Taken together, numerous studies have consistently demonstrated that oral administration of UC-II can effectively alleviate osteoarthritic diseases, especially OA and RA. Furthermore, it exhibits superior efficacy compared to current conventional drugs. Consequently, UC-II therapy may emerge as a preferred option for OA in the future.
6 Molecular mechanisms underlying the therapeutic effects of UC-II on joint diseases
UC-II can exert a therapeutic impact on joint inflammation by modulating local immunity. The oral administration of small amounts of UC-II suppresses immune responses directed against type II collagen in articular cartilage, employing a mechanism known as oral tolerance. This process is an integral component of the immune system, serving to distinguish potentially harmful foreign invaders in the gut from innocuous substances, such as dietary proteins and the commensal organisms constituting the microbiome [82]. Oral tolerance induction, characterized by an active non-response to orally administered antigens, has long been regarded as a promising strategy for treating chronic autoimmune diseases, including RA.
Numerous studies have been conducted to unravel the mechanisms of oral tolerance. The immune response to orally administered antigens occurs within in the gut-associated lymphoid tissue (GALT), comprising mesenteric lymph nodes and lymphoid tissue plaques surrounding the small intestine, known as Peyer’s patch (PP) [55]. PP contains specialized epithelial cells called microfold cells (M cells), which can facilitate the transport of antigens across the mucosal barrier. T cells engage with antigen-presenting cells in the PP, and subsequently, they are transferred to the mesenteric lymph nodes, traversing the peripheral immune system where they can exert their effects (Fig. 3).
In-depth investigations into the mechanisms of oral tolerance have predominantly centered on phenotypic changes in T cells and cytokines after the oral administration of antigens. Two discernible mechanisms of oral tolerance have been identified, namely active suppression and clonal incompetence or clonal clearance. It has been demonstrated that small antigen doses induce the suppression of immune cells, while larger doses lead to clonal incompetence or clonal clearance, with a pivotal role played by T helper cells [64]. Several factors influence the mechanism of action, including the form of the antigen, the oral antigen dosage, and the timing of administration. The principle underlying active inhibition is that small antigen doses act on regulatory Th2 and Th3 cells in gut mucosa-associated lymphoid tissues, such as PP, prompting the secretion of IL-4, IL-10, and TGF-β, thereby mediating active inhibition. The repeated administration of small antigen doses in gut-associated lymphoid tissues (GALT) has been shown to result in the generation of abundant regulatory T cells secreting TGF-β, IL-4, and IL-10. These cells are capable of suppressing Th1-mediated immune responses [64].
In contrast, the principle of clonally incompetent and clonally cleared cells involves large antigen doses acting on local Th1 and Th2 cells in mucosa-associated lymphoid tissues or being absorbed into the bloodstream to act on Th1 and Th2 cells in the thymus and spleen. The results in clonal anergy or deletion of cells, as evidenced by serum markers of inflammatory and oxidative stress showed positive changes with increased superoxide dismutase (SOD) levels and decreased high-sensitivity C-reactive protein (hs-CRP) levels.
Notably, when relatively high doses of antigen are orally administered, antigen-specific cells exhibit impaired functional properties, such as reduced production of Th1-type cytokines and limited proliferation of this cell type [65]. The oral administration of mega doses of antigen leads to the elimination of antigen-specific cells through apoptosis [63].
In addition to the previously mentioned mechanisms, it has been proposed that the suppression of arthritis through the oral administration of UC-II involves bystander suppression. Oral administration of UC-II induces the generation of regulatory T cells in gut-associated lymphoid tissues. These regulatory T cells migrated to lymphoid organs, such as mesenteric lymph nodes and the spleen, where they impeded the production of immune responses by effector cells. They inhibited Th1 through the release of inhibitory cytokines, such as TGF-β, IL-4, and IL-10, thereby exhibiting a systemic bystander effect in lesions characterized by polyarthritis. This bystander effect mechanism has been elucidated in studies where low doses of oral UC-II are transported via intestinal epithelial cells to potential immune cells in Peyer’s patches. This process converts initial T cells into regulatory T cells specifically activated by type II collagen. Activated regulatory T cells subsequently migrate from the gut-associated lymphoid tissue through the lymphatic system into the bloodstream. Upon recognizing the target in the articular cartilage, i.e., type II collagen, these regulatory T cells release anti-inflammatory cytokines, such as TGF-β, interleukin IL-4, and IL-10. These cytokines can inhibit the action of cells involved in the normal breakdown of collagen and other extracellular matrix proteins, thereby reducing joint inflammation and associated discomfort [55].
The bystander effect holds clinical significance in autoimmune diseases caused by multiple autoantigens, where the primary antigen is unclear or where all autoantigens are not yet identified. The induction of the bystander effect through oral administration of a specific antigen can suppress all inflammatory responses in the organ, where the autoantigen is located [83]. The oral administration of UC-II, proposed as a potential oral immunotherapy, is suggested to hold promise in inducing immune tolerance and thereby alleviating symptoms associated with immune-related diseases. However, immune tolerance encounters various challenges, and numerous factors may impact the efficacy of oral tolerance. The specifics of oral dosage, including the choice of dosage and the duration of the dosing cycle, have not been thoroughly elucidated in existing studies.
The impact of oral dose of UC-II on the induction of oral immune tolerance is a prominent area of research that has garnered significant attention. UC-II is believed to induce immune tolerance in the immune system, consequently mitigating symptoms of autoimmune diseases like arthritis. The critical aspect of dose selection in oral immunotherapy is underscored by evidence showing that different doses can lead to varying immune responses. Studies have indicated that there is no straightforward linear dose-response relationship, and effective minimum and maximum doses exist in oral tolerance experiments related to arthritis [66]. This observation may be reflected in the treatment mechanism of RA, with low doses inducing active cellular inhibition and high doses inducing clonal incompetence or clonal clearance.
However, there is no universally recommended dose, and a clear demarcation between low and high doses is lacking. Studies on RA treated with UC-II suggested that lower oral doses, such as 20 μg/kg, 0.1 mg/kg, and 3 mg/kg, may induce active cellular inhibition, placing 20 μg/kg to 3 mg/kg range into the category of low doses. Conversely, it has been shown that oral doses of 3 mg/kg and 25 mg/kg of UC-II treated RA through the mechanism of clonal incapacitation or clonal clearance. The same dose of 3 mg/kg has been shown to exhibit the oral administration of mega doses of antigen, leading to elimination of antigen-specific cells through apoptosis [63]. This discrepancy may be attributed to variations in the sources of UC-II, as it is derived from various animals such as chicken cattle, pig, sheep, fish [21, 84, 85], differing in amino acid composition and structure. These distinctions may influence absorption, bioactivity, biocompatibility, and tolerance in the human body.
Additionally, the dose (3 mg/kg) might be close to the cutoff point of the antigenic size dose. There are no experimental reports of oral doses exceeding 25 mg/kg, making the 3–25 mg/kg range an estimated large dose. In the study of UC-II treatment for OA, the dosage tends to be more uniform. Even a lower dose of UC-II (40 mg/d) has demonstrated a significant reduction in pain and improvement in joint function in arthritis patients. Overall, the selection of the oral UC-II dose is pivotal for inducing immune tolerance, and an accurate and effective dose must be explored through extensive experimentation.
Similarly, the duration of the oral administration cycle of UC-II holds crucial implications for its efficacy and the induction of immune tolerance. The immunomodulatory effects of UC-II require time. Following oral administration, UC-II requires a period to interact with the immune system and induce immune tolerance, ranging from as short as two to 3 weeks to as long as three to 5 months for full effectiveness. This time-dependent nature underscores the importance of not having a too brief cycle of oral UC-II administration, ensuring that the immune system has ample time to gradually adapt to UC-II and produce the desired immunomodulatory effects. However, it is essential to acknowledge that long-term treatment may raise potential safety concerns.
The side effects and safety of prolonged use of any drug garner significant attention. It has been shown that UC-II exhibits high safety in both short-term and long-term animal treatments. Acute oral toxicity tests showed no significant changes across all examined tissues, with oral LD50 in rats greater than 5000 mg/kg, causing only mild skin irritation in rabbits and minimal ocular irritation [70]. UC-II did not induce mutagenicity with or without metabolic activation. In existing long-term treatment studies, which can extend from three to 6 months, the safety and adverse reactions of prolonged drug use have garnered considerable attention. A 150-day study of UC-II for treating dog arthritis showed no adverse events after treatment, with no significant changes in the blood chemistry, body weight, heart rate, or respiratory rate [49]. Additionally, a 12-week study involving 101 knee osteoarthritis patients aged 40–65 showed that changes in vital signs and biomarkers for each individual remained within normal ranges [51]. Nevertheless, it is crucial to acknowledge the potential risks associated with long-term use, and close monitoring and evaluation are imperative for any extended treatment duration.
7 Future prospects
7.1 Enhancing efficiency in the extraction of UC-II
Research into the impact of UC-II on joint diseases is fundamentally rooted in the preservation of its non-denatured state. The significance of non-denaturing UC-II extraction extends beyond the conservation of its original structure and biological activity; it holds the potential for diverse applications. However, current extraction methods for type II collagen encounter challenges that hinder their widespread utilization.
Firstly, the extraction and purification process of type II collagen is intricate, demanding specialized equipment and technology. This complexity impedes the standardization of the extraction method, resulting in fluctuations in quality and purity. Moreover, type II collagen extracted using existing methods is highly susceptible to deformation, impacting its practical applicability.
Secondly, cost considerations are paramount. Presently, extraction methods for type II collagen, such as enzymatic hydrolysis and acidic extraction, are both costly and inefficient. These techniques typically demand substantial processing time and resources, thus hindering their scalability and commercial viability. Consequently, reducing production costs is essential to advance the widespread therapeutic use of type II collagen.
Therefore, future research should be directed towards the development of more efficient non-denaturing UC-II extraction techniques. This involves improving extraction yield and quality through the refinement of extraction conditions, enhancement of processes, and exploration of novel extraction agents. These efforts aim to maximize the retention of UC-II bioactivity and structural integrity, addressing the current limitations and paving the way for more widespread and cost-effective applications.
7.2 Impact of individual immune status on the establishment of immune tolerance
The establishment of oral tolerance is subject to various influencing factors. Beyond dosage and dosing cycles, the immune status of an individual emerges as a pivotal determinant in the immunological tolerance of oral UC-II. The immune status of an individual serves as a comprehensive reflection of their immune system, encompassing aspects such as the quantity and activity of immune cells, the levels of immune factors, and the regulatory capacity of the immune system.
The quantity and activity of an individual’s immune cells significantly influence the response to UC-II therapy. Patients with varying levels of immune system activity may experience different treatment outcomes. Those with an overactive immune system may require longer cycles to mitigate excessive immune responses, while immunosuppressed or immunocompromised patients may exhibit limited effects from UC-II.
Additionally, differences in immune factor levels can impact immune tolerance to UC-II [86, 87]. Variances in immune factor levels among patients may influence the immunomodulatory effects of UC-II. Finally, the degree of immune system regulation within an individual is a crucial factor in oral UC-II immunological tolerance. While normal immune system regulation is fundamental for maintaining immune homeostasis and tolerance, dysregulation may result in an inappropriate immune system response to UC-II [88].
Overall, an individual’s immune status plays a central role in immune tolerance to oral UC-II. A comprehensive understanding of the patient’s immune status, considering individual differences, is imperative before initiating UC-II treatment to determine the most suitable therapeutic strategy. Future studies should delve deeper into the relationship between immune status and oral UC-II therapy, aiming for a more precise and individualized therapeutic approach to enhance outcomes for patients with joint and immune-related diseases, ultimately alleviating their pain and discomfort.
The intricate relationship between oral dose, oral cycle, and individual immune status adds a layer of complexity to UC-II therapy. Determining the appropriate oral dose and cycle is paramount in treatment, often requiring customization based on the patient’s unique characteristics and the severity of disease. In certain instances, a higher oral dose may trigger an immune response and establish immune tolerance, especially in patients with dynamic immune systems. Conversely, for patients with a relatively stable immune status, a lower oral dose might suffice to achieve the desired therapeutic effect.
Moreover, a robust relationship exists between the dose and the oral dosing cycle. Higher doses of oral therapy may necessitate shorter cycles for optimal results, whereas lower doses may require longer cycles. This nuanced decision-making process should be guided by healthcare professionals, taking into account the patient’s biological response and individual needs.
Taken together, the therapeutic effectiveness of oral UC-II hinges on the complex interplay of oral dose, oral cycle, and individual immune status. When crafting a personalized treatment strategy, healthcare professionals must holistically consider these factors to ensure optimal therapeutic outcomes. To deepen our understanding of these relationships, future studies should delve further, providing more precise treatment guidelines to maximize efficacy and immune tolerance in patients with joint diseases and immune-related conditions.
7.3 Enhancing the clinical evidence for UC-II in the treatment of arthritis
While UC-II is acknowledged as a potential therapy for arthritis treatment, previous clinical trials have hinted at its potential to improve arthritis symptoms. Despite these preliminary findings, a significant scientific challenge remains due to the limited clinical evidence required to establish its efficacy and safety.
Arthritis has become a widespread health concern, significantly impacting patients’ quality of life, prompting an urgent need for more effective treatments. Despite the considerable interest in UC-II, the present study faces limitations, including restricted sample size, methodological flaws, and insufficient short-term follow-up. In particular, studies of UC-II for OA have been hindered by limited sample sizes, methodological constraints, and a lack of deeper molecular-level indicators, such as changes in marker factor levels. Clinical trials for UC-II in RA have primarily been conducted in specific animal models, and controversies surround the accuracy of these models. Furthermore, the therapeutic mechanism of UC-II remains incompletely defined, contributing to uncertainty about its precise role in joint disease treatment.
Clinical evidence for UC-II remains relatively sparse compared to traditional arthritis treatments like non-steroidal anti-inflammatory drugs (NSAIDs) and disease-modifying anti-rheumatic drugs (DMARDs). While conventional treatments are well-established in terms of efficacy and safety, UC-II is still in the early stages of research. This scarcity of clinical evidence not only impacts healthcare professionals’ clinical decision-making but also creates uncertainty for patients. To address this issue, more large-scale, high-quality clinical trials are necessary to establish the role, safety, and optimal use of UC-II in joint disease treatment. Studies with extended follow-up periods and practical clinical applications can offer comprehensive evidence to guide clinicians and patients in making informed treatment choices, ultimately maximizing the quality of life for individuals with joint disease. Future studies should intensify efforts to unveil the potential benefits of UC-II and determine its effective role in arthritis treatment.
7.4 Synergistic effects of UC-II in joint health and arthritis treatment
The remarkable efficacy of UC-II in promoting joint health and treating arthritis has prompted extensive research. Several studies have aimed to showcase the superior therapeutic effects of UC-II compared to traditional joint health medications like glucosamine and chondroitin sulfate. Traditional medications, such as glucosamine and chondroitin sulfate, are often perceived as joint preservatives, offering nutritional support essential for maintaining and repairing joint cartilage. These drugs primarily work to slow joint degeneration and supply vital components to improve joint lubrication within the synovial fluid.
In contrast, the therapeutic mechanism of UC-II is notably broader and more complex. Apart from supplying essential components for joint cartilage, UC-II possesses unique immunomodulatory properties. It plays a crucial role in balancing immune system activity, suppressing excessive immune responses, reducing inflammation, and fostering immune tolerance between the immune system and articular cartilage. This immunomodulatory effect positions UC-II as a promising option for treating immune-related diseases, e.g. RA.
The distinctions in therapeutic mechanisms between UC-II and conventional drugs make them complementary in various ways. While traditional drugs focus on joint care and support, UC-II targets the abnormally active immune system through its immunomodulatory effects, alleviating symptoms of arthritis and immune-related diseases. Considering these distinct therapeutic mechanisms, combining UC-II with conventional drugs holds the potential for synergistic effects in the treatment process. Combination therapy strategies can harness the advantages of different drugs, offering more comprehensive joint care and therapeutic effects, ultimately enhancing patients’ quality of life. However, no trials have been reported to date, and further research is needed to determine the optimal effects of multiple drug combinations. This involves studying the interactions between different drugs, establishing the ideal drug combination and dosage, and exploring the optimal timing of treatment.
8 Conclusions
UC-II is crucial for joint cartilage maintenance, with an imbalance in its synthesis and degradation leading to OA and RA. It showed significant therapeutic effects when orally administered, including pain reduction, improved joint mobility, and decreased inflammation in OA and RA patients. The immune regulatory functions help suppress autoimmune responses in arthritis. However, challenges in extraction processes and limited clinical trials on oral UC-II highlight the need for non-denaturing extraction methods and further research on dosage optimization and treatment duration to fully ascertain its efficacy and safety.
Availability of data and materials
The manuscript has no associated data.
Abbreviations
- UC-II:
-
Undenatured type II collagen
- DES:
-
Deep eutectic solvents
- IL-1:
-
Interleukin-1
- IL-6:
-
Interleukin-6
- TNF-α:
-
Tumor necrosis factor-α
- MMPs:
-
Matrix metalloproteinases
- ADAMTS-4:
-
A Disintegrin and Metalloproteinase with Thrombospondin Motifs-4
- ADAMTS-5:
-
A Disintegrin and Metalloproteinase with Thrombospondin Motifs-5
- OA:
-
Osteoarthritis
- RA:
-
Rheumatoid arthritis
- MIA:
-
Monoiodoacetate-induced arthritis
- CIA:
-
Collagen-induced arthritis
- AIA:
-
Adjuvant-induced arthritis
- MHC-II:
-
Major histocompatibility complex II
- WOMAC:
-
Western ontario and mcmaster universities osteoarthritis index
- SOD:
-
Superoxide dismutase
- hs-CRP:
-
High-sensitivity C-reactive protein
- CFA:
-
Complete Freund’s adjuvant
- IFA:
-
Incomplete Freund’s adjuvant
- SC-II:
-
Shark type II collagen
- SCC-II:
-
Soluble chicken type II collagen
- PMΦ:
-
Peritoneal macrophages
- GALT:
-
Gut-associated lymphoid tissue
- PP:
-
Peyer’s patch
- M cells:
-
Microfold cells
References
Steinmetz JD, Culbreth GT, Haile LM, Rafferty Q, Lo J, Fukutaki KG, et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5(9):e508–22.
Zhu X, Chan YT, Yung PSH, Tuan RS, Jiang Y. Subchondral bone remodeling: a therapeutic target for osteoarthritis. Front Cell Dev Biol. 2021;8.
Chen T, Weng W, Liu Y, Aspera-Werz RH, Nüssler AK, Xu J. Update on novel non-operative treatment for osteoarthritis: current status and future trends. Front Pharmacol. 2021;12.
Jain K, Mehra A, Mehta K, Dsouza L, Kumar R. Role of undenatured collagen type II and Aflapin combination in the management of osteoarthritis: a review. Int J Res Ortho. 2021;7:885.
Xu R, Wu J, Zheng L, Zhao M. Undenatured type II collagen and its role in improving osteoarthritis. Ageing Res Rev. 2023;91:102080.
Sadigursky D, Sanches MM, Garcia NM, de Oliveira CM, Matos MA. Effectiveness of the use of non-hydrolysed type II collagen in the treatment of osteoarthritis: a systematic review and meta-analysis. Braz J Health Rev. 2023;6(1):1649–60.
Avila Rodríguez MI, Rodríguez Barroso LG, Sánchez ML. Collagen: a review on its sources and potential cosmetic applications. J Cosmet Dermatol. 2018;17(1):20–6.
Wang C, Dai Y, Yang J, Chou G, Wang C, Wang Z. Treatment with total alkaloids from Radix Linderae reduces inflammation and joint destruction in type II collagen-induced model for rheumatoid arthritis. J Ethnopharmacol. 2007;111(2):322–8.
Cai X, Zhou H, Wong YF, Xie Y, Liu ZQ, Jiang ZH, Bian ZX, Xu HX, Liu L. Suppression of the onset and progression of collagen-induced arthritis in rats by QFGJS, a preparation from an anti-arthritic Chinese herbal formula. J Ethnopharmacol. 2007;110(1):39–48.
Yang S, Wang D, Gao YX. Progress in the study of non-denatured type II collagen. Food Sci. 2021;42(17):343–9.
Xue F, Zhang C, He Z, Ding L, Xiao H. Analysis of critical molecules and signaling pathways in osteoarthritis and rheumatoid arthritis. Mol Med Rep. 2013;7(2):603–7.
Cao H, Xu SY. Purification and characterization of type II collagen from chick sternal cartilage. Food Chem. 2008;108(2):439–45.
Kambic HE, McDevitt CA. Spatial organization of types I and II collagen in the canine meniscus. J Orthop Res. 2005;23(1):142–9.
Kumar P, Bansal P, Rajnish RK, Sharma S, Dhillon MS, Patel S, Kumar V. Efficacy of undenatured collagen in knee osteoarthritis: review of the literature with limited meta-analysis. Am J Transl Res. 2023;15(9):5545–55.
Elango J, Zamora-Ledezma C, Ge BL, Hou CY, Pan ZL, Bao B, et al. Paradoxical duel role of collagen in rheumatoid arthritis: cause of inflammation and treatment. Bioeng-Basel. 2022;9(7).
Akram AN, Zhang C. Extraction of collagen-II with pepsin and ultrasound treatment from chicken sternal cartilage; physicochemical and functional properties. Ultrason Sonochem. 2020;64:105053.
Gu HC, Hu JB, Ding ZS, Fan YS, Ding XH. Extraction, purification and identification of type II collagen from Agkistrodon acutus. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J Chin Mater Med. 2013;38(21):3672–5.
Cui P, Shao T, Liu W, Li M, Yu M, Zhao W, Song Y, Ding Y, Liu J. Advanced review on type II collagen and peptide: preparation, functional activities and food industry application. Crit Rev Food Sci Nutr. 2023:1–18. https://www.tandfonline.com/doi/full/10.1080/10408398.2023.2236699?scroll=top&needAccess=true.
Yuan C, Hui C, Fei X, Jinsong Y, Min Y. Preparation Technology of Collagen Type II from chicken sternal cartilage. Food Sci. 2015;36(6):24–8.
Maity PP, Dutta D, Ganguly S, Kapat K, Dixit K, Chowdhury AR, et al. Isolation and mass spectrometry based hydroxyproline mapping of type II collagen derived from Capra hircus ear cartilage. Commun Biol. 2019;2.
Che S, Du F, Liu CY, Li BF. Structural analysis of type II collagen from cartilage of Chinese sturgeon. Food Ind Sci Technol. 2018;39(04):60–3.
Zhou Y, Wu W, Zhang N, Soladoye OP, Zhang Y, Fu Y. Deep eutectic solvents as new media for green extraction of food proteins: opportunity and challenges. Food Res Int. 2022;161:111842.
Jiao X, Wang G, Li J, Wang X, Meng G, Cui J. A comparative study of methods for extracting protein from sesame residue. Biotechnol Bull. 2021;37(4):273–81.
Wahlstrom R, Rommi K, Willberg-Keyrilainen P, Ercili-Cura D, Holopainen-Mantila U, Hiltunen J, Makinen O, Nygren H, Mikkelson A, Kuutti L. High yield protein extraction from brewer’s spent grain with novel carboxylate salt - urea aqueous deep eutectic solvents. Chemistryselect. 2017;2(29):9355–63.
Liu RL, Yu P, Ge XL, Bai XF, Li XQ, Fu Q. Establishment of an aqueous peg 200-based deep eutectic solvent extraction and enrichment method for pumpkin (cucurbita moschata) seed protein. Food Anal Methods. 2017;10(6):1669–80.
Bai C, Wei Q, Ren X. Selective extraction of collagen peptides with high purity from cod skins by deep eutectic solvents. ACS Sustain Chem Eng. 2017;5(8):7220–7.
Pang J, Sha X, Chao Y, Chen G, Han C, Zhu W, Li H, Zhang Q. Green aqueous biphasic systems containing deep eutectic solvents and sodium salts for the extraction of protein. RSC Adv. 2017;7(78):49361–7.
Li N, Wang Y, Xu K, Huang Y, Wen Q, Ding X. Development of green betaine-based deep eutectic solvent aqueous two-phase system for the extraction of protein. Talanta. 2016;152:23–32.
López R, D’Amato R, Trabalza-Marinucci M, Regni L, Proetti P, Maratta A, Cerutti S, Pacheco P. Green and simple extraction of free seleno-amino acids from powdered and lyophilized milk samples with natural deep eutectic solvents. Food Chem. 2020;326:126965.
Li G, Row KH. Air assisted dispersive liquid-liquid microextraction (aa-dllme) using hydrophilic-hydrophobic deep eutectic solvents for the isolation of monosaccharides and amino acids from kelp. Anal Lett. 2020;53(2):188–202.
Chao Y, Ding H, Pang J, Jin Y, Li X, Chang H, Jiang W, Chen G, Han C, Zhu W. High efficient extraction of tryptophan using deep eutectic solvent-based aqueous biphasic systems.Indian. J Pharm Sci. 2019;81(3):448–55.
Balaraman HB, Rathnasamy SK. Selective purification of protease from ginger and sodom apple by ultrasound assisted liquid-liquid microextraction using natural deep eutectic solvent. Microchem J. 2019;150:104132.
Marchel M, Coroadinha AS, Marrucho IM. Novel acidic deep eutectic solvent-based aqueous biphasic systems for efficient extraction of pepsin. ACS Sustain Chem Eng. 2020;8(33):12400–8.
Xu P, Wang Y, Chen J, Wei X, Xu W, Ni R, Meng J, Zhou Y. Development of deep eutectic solvent-based aqueous biphasic system for the extraction of lysozyme. Talanta. 2019;202:1–10.
Meechai R. A literature review of undenatured type ii collagen (uc-ii) in joint health and disease. Int J Curr Sci Res Rev. 2022;5(10).
Martel-Pelletier J, Boileau C, Pelletier J-P, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22(2):351–84.
Gentile P, De Angelis B, Agovino A, Orlandi F, Migner A, Di Pasquali C, Cervelli V. Use of platelet rich plasma and hyaluronic acid in the treatment of complications of achilles tendon reconstruction. World J Plast Surg. 2016;5(2):124–32.
Deng Z, Lin Z, Zhong Q, Lu M, Fang H, Liu J, Duan L, Chen L, Wang L, Wang D, Li W. Interleukin 1 beta-induced chloride currents are important in osteoarthritis onset: an in vitro study. Acta Biochim Biophys Sin. 2021;53(4):400–9.
Araki Y, Mimura T. Matrix metalloproteinase gene activation resulting from disordred epigenetic mechanisms in rheumatoid arthritis. Int J Mol Sci. 2017;18(5):905.
Agere SA, Akhtar N, Watson JM, Ahmed S. Rantes/ccl5 induces collagen degradation by activating mmp-1 and mmp-13 expression in human rheumatoid arthritis synovial fibroblasts. Front Immunol. 2017;8.
Shiozawa K, Yamane T, Murata M, Yoshihara R, Tsumiyama K, Imura S, Shiozawa S. MMP-3 as a predictor for structural remission in RA patients treated with MTX monotherapy. Arthritis Res Ther. 2016;18(1):55.
Bagi C, Berryman E, Teo S, Lane N. Oral administration of undenatured native chicken type II collagen (UC-II) diminished deterioration of articular cartilage in a rat model of osteoarthritis (OA). Osteoarthr Cartil. 2017;25(12):2080–90.
Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–707.
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–59.
Marita C, Emma S, Damian H, Sandra N, Ilana A, Marlene F, Lisa B, Sean W, Francis G, Catherine LH, Laura LL, Graeme J, Flavia C, Richard O, Theo V, Rachelle B, Anthony W, Lyn M. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323.
Lin Y-J, Anzaghe M, Schülke S. Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells. 2020;9(4):880.
Leblond A, Allanore Y, Avouac J. Targeting synovial neoangiogenesis in rheumatoid arthritis. Autoimmun Rev. 2017;16(6):594–601.
Weyand CM, Goronzy JJ. The immunology of rheumatoid arthritis. Nat Immunol. 2021;22(1):10–8.
Chen S-J, Lin G-J, Chen J-W, Wang K-C, Tien C-H, Hu C-F, et al. Immunopathogenic mechanisms and novel immune-modulated therapies in rheumatoid arthritis. Int J Mol Sci. 2019;20.
Nygaard G, Firestein GS. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat Rev Rheumatol. 2020;16(6):316–33.
Tu J, Wang X, Gong X, Hong W, Han D, Fang Y, Guo Y, Wei W. Synovial macrophages in rheumatoid arthritis: the past, present, and future. Mediat Inflamm. 2020;2020:1583647.
Sugihara T, Harigai M. Targeting low disease activity in elderly-onset rheumatoid arthritis: current and future roles of biological disease-modifying antirheumatic drugs. Drugs Aging. 2016;33(2):97–107.
Gupta RC, Canerdy TD, Lindley J, Konemann M, Minniear J, Carroll BA, Hendrick C, Goad JT, Rohde K, Doss R, Bagchi M, Bagchi D. Comparative therapeutic efficacy and safety of type-II collagen (uc-II), glucosamine and chondroitin in arthritic dogs: pain evaluation by ground force plate. J Anim Physiol Anim Nutr. 2012;96(5):770–7.
Fan R, Kang J, Hao Y, Liu X, Hu J, Mao R, Liu R, Zhu N, Xu M, Li Y. Undenatured type II collagen prevents and treats osteoarthritis and motor function degradation in T2DM patients and db/db mice. Food Funct. 2021;12(10):4373–91.
Orhan C, Juturu V, Sahin E, Tuzcu M, Ozercan IH, Durmus AS, et al. Undenatured type II collagen ameliorates inflammatory responses and articular cartilage damage in the rat model of osteoarthritis. Frontiers in veterinary. Science. 2021;8.
Yaguang Z, Hongyu Z, Aiqing L, Shuang L, Guoqing Z, Haiyan W. The effects of Undenatured type II collagen on inflammatory mediators and oxidative stress in an osteoarthritis rat model. IOP Conf Ser. 2020;598.
Yoshinari O, Moriyama H, Shiojima Y. An overview of a novel, water-soluble undenatured type II collagen (NEXT-II). J Am Coll Nutr. 2015;34(3):255–62.
Peal A, D’Altilio M, Simms C, Alvey M, Gupta RC, Goad JT, Canerdy TD, Bagchi M, Bagchi D. Therapeutic efficacy and safety of undenatured type-II collagen (UC-II) alone or in combination with (−)-hydroxycitric acid and chromemate in arthritic dogs. J Vet Pharmacol Ther. 2007;30(3):275–8.
Gupta RC, Canerdy TD, Skaggs P, Stocker A, Zyrkowski G, Burke R, Wegford K, Goad JT, Rohde K, Barnett D, DeWees W. Therapeutic efficacy of undenatured type-II collagen (UC-II) in comparison to glucosamine and chondroitin in arthritic horses. J Vet Pharmacol Ther. 2009;32(6):577–84.
Lugo JP, Saiyed ZM, Lau FC, Molina JP, Pakdaman MN, Shamie AN, Udani JK. Undenatured type II collagen (UC-II®) for joint support: a randomized, double-blind, placebo-controlled study in healthy volunteers. J Int Soc Sports Nutr. 2013;10(1):48.
Crowley DC, Lau FC, Sharma P, Evans M, Guthrie N, Bagchi M, Bagchi D, Dey DK, Raychaudhuri SP. Safety and efficacy of undenatured type II collagen in the treatment of osteoarthritis of the knee: a clinical trial. Int J Med Sci. 2009;6(6):312–21.
Tjandra O, Gunawan S, Johan J, Lie FF, Rumawas ME, Limarta A. Efficacy and safety of Undenatured type II collagen in the treatment of osteoarthritis of the knee: a randomized, double-blind, Placebo-controlled Trial. Indones Biomed J. 2023;15(3):277–86.
Chen LJ, Bao B, Bu YS, Wang N, Xie J, Wu WH. Gavage-induced immune tolerance with jaws type II collagen for the treatment of rheumatoid arthritis. Pharm Serv Res. 2013;13(4):265–9.
Zhao Y, Cai MY, Wang X, Wei DP. Effect of oral type II collagen on TGF-β and IL-12 in adjuvant arthritis. J Guiyang Med Coll. 2010;35(1):50–2.
Zhao Y, Cai MY, Wang X, Wei DP. Effect of type II collagen on immune function in arthritic rats. J Clin Exp Med. 2009;8(8):6–7.
Wang X, Wei DP, Cai MY, Zhao Y. Effects of oral administration of chicken type II collagen on arthropathy and autoimmune function in rats with adjuvant arthritis (in English). China Clin Rehabil. 2004;24:5162–3.
Lu YX, Chen MZ. Immunotherapeutic effects of soluble chicken type II collagen on collagenous arthritis in rats. Chin J Pharmacol Toxicol. 2004;5:365–70.
Wang NP, Shen YX, Wang H, Zhang LL, Yue L, Wei W. Therapeutic effect of collagen type II oral spray on collagenous arthritis in mice. Chin J Pharmacol Toxicol. 2002;06:449–53.
Qian XJ, Zhao WM, Hu YX, Jiao Y, Du W, Wei W. Oral administration of type II collagen induces immune tolerance in rats with adjuvant arthritis. Chinese. J Rheumatol. 2002;5:331-334–87.
Wang L, Han XF, Ma BL, Zhang JY, Bai J, Hao AM. Oral administration of chicken type II collagen for the treatment of experimental rheumatoid arthritis in rats. Shanghai J Immunol. 2001;4:226–8.
Stabile M, Lacitignola L, Samarelli R, Fiorentino M, Crovace A, Staffieri F. Evaluation of clinical efficacy of undenatured type II collagen supplementation compared to cimicoxib and their association in dogs affected by natural occurring osteoarthritis. Res Vet Sci. 2022;151:27–35.
Schoen C, Knaub K, Alt W, Durkee S, Saiyed Z, Juturu V. UC-II undenatured type II collagen for knee joint flexibility: a multicenter, randomized, double-blind, placebo-controlled clinical study. J Integr Complement Med. 2022;28(6):540–8.
Sadigursky D, Stolze Magnavita VF, Couto De Sa CK, Monteiro HDS, Melro Braghiroli OF, Almeida Matos MA. Undenatured collagen type II for the treatment of osteoarthritis of the knee. Acta Ortop Bras. 2022;30(2).
Luo C, Su W, Song Y, Srivastava S. Efficacy and safety of native type II collagen in modulating knee osteoarthritis symptoms: a randomised, double-blind, placebo-controlled trial. J Exp Orthop. 2022;9(1).
Li Z, Bai X, Fan Y, Jia Q, Zhang H, Hou H. Structure of type II collagen from sturgeon cartilage and its effect on adjuvant-induced rheumatoid arthritis in rats. Food Funct. 2022;13(11):6152–65.
Galhom RA, Korayem HE, Ibrahim MA, Abd-Eltawab Tammam A, Khalifa MM, Rashwan EK, et al. Urine-derived stem cells versus their lysate in ameliorating erectile dysfunction in a rat model of type 2 diabetes. Front Physiol. 2022;13.
Yan F, Li H, Zhong Z, Zhou M, Lin Y, Tang C, Li C. Co-delivery of prednisolone and curcumin in human serum albumin nanoparticles for effective treatment of rheumatoid arthritis. Int J Nanomedicine. 2019;14:9113–25.
Kumar A, Dhaliwal N, Dhaliwal J, Dharavath RN, Chopra K. Astaxanthin attenuates oxidative stress and inflammatory responses in complete Freund-adjuvant-induced arthritis in rats. Pharmacol Rep. 2020;72(1):104–14.
Rosloniec EF, Whittington K, Proslovsky A, Brand DD. Collagen-induced arthritis mouse model Current Protocols. 2021;1(12):e313.
Bessis N, Decker P, Assier E, Semerano L, Boissier MC. Arthritis models: usefulness and interpretation. Semin Immunopathol. 2017;39(4):469–86.
Jung SM, Lee J, Baek SY, Lee J, Jang SG, Hong S-M, Park J-S, Cho M-L, Park S-H, Kwok S-K. Ethyl pyruvate ameliorates inflammatory arthritis in mice. Int Immunopharmacol. 2017;52:333–41.
Favazzo LJ, Hendesi H, Villani DA, Soniwala S, Dar QA, Schott EM, Gill SR, Zuscik MJ. The gut microbiome-joint connection: implications in osteoarthritis. Curr Opin Rheumatol. 2020;32(1):92–101.
Park KS, Park MJ, Cho ML, Kwok SK, Ju JH, Ko HJ, Park SH, Kim HY. Type II collagen oral tolerance; mechanism and role in collagen-induced arthritis and rheumatoid arthritis. Mod Rheumatol. 2009;19(6):581–9.
Song RR, Bao B, Bu YS, Wang YX, Chen LJ, Wu WH. Physicochemical properties of blue shark cartilage type II collagen. Food Sci. 2013;34(09):24–7.
Guo XY, He L, Wei XJ, Wang NP. Extraction method and structural analysis of squid cartilage type II collagen. Adv Biomed Eng. 2016;37(1):1–5.
Yoshinari O, Marone PA, Moriyama H, Bagchi M, Shiojima Y. Safety and toxicological evaluation of a novel, water-soluble undenatured type II collagen. Toxicol Mech Methods. 2013;23(7):491–9.
Bagchi D, Misner B, Bagchi M, Kothari SC, Downs BW, Fafard RD, et al. Effects of orally administered undenatured type II collagen against arthritic inflammatory diseases: a mechanistic exploration. Int J Clin Pharmacol Res. 2002, 101;22(3–4):–110.
Bakilan F, Armagan O, Ozgen M, Tascioglu F, Bolluk O, Alatas O. Effects of native type ii collagen treatment on knee osteoarthritis: a randomized controlled trial. Eur J Med. 2016;48(2):95–101.
Acknowledgements
This work was funded by National Natural Science Foundation of China (32101980), Natural Science Foundation of Chongqing (CSTB2023NSCQ-MSX0304), Science and Technology Research Program of Chongqing Municipal Education Commission (KJQN202100225), National Center of Technology Innovation for Pigs (NCTIP-XD/B02) and Innovation Training Program for College Students at Southwest University (202310635054).
Author information
Authors and Affiliations
Contributions
Yuhao Zhou, Writing-original draft; Yuer Zhang: Writing-original draft; Hongjie Dai: Writing-Review & Editing; Yuhao Zhang: Writing-Review & Editing; Yu Fu: Conceptualization-Lead, Funding acquisition-Lead, Supervision-Lead, Writing-Review & Editing.
Corresponding author
Ethics declarations
Consent for publication
All authors have given approval to the final version of the manuscript.
Competing interests
The authors declare no competing financial interests in this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, Y., Zhang, Y., Dai, H. et al. The potential of undenatured type II collagen against arthritis: a review. Collagen & Leather 6, 17 (2024). https://doi.org/10.1186/s42825-024-00160-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42825-024-00160-y