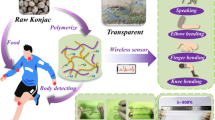
Abstract
The construction of biomass-based conductive hydrogel e-skins with high mechanical properties is the research hotspot and difficulty in the field of biomass materials. Traditional collagen-based conductive hydrogels, constructed by the typical “bottom–up” strategy, normally have the incompatible problem between high mechanical property and high collagen content, and the extraction of collagen is often necessary. To solve these problems, inspired by the high mechanical properties and high collagen content of animal skins, this work proposed a “top–down” construction strategy, in which the extraction of collagen was unnecessary and the skin collagen skeleton (SCS) with the 3D network structure woven by natural collagen fibers in goatskin was preserved and used as the basic framework of hydrogel. Following a four-step route, namely, pretreatment → soaking in AgNPs (silver nanoparticles) solution → soaking in the mixed solution containing HEA (2-hydroxyethyl methacrylate) and AlCl3 → polymerization, this work successfully achieved the fabrication of a new skin-based conductive hydrogel e-skin with high mechanical properties (tensile strength of 2.97 MPa, toughness of 6.23 MJ·m−3 and breaking elongation of 428%) by using goatskin as raw material. The developed skin hydrogel (called PH@Ag) possessed a unique structure with the collagen fibers encapsulated by PHEA, and exhibited satisfactory adhesion, considerable antibacterial property, cytocompatibility, conductivity (3.06 S·m−1) and sensing sensitivity (the maximum gauge factor of 5.51). The PH@Ag e-skin could serve as strain sensors to accurately monitor and recognize all kinds of human motions such as swallowing, frowning, walking, and so on, and thus is anticipated to have considerable application prospect in many fields including flexible wearable electronic devices, health and motion monitoring.
Graphical abstract

Similar content being viewed by others
1 Introduction
Conductive hydrogels, “soft” and “wet” functional materials with three-dimensional (3D) network structure formed by physical or (and) chemical crosslinking of synthetic or natural polymers [1, 2], have attracted considerable research interests owing to their extensive application prospects and demands in the fields of flexible wearable electronic devices [3, 4], electronic skin [5, 6], health monitoring [7, 8], human–computer interaction [9], artificial intelligence [10], soft robots [11], biosensors [12], supercapacitors [13], drug delivery [14], etc. Currently, it is a general trend to adopt natural biomass-based polymers with good biocompatibility and biodegradability (such as protein, polysaccharide, etc.) as raw materials to prepare conductive hydrogels with high and stable mechanical properties [15, 16].
Collagen, often extracted from animal skins, possesses good biocompatibility, biodegradability, low immunogenicity and other merits [17, 18], and has the innate advantages of constructing conductive hydrogels as flexible sensing materials [19,20,21]. Even so, collagen-based conductive hydrogels as flexible sensing materials have very limited application performance and range [22,23,24,25]. This is mainly because the shortcomings of collagen-based conductive hydrogels, poor mechanical properties, have not been completely solved, and it is difficult for them to possess both high mechanical properties and high collagen content, and so it is challenging to meet the application requirements of flexible sensing materials [26, 27]. Furthermore, the complex and low efficiency of collagen extraction and the poor water solubility of collagen lead to the harsh preparation conditions of hydrogels, which further aggravates this prominent problem. For example, Zhang [28, 29] and Gao [30] reported the fabrication of collagen-based conductive composite hydrogels and their application of monitoring human movement as a flexible strain sensors. These collagen-based conductive hydrogels possessed a variety of functions, such as self-healing, adhesion and stimuli-responsiveness, and exhibited good tensile property (900–1050% of elongation at break), but poor mechanical strength (only 88–123 kPa of tensile strength). Compared with synthetic polymer (such as polyvinyl alcohol) conductive hydrogels, their mechanical properties still have a big gap. Therefore, it is very important and urgent to study the construction of collagen-based conductive hydrogels with high mechanical properties (including high stretchability, high strength and high toughness) and high collagen content, which has important practical significance for promoting the high-value utilization of collagen-based functional materials.
At present, the preparation method of collagen-based conductive hydrogels still follows the traditional “bottom–up” strategy typically used in the preparation of synthetic polymer conductive hydrogels [22,23,24,25,26,27,28,29,30]. First, skin collagen skeleton (SCS) was obtained through the pretreatment of animal skin in which the non-collagen components are removed; collagen is then extracted from SCS by using acid, enzyme or other methods; the collagen-based conductive hydrogels with 3D cross-linking network structures of collagen molecules (or fibers) were finally constructed by using chemical and (or) cross-linking treatments [22,23,24,25,26,27,28,29,30]. Notably, the natural 3D network structure of collagen fibers in animal skin is completely destroyed during the extraction process of collagen [17,18,19,20,21]. However, the stability of the re-constructed 3D network structures of collagen molecules (or fibers) in the resulting collagen-based conductive hydrogels is far less than that of the natural 3D network structure of collagen fibers in animal skins [26, 28,29,30], which is the main reason for the poor mechanical properties of collagen-based conductive hydrogels. On the contrary, if collagen was not extracted from SCS and the natural 3D woven network structure of collagen fibers in SCS was retained, and the SCS was further used as the basic framework of hydrogel, can collagen-based conductive hydrogels with high mechanical properties and high collagen content be constructed?
To confirm this possibility, inspired by the high mechanical properties and high collagen content of animal skins, this work put forward a new “top–down” strategy (Scheme 1a) in which skin-based conductive hydrogels with high mechanical properties and high collagen content were successfully fabricated by using goatskin as raw material. Concretely, goatskin was firstly pretreated following the typical leather-making preparation process to produce the pickled goatskin (also called naked goatskin) as SCS. The soaking treatment in the AgNPs (silver nanoparticles) solution was then conducted to endow SCS with good antimicrobial property. The resulting SCS (called P@Ag) was impregnated in the mixed solution containing 2-hydroxyethyl methacrylate (HEA) monomer and AlCl3 as tanning agent, and HEA and Al3+ ions permeated and distributed evenly among collagen fibers of SCS after the soaking treatment. Finally, the in situ polymerization of HEA was conducted to provide the skin-based conductive hydrogel (called PH@Ag) containing SCS as the basic framework.
In this hydrogel system, the key structure of PHEA-encapsulated collagen fibers was formed, and the Al3+ coordination allowed stronger binding interactions between collagen fibers-collagen fibers, PHEA-PHEA, and collagen fibers-PHEA. The effective combination of goatskin collagen skeleton (GSCS) and polymeric hydrogel PHEA not only gave PH@Ag strong tensile strength (2.97 MPa), fatigue resistance (100 times tensile cycle), and toughness (6.23 MJ·m−3), but also provided excellent stretchability (428%) comparable to that of conventional polymer hydrogels. In addition, the hydroxyl-rich PHEA provided good adhesion ability (shear stress from 5.2 to 12 kPa based on the different interfaces), allowing PH@Ag to stably adhere to various substrate materials such as human skin, plastics, paper, and metals. The antibacterial ability of PH@Ag was greatly enhanced by adding AgNPs, which thus prolongs its lifetime. The goatskin-based PH@Ag also exhibited good cytocompatibility, even exceeding 100% cell viability when cultured with the maximum concentration of its extracts (1 mg·mL−1). It is worth noting that the ionic conductor Al3+ ions built up conductive pathways, allowing PH@Ag to exhibit significant stability, electrical conductivity and sensitivity in sensing tests (conductivity of 3.06 S·m−1 with the maximal gauge factor (GF) of 5.51), and its sensing accuracy could meet the monitoring and recognizing requirements of human motions such as swallowing, frowning, walking, and so on. In summary, this work provided a feasible novel “top–down” strategy to fabricate a novel skin hydrogel functional material with satisfying mechanical properties, good adhesion, antimicrobial property, electrical conductivity, and cytocompatibility. The obtained real “e-skin” is expected to have remarkable potential in many fields such as motion and health monitoring.
2 Experimental
2.1 Materials
Pickled goatskin was purchased from Qingyang District Century Fur Business Department (Chengdu, China); Tara tannin was provided by Ting Jiang New Materials Co, Ltd (Chengdu, China); 2-hydroxyethyl acrylate (HEA) was bought from Energy Chemical (Shanghai, China); aluminum chloride (AlCl3), silver nitrate (AgNO3), and ammonium persulfate (APS) were bought from Chron Chemicals (Chengdu, China). Deionized (DI) water was used as the solvent for all the above materials and the other chemicals were from commercial sources.
2.2 Characterizations
AgNPs were characterized using UV–visible (UV–vis) spectrophotometry (SHIMADZU, UV600, Japan), dynamic light scattering (DLS, Malvern Panalytical, Zetasizer Nano ZS, UK), and transmission electron microscopy (TEM, JEOL, JEM-2100Plus, Japan). The structure of PH@Ag was characterized by using scanning electron microscopy (SEM, ThermoFisher, Helios G4 UC, USA), Fourier transform infrared spectroscopy (FTIR, ThermoFisher Nicolet iS10, USA), X-ray Diffraction (XRD, Malvern Panalytical, EMPYREAN, Netherlands), and X-ray photoelectron spectroscopy (XPS, Kratos, AXIS Ultra DLD, UK). The shrinkage temperature (Ts) of goatskin was determined using a leather shrinkage temperature tester (TX045, TST Instruments (Fujian) Co. Ltd. China). A universal material testing machine (INSTRON 5967, USA) was used to test the mechanical and adhesion properties of all the materials. An electrochemical workstation (CHI760E, China) was adopted to test the electrical properties and human motion monitoring of PH@Ag strain sensors. The images of Hela cells were recorded by using a laser scanning confocal microscope (LSCM, ZEISS, LSM880, Germany).
2.3 Preparation of AgNPs
As shown in Additional file 1: Table S1, AgNO3 with different concentrations were reduced by Tara tannin, and the reduction extents at different pH and temperatures were investigated, and the particle size and 15 day stability of AgNPs were also tested. Concretely, the solution A was prepared by dissolving Tara tannin (0.05 g) in 10 mL of DI water, and AgNO3 (0.05–0.25 g) was also dissolved in 10 mL of DI water to provide the solution B. Under the stirring condition, the solution B was slowly (about 0.1 mL·s−1) added into the solution A at different temperatures (0, 20, 40, 60, and 80 °C, respectively) and pH (2, 4, 6, 8, and 10, respectively) environments. After that, the obtained mixtures were further stirred for 30 min, and the resulting AgNPs solutions were sealed and stored at room temperature (25 °C or so) for subsequent characterization and application in the fabrication of skin hydrogels.
2.4 Pretreatment of pickled goatskin
The pickled goat pelt pieces (40 × 15 × 2 mm) were obtained from the back area of pickled goatskin, and their weights were used as the reference for the dosage of other chemicals. The pelt pieces were immersed in the NaCl solution (10 wt%) and the liquid ratio was 60 wt%. Under the stirring condition, NaHCO3 solution was added drop-by-drop to neutralize the acids in goat pelt pieces, and their final pH values were improved to 5.5–6.0. Then, the de-pickled goat pelt pieces were soaked in DI water for 48 h to remove the excess NaCl, and during this period, DI water was renewed every 6 h. Finally, the goat pelt samples were freeze-dried and stored at room temperature for subsequent experiments.
2.5 Al-tanning of goatskin pelt
The hydrothermal stability of goat pelt should be improved to prevent its curling (owing to the thermal denaturation of collagen fibers) during the subsequent polymerization reaction. The freeze-dried pelt pieces were immersed in different concentrations of AlCl3 solutions as shown in Additional file 1: Table S2, and stirred in a vacuum environment (the atmospheric pressure intensity is 20 kPa) for 15 min, and this vacuum operation was further repeated three times. Subsequently, the Ts values of pelts were measured by using the leather shrinkage temperature tester.
2.6 Preparation of PH@Ag
As shown Scheme 1a, a freeze-dried pelt piece (40 × 15 × 2 mm) was immersed in the afore-prepared AgNPs solution (about 100 mL) at room temperature for 15 min under a vacuum environment (the atmospheric pressure intensity is 20 kPa), and the vacuum soaking treatment was repeated for three times to get P@Ag that was further freeze-dried for subsequent experiments. Several soaking solutions containing HEA, AlCl3, and APS were prepared, respectively, following the formulas shown in Additional file 1: Table S3. Then, the above freeze-dried P@Ag pieces were immersed in the obtained soaking solutions (about 100 mL) at room temperature for 15 min under a vacuum environment (the atmospheric pressure intensity is 20 kPa), and the vacuum soaking treatment was also repeated three times to provide P@Ag&HEA. Finally, the in situ polymerization of HEA in the P@Ag&HEA piece was conducted at 65 °C for 3 h to produce the skin hydrogel PH@Ag. Specifically, P@Ag&HEA was covered with aluminum foil and placed in a culture dish that was then floated on the water surface of a water bath (65 °C). Notably, P@Ag&HEA was not contacted with water during the whole heating process. The prepared skin hydrogels by using the four soaking solutions (Additional file 1: Table S3) were named PH@Ag-1, PH@Ag-2, PH@Ag-3, and PH@Ag-4, respectively. In addition, PHEA hydrogels with and without AlCl3 were also prepared for comparison through the polymerization reaction of HEA under similar conditions.
2.7 Mechanical property test
The mechanical properties of PH@Ag were tested by using a universal material testing machine (INSTRON 5967, USA). A rectangular sample (40 × 15 × 2 mm) was installed on the machine and tested for tensile strength and cyclic stretching strength at moving rates of 20 mm·min−1 and 200 mm·min−1, respectively. The test data were used to calculate the nominal tensile stress and tensile strain according to Additional file 1: Eqs. S1 and S2 (all the relevant Equations were listed in Additional file 1: Table S4). The hydrogel was a typical elastic material and would deform significantly (deformation > 10%) under the action of external forces, so a conversion was conducted to obtain a real stress strain for calculation [31,32,33]. Using Additional file 1: Eqs. S1 and S2, and the volume conservation Additional file 1: Eq. S3, the real tensile stress Additional file 1: Eq. S4 and the real tensile strain Additional file 1: Eq. S5 could be deducted. The stress–strain equation of PH@Ag was calculated by using Additional file 1: Eq. S6 and its toughness was calculated by using Additional file 1: Eq. S7. Cylindrical (the diameter was 20 mm and the thickness was 4 mm) PH@Ag and PHEA samples were used to test the rheology, and their storage modulus (Gʹ), loss modulus (Gʺ), and linear viscoelastic region were recorded.
2.8 Adhesive property test
Similarly, the shear adhesion properties as well as the repetitive adhesion properties of the materials were tested by using the universal material testing machine. Specifically, a thin sheet of PH@Ag (10 × 10 mm) was placed between two substrates, and then the two substrates were stretched by the machine at a moving speed of 10 mm·min−1. The maximum shear tensile strength was calculated according to Additional file 1: Eq. S1.
2.9 Antibacterial and cytocompatibility test
Antibacterial abilities of goat pelt and PH@Ag against E. coli (Gram-negative) and S. aureus (Gram-positive) were tested using the typical inhibition zone method [34]. Specifically, the mother solution of E. coli (or S. aureus) was transferred into Luria–Bertani broth and cultured at 37 °C for 24 h. The activated strains were inoculated into a sterilized agar medium, and the circular (2.5 cm of diameter) goat pelt and PH@Ag samples were placed in the center of the medium and then incubated at constant temperature (25 °C) and relative humidity (50%) for 24 h to observe the inhibition zones.
The cytocompatibility of PH@Ag, PHEA, and goat pelt was characterized by the CCK-8 method [35]. For the tests, 100 mg of the freeze-dried PH@Ag, PHEA, and goat pelt were soaked in the PBS (phosphate buffer saline) solution for 72 h. Subsequently, the extracts were prepared according to the concentration dilution method with the concentrations of 1, 0.8, 0.6, 0.4, 0.2, and 0.1 mg·mL−1, respectively, and the standard medium (the pure PBS solution) was used as the control group. The HeLa cells at logarithmic growth stage were taken for cell counting. According to the above groups, 5000 cells were cultured in each orifice plate for 24 h. After that, the orifice plate was cleaned through the PBS solution, and 100 μL (10 wt%) CCK-8 medium was added and cultured at 37 °C with 5% CO2 for 2 h. Subsequently, the absorbance of the sample at 450 nm was measured through an enzyme marker, then the cell viability could be calculated. In addition, the HeLa cells cultured with 1 mg·mL−1 PH@Ag, PHEA, and goat pelt extracts, respectively, were observed by using LSCM.
2.10 Conductivity test and motion monitoring
The electrical properties of PH@Ag were measured by using an electrochemical workstation with a constant voltage of 5 V. The electrical conductivity was calculated by using Additional file 1: Eq. S8. The relative resistance was used to describe the conductivity change in PH@Ag for all analyses and it was calculated by using Additional file 1: Eq. S9. In addition, the relationship between tensile strain and relative resistance could be established by using Additional file 1: Eq. S10. The gauge factor (GF) could be calculated by deriving Additional file 1: Eq. S10, shown in Additional file 1: Eq. S11. In particular, the response time of PH@Ag when connected to the electrochemical workstation could be obtained by recording the instantaneous current change when the deformation occurred.
3 Results and discussion
3.1 Preparation and structure characterization of PH@Ag
The preparation of conventional conductive hydrogels usually started with monomers that were then polymerized to form the cross-linking macromolecular structures, or polymers that are cross-linked by various cross-linking agents to produce the final three-dimensional (3D) network structures. These are typical “bottom–up” strategies. On the contrary, in this work, a “top–down” strategy was proposed to fabricate a novel skin hydrogel, in which the natural 3D woven network structure of collagen fibers in goatskin was preserved and served as the basic 3D skeleton of the final hydrogel. The HEA monomers, located in collagen fiber gaps, took place in situ polymerization to form a unique structure of PHEA (poly(2-hydroxyethyl acrylate))-encapsulated collagen fibers. Owing to the presence of the tough SCS, this special structure of skin hydrogel led to a significant improvement in its mechanical properties. The specific fabrication route was shown in Scheme 1a. The goat pelt used in this work was naked goatskin which was called pickled goatskin in leather industry, and most of the non-collagenous components in raw goatskin were removed in the leather-making preparation processes that generally includes soaking, fleshing, unhairing, liming, deliming, enzyme bating, pickling and other physical and chemical treatment procedures (Additional file 1: Scheme S1). Thus, the starting goat pelt could be regarded as a special 3D network weaved by collagen fibers, namely SCS. The original pH of pickled goat pelt was about 2.8–3.0, and if it was not pretreated, it would be extremely susceptible to swelling exposed to a neutral solution due to the different osmotic pressure, which is called acid-swelling in leather industry [36]. Therefore, the priority was to adjust the pH of goat pelt. As displayed in Scheme 1a, the de-pickling treatment was conducted in Step (I) by using sodium bicarbonate (NaHCO3) solution in the presence of NaCl, and the final pH of goat pelt was regulated to 5.5–6.0, and it could be called naked goatskin.
In general, the naked goatskin exhibited poor resistance to bacteria and proteases, which made the freeze-drying and AgNPs-treatment necessary. Specifically, in Step (II), the naked goatskin was freeze-dried and then immersed in the solution containing AgNPs. The vacuum environment ensured that AgNPs could be fully dispersed in the interior of naked goatskin, and then the AgNPs-loaded goatskin was once again freeze-dried and preserved. In Step (III), hydrothermal stability is one of the most important parameters of goatskin [37], and this property is usually tested by placing goatskin in gradually warming water, and the temperature at which it takes place shrinkage significantly is measured. This temperature is also known as the shrinkage temperature in leather industry, denoted as Ts. Unfortunately, the Ts of goat pelt was low (about 40–50 °C), which led to the poor mechanical property after heating treatment, so tanning of goat pelt is also a highly essential step. As shown in Additional file 1: Table S2, the naked goatskin was tanned with different concentrations of Al3+ salt. When its concentration reached 12 g·L−1, goatskin had the maximum Ts of about 72 °C (Additional file 1: Fig. S1). Considering that Al3+ could also acted as a conductive medium, 16 g·L−1 of Al3+ was chosen as the final concentration for tanning. Based on Step (III), several HEA&Al solutions were prepared, as shown in Additional file 1: Table S3, and the goatskin-based hydrogel samples prepared from solutions 1–4 were named PH@Ag-1, PH@Ag-2, PH@Ag-3, and PH@Ag-4, respectively. The naked goatskin samples were immersed in the above solutions, and the vacuum environment was maintained to ensure the complete dispersion of all the solution components. In Step (IV), after the repeated vacuum impregnation, HEA monomers, initiator and Al3+ ions could fill the entire collagen fiber gaps and the in situ polymerization was carried out at 65 °C, in which the SCS acted as the fulcrums, and the special structure of PHEA-encapsulated collagen fibers gradually formed.
PH@Ag, the final skin hydrogel, was tough and easily stretched, and exhibited completely different mechanical properties from the naked goatskin and PHEA hydrogel. The main factors affecting the mechanical property of PH@Ag could be the macroscopic encapsulation structure, the microscopic hydrogen bonding, van der Waals force and metal coordination, as well as the destruction of collagen by heating treatment. As shown in Scheme 1b, HEA took place in situ polymerization on the surfaces of collagen fibers, forming an encapsulated structure, and the coordination of Al3+ among collagen-collagen, PHEA-PHEA, and collagen-PHEA made their connection stronger. The heating treatment could inevitably lead to the loosening of collagen fibers, so PH@Ag became easier to be stretched but had less strength owing to the losing of the tight structure [38].
To prove the above proposition, several characterizations of PH@Ag were performed. As shown in Fig. 1a, the naked goatskin exhibited a highly dense structure woven by collagen fibers, and small collagen fiber bundles (diameter of about 50–60 μm) could further be combined to form a larger fibrous structure. However, when the naked goatskin was heated directly without any tanning treatment, its bundle fiber structure could be significantly damaged, as shown in Additional file 1: Fig. S2. It was worth noting that, in Fig. 1b, the collagen fibers structure of goatskin treated with the HEA&Al solution did not change significantly after heating of 1 h at 65 °C, and the HEA gradually polymerized in situ on the surfaces of collagen fibers, forming the PHEA layers. (More SEM characterizations were listed in Additional file 1: Fig. S3a). The final PH@Ag showed the characteristic porous structure of typical hydrogel when the reaction time was increased to 3 h (Additional file 1: Fig. S3b). Although most of collagen fibers were covered by PHEA, the structure of collagen fibers encapsulated by PHEA could be still observed, as shown in Fig. 1c. Hence, these SEM results fully confirm the speculation that HEA polymerized in situ on collagen fibers to encapsulate them. Furthermore, the SEM-based mapping images (Additional file 1: Fig. S4) indicate that the elements Al and Ag were uniformly distributed in the inner of PH@Ag.
The FTIR spectra, shown in Fig. 2a, reveal that PH@Ag has the characteristics of both the naked goatskin and PHEA. The characteristic peaks corresponding to the amide bond (–NHCO–) of collagen appeared at 1628 cm−1 (amide I) and 1538 cm−1 (amide II), respectively [39]. The peak for the unique ester bond (O=C–O) of PHEA appeared at 1720 cm−1. Notably, the above peaks were also observed in the PH@Ag’s FTIR spectrum, which indicates the existence of collagen and PHEA in PH@Ag. Furthermore, the hydrogen bonding peak of the naked goatskin was not as significant as those of PHEA (3382 cm−1) and PH@Ag (3330 cm−1), and it is worth noting that the hydrogen bonding peak of PH@Ag shifted to the lower wavenumbers, indicating that its hydrogen bonding was enhanced.
The triple helix structure of collagen fibers could be completely recorded by the XRD spectrum. In the XRD spectrum of the naked goatskin (Fig. 2b), the signals at 8.2° and 31.7° are both characteristic peaks for collagen [40]. The peak at 8.2° indicates the lateral distance between the molecular chains of collagen fibers, while the peak at 31.7° exhibits the axial period corresponding to one rotation height of collagen helix, which is the typical triple helix structure [41]. The peak at 21.2° is resulted from the amorphous structure of the non-crystal (the diffuse reflection caused by collagen fibers or PHEA) domain. Notably, the peaks at 8.2° and 31.7° were also observed in the XRD spectra of PH (skin hydrogel without AgNPs) and PH@Ag, suggesting that collagen still kept an intact triple helix structure. Interestingly, however, their intensities are weaker than that of goatskin, which is possibly due to the encapsulation structure. The detection thickness of XRD is usually in the range of 5–10 nm, thus leading to a lower intensity of the triple helix structure, and a similar situation could be found in the N1s XPS spectrum, which hints at the existence of the encapsulated structure from the side. In addition, the characteristic peaks of AgNPs appeared at 38° (Ag111), 44.1° (Ag200), 64.3° (Ag220) and 77° (Ag311), respectively, in the XRD spectrum of PH@Ag [42].
To investigate the coordination interactions of Al3+ ions, the XPS characterization shown in Fig. 2c was performed for PHEA, naked goatskin and PH@Ag, and their C1s, O1s and N1s XPS spectra were recorded, too. In particular, both Ag3d (362.08 eV) and Al2p (75.08 eV) peaks appeared in the XPS spectrum of PH@Ag, indicating the successful introduction of both AgNPs and Al3+ ions. As displayed in Fig. 2d, the C1s spectrum of goatskin and PH@Ag could be classified into three peaks corresponding to C–C, C–N, and O=C–C bonds, respectively. It should be noted especially that PHEA contains no N element, therefore, there is no C–N peak. Notably, the C–N (285.9 eV) and O=C–C (287.9 eV) peaks of PH@Ag showed significant right shifts of 1.1 eV and 0.6 eV, respectively, indicating that Al3+ ions took place the coordination interactions with these structures and the coordination with O=C–C was more dominant [43]. Similar conclusions could also be drawn in the O1s and N1s spectra. As exhibited in Fig. 2e and f, the peaks corresponding to the O=C (531.5 eV), O-C (532.8 eV), and N–C (400.3 eV) bonds also showed different degrees of right shifts in the XPS spectrum of PH@Ag, further illustrating the Al3+ coordination with them [44]. In summary, the FTIR, XRD and XPS results confirm the formation of the encapsulated structure, the stronger hydrogen bonding, and the coordination interactions in PH@Ag, which could significantly alter its mechanical properties.
3.2 Mechanical property of PH@Ag
In this work, the natural SCS structure from goatskin served as the framework of PH@Ag, which could significantly improve its mechanical properties. As exhibited in Fig. 3a, PH@Ag can easily withstand the 250 g of weight, and also can be stretched to 2.5 times its original length (4 cm). Quantitative mechanical property tests were further performed, the results are shown in the Fig. 3b and c. According to Fig. 3b, it can be found that the naked goatskin and PHEA hydrogel are two different extremes in the stress–strain curves. One had the strongest tensile stress (9.91 Mpa), while the other showed the greatest tensile strain (717%). It is obvious that all the groups of PH@Ag hydrogels combined the respective advantages of goatskin and PHEA hydrogel. In particular, PH@Ag-3 exhibited the best comprehensive mechanical properties with tensile stress of 2.97 MPa and tensile strain of 428%. One possible reason is that the heating treatment mentioned before could damage the collagen structure, which was inevitable, leading to a decrease in mechanical properties as shown in Additional file 1: Fig. S5. So, the tensile strengths of all the PH@Ag hydrogels were much lower than that of the naked goatskin. On the other hand, the tensile strength and strain of PH@Ag could be improved along with the increase of PHEA content when PHEA was a variable in the preparation. Notably, the concentration of HEA in PH@Ag-4 was the highest, but its mechanical property was poorer than that of PH@Ag-3. Meantime, the toughness values were also calculated and shown in Fig. 3d. Interestingly, PH@Ag reached the maximum toughness (6.23 MJ·m−3), which is higher than anyone else sample, so it was used for the subsequent tests and simplified into PH@Ag.
Mechanical properties of PH@Ag. a Photos of PH@Ag sheets with a weight of 250 g and manually stretched. b The illustration of test installation. Stress–strain curves (c) and toughness (d) of naked goatskin (pelt), PH@Ag, and PHEA hydrogel. Rheological (e) and cyclic tensile stress–strain (f) curves of PH@Ag and PHEA hydrogel. Cyclic tensile stress–strain curves under 100 times (g) and the self-recovery (h) performance of PH@Ag
Furthermore, Fig. 3e and f exhibited the mechanical performance of PH@Ag and PHEA under rheological and cyclic tensile tests. The naked goatskin is strain-limited and could not participate in these tests. It is clear that PH@Ag not only exhibited a stronger storage modulus (Gʹ, 1.03 × 104 Pa), but also appeared a gel-sol transition point at a higher strain (> 3000%). Notably, the typical feature of hydrogel was observed in the tensile cycle curves of both PH@Ag and PHEA hydrogel, which is the dissipation caused by the breaking of hydrogen bonds and van der Waals force under the first cycle. Compared with PHEA hydrogel, PH@Ag exhibited the highly repetitive cyclic curve, lower dissipation (19.9% of PH@Ag versus 51.5% of PHEA), less elastic deformation (5–8% of PH@Ag versus 10–22% of PHEA), and a more stable cyclic stretching behavior. These results prove that PH@Ag has better mechanical properties and could be adapted to a wider range of usage conditions than the naked goatskin and PHEA hydrogel. Not only that, PH@Ag also had good reusability and satisfactory self-recovery properties. Figure 3g shows the 100 times of cyclic tensile stress–strain curves for PH@Ag. It is worth noting that all the cyclic stress curves of PH@Ag were almost stable around 230 kPa and the fluctuation percentage of stress was less than 13.04%, and its maximum elastic strain was still less than 8%. In addition, the good self-recovery of PH@Ag could be observed distinctly when the strain was increased to 150% (Fig. 3h). There was a significant decrease in the maximum stress when the second stretching was performed immediately, however, with the waiting time from 20 to 60 s, the stress–strain curves gradually coincided with the original one, and the recoveries reached 96.3%, 97.7%, and 98.9%, respectively (Fig. 3h).
3.3 Adhesive property of PH@Ag
Owing to the introduction of PHEA, PH@Ag was also endowed with satisfactory adhesion ability to some common materials shown in Fig. 4a, including iron, wood, paper, plastic (polymethyl methacrylate, PMMA), human skin and nitrile, which meets the basic requirements for the strain sensor application. It is clear from Fig. 4b that the naked goatskin showed no adhesion, while PHEA hydrogel exhibited good adhesive ability. Therefore, we assumed that the adhesion property of PH@Ag was mainly provided by PHEA, and the possible mechanism is shown in Fig. 4c. PHEA contains a large number of hydroxyl groups (-OH), and can involve in hydrogen bonding and metal coordination, which could be the main reason for its adhesion to the various substrates [45]. The cyclic adhesion tests (cycle 10 times) were performed as displayed in Fig. 4d. The PH@Ag exhibited the best adhesion strengths to materials containing cellulose such as wood (12.5 kPa) and paper (10.8 kPa), and the worst adhesion to plastics (5.2 kPa), however, it is satisfied that the adhesion to pigskin reached 10.2 kPa, which could meet the basic requirement for most of sensing applications.
Adhesion property of PH@Ag. Photos of PH@Ag sheets adhering to the surfaces of iron, wood, paper, plastic (PMMA), human skin and nitrile glove (a). Adhesion strengths (b) of naked goatskin, PH@Ag and PHEA hydrogel to plastic (PMMA), iron, pigskin, paper and wood. Possible adhesion mechanism (c) and cyclic adhesion strengths (d) of PH@Ag for different substrates
3.4 Antibacterial property and cytocompatibility
The abundant porous structures, water and nutrients in hydrogels create an ideal living environment for microorganisms. So, the corrosion of microorganisms could greatly limit the lifetime of hydrogels if not given antibacterial treatment. Adding AgNPs is a simple and efficient means to kill bacteria. In this work, AgNPs were prepared by using the cheap Tara tannin, an important vegetable tanning agent in leather industry, as the reducing agent and stabilizer. As shown in Additional file 1: Table S1, a series of formulas for the preparation of AgNPs by uisng Tara tannin were tested and the obtained AgNPs were characterized in detail. As shown Fig. 5a and 5b, AgNPs could only be successfully prepared by Tara tannin in an alkaline environment from 0 to 80 °C [46]. Considering the particle size and experimental operation, 20 °C was adopted as the ideal temperature, and the average size of AgNPs prepared at this temperature was about 15 nm by DLS (Fig. 5c). The feed molar ratio of Tara/AgNO3 also had obvious effect on the formation (Fig. 5d), particle size (Fig. 5e) and stability (Fig. 5f) of AgNPs. Based on these results, the ratio of 1:5 was adopted as the optimal feed molar ratio of Tara/AgNO3. The resulting AgNPs exhibited the characteristic XRD pattern (Fig. 5g) at the peak of 38.54° (Ag111), 48.04° (Ag200), 64.31° (Ag220), and 77.75° (Ag311) with the average particle size of 11.1 nm by TEM (Fig. 5h), and was used as the antibacterial material for the preparation of PH@Ag. Using the classical inhibition zone method [34], the antibacterial activity of PH@Ag was evaluated against Escherichia coli (E. coli, Gram-negative) and Staphylococcus aureus (S. aureus, Gram-positive), in which the naked goatskin was used as the control group. As displayed in Fig. 5i, circular bacteriostatic rings were observed for the PH@Ag sample under both E. coli and S. aureus environments, while the naked goatskin exhibited no obvious inhibition zone. The naked goatskin resulted in very small inhibition area of 2.3 cm2 and 3.0 cm2 against E. coli and S. aureus, respectively. This slight antibacterial activity is probably attributed to the residual acids. On the contrary, owing to the addition of AgNPs, PH@Ag showed the satisfactory antibacterial performance, and its inhibitory area reached 29.3 cm2 for E. coli and 33.5 cm2 for S. aureus, respectively.
Characterization of AgNPs and antibacterial property of PH@Ag. a, b UV–vis spectra of AgNPs prepared under different pH and temperature conditions. c Hydrated particle size by DLS of AgNPs prepared at different temperatures. d UV–vis spectra of AgNPs prepared at different Tara/AgNO3 ratios. e Hydrated particle size by DLS of AgNPs prepared at different Tara/AgNO3 ratios. f Stability by UV–vis of AgNPs prepared at different Tara/AgNO3 ratios over 15 d. g XRD pattern of AgNPs prepared at 1:5 Tara/AgNO3 ratio. h TEM and HRTEM images of AgNPs prepared at 1:5 Tara/AgNO3 ratio. Inhibition zones (i) and area (j) of naked goatskin and PH@Ag against E. coli and S. aureus
The cytocompatibility of PH@Ag was evaluated by using the typical the CCK-8 method [35]. The extracts from different concentrations of naked goatskin, PH@Ag and PHEA hydrogel were used to culture HeLa cells, and the cell viability was recorded and shown in Fig. 6a. Interestingly, it was found that whether the extracts were from goatskin or PH@Ag, all the viability of HeLa cells exceeded 95% at all the tested concentrations and even exceeded 100% at the high concentrations (0.8 and 1.0 mg·mL−1). However, the viability of HeLa cells cultured by the PHEA extracts was unremarkable, and the average viability was 95% at all the concentrations, similar to the results of control group (cultured with the pure PBS solution), reflecting the general normal cell death process. It has been widely reported that collagen exhibited the ability to promote cell growth [47], which may be the main reason for the above phenomenon. The HeLa Cells cultured with 1 mg·mL−1 of extract were observed by using a laser scanning confocal microscope (LSCM) to further evaluate their cytocompatibility. As shown in Fig. 6b, red and green fluorescence represent dead and living cells, respectively. Goat pelt as a kind of animal biomass has good cytocompatibility, which is why the cell viability under this extract was higher than the control group. Meanwhile, the PH@Ag followed the characteristics of the naked goatskin and still offered the same good cytocompatibility, indicating that PH@Ag has little risk when contacting with human bodies.
3.5 Conductivity and motion-sensing property
Conductivity is an essential feature of the flexible motion-sensing devices, so it is highly necessary to evaluate the conductivity of PH@Ag. Owing to the introduction of AlCl3, PH@Ag exhibited good electrical conductivity, and its possible conductive mechanism is that the directional movement of Al3+ and Cl− ions under the electric field generated the current. Figure 7a preliminarily confirmed the conductive performance of PH@Ag in the conventional circuit. The red LED (light-emitting diode) was lit up and its brightness dimmed as PH@Ag was stretched by hand. Additionally, the PH@Ag was linked to the electrochemical workstation and its resistance signal was detected under the constant voltage of 5 v for the further conductivity investigation. As shown in Fig. 7b, the response time of PH@Ag was less than 23 ms, and this good response sensitivity paves the way for its subsequent applications. Figure 7c recorded the relative resistance change of PH@Ag (5 × 0.3 × 1 cm) in the range of 0–200% strain. The conductivity of PH@Ag can be calculated as 3.06 S·m−1, which is much higher than that of PHEA without AlCl3 (0.17 S·m−1) and the naked goatskin (almost 0 S·m−1). It could be found that the strain-relative resistance curve met the fitting equation of a = 0.0087ε2 + 2.03ε + 0.601, in which the relatedness reached 0.999. The functional relationship between the gauge factor (GF) and strain applied can be defined when deriving the above equation, and the equation of GF = 0.017ε + 2.03 (Fig. 7d) could be obtained. From these two equations and curves, it can be concluded that the conductivity of PH@Ag is highly sensitive to the strain, even a minor strain of 10% (The actual length of change was about 0.5 cm) could lead to the significant change in its relative resistance. Usually, the strain of most movements was generally in the range of 0–200%, while PH@Ag had a fairly stable GF value (highly linear from 2.03 to 5.51) in this scope. So, its conductive sensitivity can fully meet the basic requirements for motion monitoring. In addition, the anti-fatigue of PH@Ag was studied. We performed rapid cyclic stretching of PH@Ag within 500 s and got the relative resistance-strain curve shown in Fig. 7e. It is clear that the resistance change of PH@Ag exhibited very stable throughout the whole process. Whether it was in the range of 0–50 s or 400–450 s, the frequency was highly matched to the movement, proving that PH@Ag possesses good fatigue resistance capability and could adapt to the environment of constant behavior monitoring.
Based on its good mechanical properties, self-adhesion ability, and stable conductivity and sensitivity, the PH@Ag is expected to have a potential capability to monitor human movements. Further, it was adhered to the different parts of human body as strain sensors for monitoring application. As illustrated in Fig. 8, the motion behaviors are divided into three categories, which are small movements like swallowing and frowning (the actual strain is lower than 10%), medium movements like wrist, elbow and finger turning (the actual strain is about 15–25%), and large movements like knee bending (the actual strain is more than 35%). It is unsurprised that all the three kinds of motion behaviors could be clearly and completely recorded by PH@Ag sensors. On the one hand, PH@Ag strain sensor could still sensitively detect the swallowing and frowning signals of the volunteer, although the relative resistance changes were very small. On the other hand, the medium and large behaviors that were detected by PH@Ag sensors maintained the signal integrity and continuity without signal breakage. Additionally, it is interesting that different behaviors had their unique signal shapes, which could potentially be used as the characteristics to distinguish and identify different motion behaviors. For example, Fig. 8a and b showed that both swallowing and frowning had three peaks a, b, and c, however, it is clear that peak a in swallowing curve was lower. Similarly, wrist turning had a sharper peak b, while knee bending exhibited only a smooth peak a, and part b of the finger-bending curve was flatter than that elbow-bending curve. The above-mentioned methods of analyzing different signals could tentatively identify the motion behaviors of the volunteer, and finally, achieve the purpose of motion identification. In short, the above test results indicate that PH@Ag does possess the great potential to act as a novel flexible strain sensor, an “e-skin”, and to contribute to human motion monitoring and identification.
4 Conclusion
In summary, we proposed and successfully confirmed that the “top–down” strategy, in which the extraction of collagen was unnecessary and the goatskin collagen skeleton with the three-dimensional network structure woven by natural collagen fibers was preserved and used as the basic framework of hydrogel, was feasible to construct conductive skin hydrogel, a real electronic skin, with high mechanical properties. The fabricated skin hydrogel PH@Ag inherited the advantages of both goatskin (high mechanical strength) and PHEA polymeric hydrogel (high stretching property), and exhibited excellent physical and mechanical properties such as high tensile strength (2.97 MPa), high toughness (6.23 MJ·m−3), high breaking elongation (428%) and good anti-fatigue performance. Its comprehensive mechanical properties are much better than those of known collagen-based conductive hydrogels and most of synthetic polymer-based ones (Additional file 1: Fig. S8 and Table S5). The PH@Ag not only remained the typical characteristics of conventional conductive hydrogels but also had the unique microstructure of PHEA-encapsulated collagen fibers. In addition, PH@Ag possessed satisfactory adhesion ability to many substrates (e.g. human skin, wood, plastic, paper, etc.) owing to the presence of abundant hydroxyl groups from PHEA, and also exhibited good antibacterial and cytocompatibility properties. The introduction of Al3+ salt not only endowed PH@Ag with high hydrothermal stability, which is crucial for the preservation of skin collagen skeleton, owing to its good tanning and crosslinking interactions, but also provided PH@Ag good conductive property (3.06 S·m−1 of conductivity). Notably, PH@Ag showed the considerable sensing sensitivity with the maximum GF of 5.51, and could, as strain sensors, meet the detection requirements for all kinds of human motions (e.g. swallowing, frowning, walking, etc.). So, the PH@Ag skin hydrogel electronic skin is anticipated to possess eye-catching application prospect in many fields such as flexible wearable electronic devices, health and motion monitoring, and so on. All in all, the research work has important theoretical significance for enriching the design theory and preparation strategy of collagen-based conductive hydrogel electronic skin, and also possesses practical significance for promoting the development of collagen-based biomaterials and the high-value utilization of animal skins.
Availability of data and materials
All the data needed to evaluate the conclusions in the paper are present in the paper and the Additional file.
Abbreviations
- 3D:
-
Three-dimensional
- AgNPs:
-
Silver nanoparticles
- APS:
-
Ammonium persulfate
- DI:
-
Deionized
- DLS:
-
Dynamic light scattering
- E. coli :
-
Escherichia coli
- FTIR:
-
Fourier transform infrared spectroscopy
- GF:
-
Gauge factor
- GSCS:
-
Goatskin collagen skeleton
- HEA:
-
2-Hydroxyethyl methacrylate
- HEA&Al:
-
2-Hydroxyethyl methacrylate and AlCl3
- LED:
-
Light-emitting diode
- LSCM:
-
Laser scanning confocal microscope
- P@Ag:
-
Skin loaded with silver nanoparticles
- P@Ag&HEA:
-
Skin loaded with silver nanoparticles and 2-hydroxyethyl methacrylate
- PBS:
-
Phosphate buffer saline
- PHEA:
-
Poly(2-hydroxyethyl methacrylate)
- PH@Ag:
-
Skin hydrogel containing poly(2-hydroxyethyl methacrylate) and silver nanoparticles
- PMMA:
-
Polymethyl methacrylate
- S. aureus :
-
Staphylococcus aureus
- SCS:
-
Skin collagen skeleton
- SEM:
-
Scanning electron microscopy
- TEM:
-
Transmission electron microscopy
- Ts:
-
Shrinkage temperature
- UV–vis:
-
UV–visible
- XPS:
-
X-ray photoelectron spectroscopy
- XRD:
-
X-ray diffraction
References
Zhu TX, Ni YM, Biesold GM, Cheng Y, Ge MZ, Li HQ, Huang JY, Lin ZQ, Lai YK. Recent advances in conductive hydrogels: classifications, properties, and applications. Chem Soc Rev. 2023;52(2):473–509.
Wang ZW, Cong Y, Fu J. Stretchable and tough conductive hydrogels for flexible pressure and strain sensors. J Mater Chem B. 2020;8(16):3437–59.
Wang LR, Xu TL, Zhang XJ. Multifunctional conductive hydrogel-based flexible wearable sensors. TRAC-Trend Anal Chem. 2021;134: 116130.
Chen Z, Chen YJ, Hedenqvist MS, Chen C, Cai C, Li H, Liu HZ, Fu J. Multifunctional conductive hydrogels and their applications as smart wearable devices. J Mater Chem B. 2021;9(11):2561–83.
Yin YD, Rogers JA. Introduction: smart materials. Chem Rev. 2022;122(5):4885–6.
Rogers JA. Wearable electronics nanomesh on-skin electronics. Nat Nanotechnol. 2017;12(9):839–40.
Ke T, Zhao L, Fan X, Gu HB. Rapid self-healing, self-adhesive, anti-freezing, moisturizing, antibacterial and multi-stimuli-responsive PVA/starch/tea polyphenol-based composite conductive organohydrogel as flexible strain sensor. J Mater Sci Technol. 2023;135:199–212.
Ling QJ, Liu WT, Liu JC, Zhao L, Ren ZJ, Gu HB. Highly sensitive and robust polysaccharide-based composite hydrogel sensor integrated with underwater repeatable self-adhesion and rapid self-healing for human motion detection. ACS Appl Mater Interfaces. 2022;14(21):24741–54.
Zhang H, Zhang DZ, Wang ZH, Xi GS, Mao RY, Ma YH, Wang DY, Tang MC, Xu ZY, Luan HX. Ultrastretchable, self-healing conductive hydrogel-based triboelectric nanogenerators for human-computer interaction. ACS Appl Mater Interfaces. 2023;15(4):5128–38.
Deng ZX, Yu R, Guo BL. Stimuli-responsive conductive hydrogels: design, properties, and applications. Mater Chem Front. 2021;5(5):2092–123.
Lo CY, Zhao YS, Kim C, Alsaid Y, Khodambashi R, Peet M, Fisher R, Marvi H, Berman S, Aukes D, He XM. Highly stretchable self-sensing actuator based on conductive photothermally-responsive hydrogel. Mater Today. 2021;50:35–43.
He XY, Sun N, Jia H, Hou MM, Tan ZP, Lu XQ. Antifouling electrochemical biosensor based on conductive hydrogel of DNA Scaffold for ultrasensitive detection of ATP. ACS Appl Mater Interfaces. 2022;14(36):40624–32.
Cheng T, Zhang YZ, Wang S, Chen YL, Gao SY, Wang F, Lai WY, Huang W. Conductive hydrogel-based electrodes and electrolytes for stretchable and self-healable supercapacitors. Adv Funct Mater. 2021;31(24):2101303.
Ganguly S, Ray D, Das P, Maity PP, Mondal S, Aswal VK, Dhara S, Das NC. Mechanically robust dual responsive water dispersible-graphene based conductive elastomeric hydrogel for tunable pulsatile drug release. Ultrason Sonochem. 2018;42:212–27.
Lee HR, Kim CC, Sun JY. Stretchable ionics-A promising candidate for upcoming wearable devices. Adv Mater. 2018;30(42):1704403.
Zhao XH. Designing toughness and strength for soft materials. P Natl Acad Sci USA. 2017;114(31):8138–40.
Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–58.
Darvish DM. Collagen fibril formation in vitro: from origin to opportunities. Mater Today Bio. 2022;15: 100322.
Ling QJ, Fan X, Ling MJ, Liu JC, Zhao L, Gu HB. Collagen-based organohydrogel strain sensor with self-healing and adhesive properties for detecting human motion. ACS Appl Mater Interfaces. 2023;15(9):12350–62.
Roshanbinfar K, Vogt L, Greber B, Diecke S, Boccaccini AR, Scheibel T, Engel FB. Electroconductive biohybrid hydrogel for enhanced maturation and beating properties of engineered cardiac tissues. Adv Funct Mater. 2018;28(42):1803951.
Ravichandran R, Martinez JG, Jager EWH, Phopase J, Turner APF. Type I Collagen-derived injectable conductive hydrogel scaffolds as glucose sensors. ACS Appl Mater Interfaces. 2018;10(19):16244–9.
Vijayavenkataraman S, Vialli N, Fuh JYH, Lu WF. Conductive collagen/polypyrrole-b-polycaprolactone hydrogel for bioprinting of neural tissue constructs. Int J Bioprint. 2019;5(21):31–43.
Ha JH, Lim JH, Kim JW, Cho HY, Jo SG, Lee SH, Eom JY, Lee JM, Chung BG. Conductive GelMA-collagen-AgNW blended hydrogel for smart actuator. Polymers. 2021;13(8):1217.
Lei H, Fan DD. Conductive, adaptive, multifunctional hydrogel combined with electrical stimulation for deep wound repair. Chem Eng J. 2021;421: 129578.
Xu XZ, Wang L, Jing JH, Zhan JF, Xu CG, Xie WK, Ye SM, Zhao Y, Zhang C, Huang F. Conductive collagen-based hydrogel combined with electrical stimulation to promote neural stem cell proliferation and differentiation. Front Bioeng Biotechnol. 2022;10: 912497.
Yue OY, Wang XC, Hou MD, Zheng MH, Bai ZX, Cui BQ, Cha SY, Liu XH. Skin-inspired wearable self-powered electronic skin with tunable sensitivity for real-time monitoring of sleep quality. Nano Energy. 2022;91: 106682.
Ren ZJ, Ke T, Ling QJ, Zhao L, Gu HB. Rapid self-healing and self-adhesive chitosan-based hydrogels by host-guest interaction and dynamic covalent bond as flexible sensor. Carbohydr Polym. 2021;273: 118533.
Zhang M, Deng F, Tang LL, Wu H, Ni YH, Chen LH, Huang LL, Hu XQ, Lin S, Ding CC. Super-ductile, injectable, fast self-healing collagen-based hydrogels with multi-responsive and accelerated wound-repair properties. Chem Eng J. 2021;405: 126756.
Zhang M, Yang QL, Hu TS, Tang LL, Ni YH, Chen LH, Wu H, Huang LL, Ding CC. Adhesive, antibacterial, conductive, anti-UV, self-healing, and tough collagen-based hydrogels from a pyrogallol-Ag self-catalysis system. ACS Appl Mater Interfaces. 2022;14(7):8728–42.
Gao Y, Wang YR, Xia S, Gao GH. An environment-stable hydrogel with skin-matchable performance for human-machine interface. Sc China Mater. 2021;64(9):2313–24.
Uchida M, Sengoku T, Kaneko Y, Okumura D, Tanaka H, Ida S. Evaluation of the effects of cross-linking and swelling on the mechanical behaviors of hydrogels using the digital image correlation method. Soft Matter. 2019;15(16):3389–96.
Wang E, Batra S, Cakmak M. A real time study on drying and the mechano-optical behavior of polyvinyl alcohol films in solid and swollen state. Polymer. 2015;67:200–7.
Zhang R, Fu Q, Zhou K, Yao Y, Zhu X. Ultra-stretchable, tough and self-healable poly(acrylic acid) hydrogels cross-linked by self-enhanced high-density hydrogen bonds. Polymer. 2020;199: 122603.
Song B, Fan X, Gu HB. Chestnut-tannin-crosslinked, antibacterial, antifreezing, conductive organohydrogel as a strain sensor for motion monitoring, flexible keyboards, and velocity monitoring. ACS Appl Mater Interfaces. 2023;15(1):2147–62.
Liu JC, Bao S, Ling QJ, Fan X, Gu HB. Ultra-fast preparation of multifunctional conductive hydrogels with high mechanical strength, self-healing and self-adhesive properties based on Tara Tannin-Fe3+ dynamic redox system for strain sensors applications. Polymer. 2022;240: 124513.
Huang YW, Xiao HZ, Pu HL, Xue N, Hao BC, Huang X, Shi B. Self-driven directional dehydration enabled eco-friendly manufacture of chrome-free leather. J Leather Sci Eng. 2022;4:17.
Sun Q, Zeng YH, Yu Y, Wang YN, Shi B. An exploration of enhancing thermal stability of leather by hydrophilicity regulation: effect of hydrophilicity of phenolic syntan. J Leather Sci Eng. 2022;4:22.
Yang PY, He XC, Zhang WJ, Qiao YX, Wang F, Tang KY. Study on thermal degradation of cattlehide collagen fibers by simultaneous TG-MS-FTIR. J Therm Anal Calorim. 2017;127(3):2005–12.
Liu Y, Song B, Zhang J, Gaidau C, Gu HB. Aluminum tanning of hide powder and skin pieces under microwave irradiation. J Leather Sci Eng. 2020;2:23.
Sionkowska A, Adamiak K, Musial K, Gadomska M. Collagen based materials in cosmetic applications: a review. Materials. 2020;13(19):4217.
Giraud-Guille MM, Besseau L, Chopin C, Durand P, Herbage D. Structural aspects of fish skin collagen which forms ordered arrays via liquid crystalline states. Biomaterials. 2000;21(9):899–906.
Wei SS, Xu XY, Liu YJ, Yang JM. Preparation of hydrophobic nano-silver colloid and aqueous nano-silver colloid by phase transfer. Mater Chem Phys. 2011;126(1–2):12–5.
Hou J, Wang W, Zhou TY, Wang B, Li HY, Ding L. Synthesis and formation mechanistic investigation of nitrogen-doped carbon dots with high quantum yields and yellowish-green fluorescence. Nanoscale. 2016;8(21):11185–93.
Fan X, Zhao L, Ling QJ, Gu HB. Tough, self-adhesive, antibacterial, and recyclable supramolecular double network flexible hydrogel sensor based on PVA/chitosan/cyclodextrin. Ind Eng Chem Res. 2022;61(10):3620–35.
Li SN, Cong Y, Fu J. Tissue adhesive hydrogel bioelectronics. J Mater Chem B. 2021;9(22):4423–43.
He HX, Astruc D, Gu HB. Green fabrication of hydrogel-immobilized Au@Ag nanoparticles using tannic acid and their application in catalysis. New J Chem. 2021;45(15):6914–27.
Chen PF, Tao JD, Zhu SA, Cai YZ, Mao QJ, Yu DS, Dai J, Ouyang HW. Radially oriented collagen scaffold with SDF-1 promotes osteochondral repair by facilitating cell homing. Biomaterials. 2015;39:114–23.
Acknowledgements
The authors thank the valuable help of Dr. Jinwei Zhang from the College of Biomass Science and Engineering of Sichuan University, and Hui Wang from the Analytical & Testing Center of Sichuan University. We thank eceshi (www.eceshi.com) for the great help in XPS analysis and cell imaging experiments.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 21978180), the Université de Bordeaux and the Centre National de la Recherche Scientifique (CNRS).
Author information
Authors and Affiliations
Contributions
JL performed all the experiments and was a major contributor in data analyses and writing of this manuscript. XF made substantial contributions to the design and implement of the experiments in this work. DA made substantial contributions to the design and implement of the experiments in this work. HG is the superior of this work and made great contribution to the conception of the study, the discussion of the results and the revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Main components for the preparation of AgNPs. Table S2. Effect of AlCl3 concentration on the Ts of the naked goatskin. Table S3. Main components for the preparation of hydrogels PH@Ag and PHEA. Table S4. Main equations involved in the whole experiments. Scheme S1. Schematic process flow of leather-making. Fig. S1. Shrinkage temperatures of goat pelts tanned by different concentrations of AlCl3 solutions. Fig. S2. SEM images of naked goatskin beforeand after heating treatment of 65 °C for 1 h. Fig. S3. SEM images of goat pelt soaked in HEA&Al3+ solution after heating treatment of 65 °C for 1 hand 3h. Fig. S4. Distributions of elements N, Ag, and Al in PH@Ag through SEM-based mapping characterization. Fig. S5. Tensile stress-strain curves of goat pelt after heating treatment of 65 °C for different time. Fig. S6. Illustration of vacuum immersion methodand the absorption efficiency of goatskin piece to the pre-gel solution in vacuumand atmospheric pressureimmersion. Fig. S7. AgNPs solution prepared using the strategy in this work. Fig. S8. Mechanical property comparison of PH@Ag and other hydrogel-based materials. Table S5. Mechanical property comparison of PH@Ag and other hydrogel-based materials.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, J., Fan, X., Astruc, D. et al. Robust conductive skin hydrogel e-skin constructed by top–down strategy for motion-monitoring. Collagen & Leather 5, 17 (2023). https://doi.org/10.1186/s42825-023-00123-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42825-023-00123-9