Abstract
Background
To compare the preventive effects of levetiracetam and valproate on late-onset post-traumatic seizures in patients with traumatic brain injury (TBI).
Methods
A total of 95 patients with TBI were recruited from 2017 to 2020. They were randomized into three groups: levetiracetam (LEV) group (n = 30) receiving LEV treatment (500 mg, bid, po); valproate group (n = 32) receiving sodium valproate (500 mg/d, once daily, po); and control group (n = 33) receiving no anti-seizure medication. LEV and valproate were given to corresponding groups within seven days after TBI, and the administration lasted for one month. The incidence of epilepsy and adverse events were evaluated at 7 days and 12 months post-TBI.
Results
The cumulative incidences of late post-traumatic seizures at the 12-month follow-up in the LEV, valproate, and control groups were 3.33%, 12.50% and 15.63%, respectively. The cumulative incidence of late post-traumatic seizures in the LEV group was significantly lower than those in the valproate and control groups (P < 0.05). The cumulative incidence of late post-traumatic seizure in the valproate group was not significantly different from that in the control group (P > 0.05).
Conclusions
LEV can reduce the cumulative incidence of late post-traumatic seizures, whereas valproate can not.
Similar content being viewed by others
Background
With increased incidence of traffic accidents, traumatic brain injury (TBI) has become a common and frequently occurring condition, and one of the most common etiologies leading to secondary epilepsy [1, 2]. The incidence of post-traumatic seizures (PTS) after severe TBI ranges 10–20% [3]. Two types of PTS have been identified: early post-traumatic seizures (EPTS) and late post-traumatic seizures (LPTS). EPTS occur within one week post-TBI, whereas LPTS usually occur over one week after TBI [4]. Focal epilepsy and generalized epilepsy are more common after cerebral trauma, while mixed epilepsy, psychomotor epilepsy or non-convulsive epilepsy are less common.
LPTS is one of the most medication-resistant epilepsies and has serious impacts on the physical and mental health of patients with TBI [5]. Risk factors for PTS include old age, penetrating injury, and a higher degree of injury (eg, an intracranial hemorrhage, a midline displacement greater than 5 mm, and a longer duration of coma). Several clinical trials have established that traditional anti-seizure medications (ASMs) can prevent EPTS but not LPTS [6-8]. Valproate acid (VPA) is a commonly used medication for epileptic seizures, but its administration is associated with neurotoxic effects. In contrast, no such adverse effects have been reported for levetiracetam (LEV) treatment. In addition, clinical studies have demonstrated favorable side effects and pharmacokinetic data, as well as high efficacy of LEV. LEV is a broad-spectrum ASM that is widely used for the treatment of partial and generalized epilepsy. However, whether LEV can prevent or reduce LPTS remains unclear [9-12]. VPA and phenytoin are commonly used to prevent PTS in neurosurgery departments in China. In an earlier investigation, the rates of LPTS did not differ significantly between the VPA and phenytoin treatment groups (P > 0.05) [13]. In this study, we aimed to evaluate LEV prevention of LPTS in comparison to the effect of VPA.
Methods
Subjects
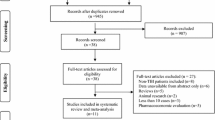
This study was approved by the Institutional Review Board at the First Hospital of Shanxi Medical University, and informed consent was obtained from all patients. Ninety-five patients with TBI who were admitted in the Neurosurgery and Emergency Departments of the First Hospital of Shanxi Medical University were included in this study (32 females, 63 males; mean age 44.6 ± 2.17 years, range 20–65 years). The inclusion criteria were: (1) age of 18–70 years; (2) seizures within 30 min after TBI with at least one of the following conditions detected by CT: brain contusion, subdural hematoma, epidural hematoma, and intracranial hematoma. The exclusion criteria were as follows: (1) a family history of epilepsy; (2) patients with epilepsy before TBI; (3) pregnancy and lactation; (4) patients who did not receive ASM treatment within seven days after TBI; (5) patients with any of the following medical conditions: hypertension, diabetes, stroke, renal and hepatic dysfunctions, and malignant tumor.
Of the 95 patients, 25 had simple brain contusion (SBC), 27 had SBC and subdural hematoma, 18 had SBC and epidural hematoma, and 25 had SBC and multiple intracranial hematomas. Glasgow coma score (GCS) [14] was assessed within 4 h post-TBI (Table 1). There were 16 severe (GCS 3–7), 33 moderate (GCS 8–12), and 46 mild cases (GCS 13–15).
Pharmacological treatment with ASMs
The 95 patients were randomized into three groups by a random number table: (1) the LEV group, in which they were treated with 500 mg LEV twice daily after brain injury, for one month; (2) the VPA group, in which they were treated with 500 mg VPA once daily after brain injury, for one month; and (3) the control group, in which they received no ASM treatment. In the VPA group, although the minimum dose of sodium valproate was recommended to be 600 mg/day, it was administered at 500 mg/day for convenience as the sodium valproate sustained-release tablets were 500 mg each. If the patients could not take drugs orally, the medication was given through a nasogastric tube. The medication was started at an average of 4.5 days after brain injury.
The seizure frequency of LPTS was recorded for 12 months after brain injury.
Statistical analysis
Data of age, GCS, and the number of diagnoses were analyzed with One-way ANOVA test. Pearson χ2 test was applied to compare the EPTS, brain injuries, and the cumulative incidence of LPTS among groups at 12 months. All analyses were made using the SPSS 17.0 software. P < 0.05 was considered as statistically significant.
Results
Patient follow-up
All patients in the LEV and VPA groups completed the12-month follow-up. One patient in the control group was lost to follow-up due to the wrong telephone number provided. Both LEV and VPA were well tolerated by the patients. One patient experienced brief dizziness during the LEV treatment, but it was improved a few days later. One patient experienced mild insomnia during the VPA treatment, which diminished three days later. Another patient in the VPA group had mild gastrointestinal discomfort that improved one day later.
Cumulative incidence of seizures
The incidence of LPTS was significantly higher in the LEV group than in the VPA group (3.33% vs 12.50%, χ2 = 4.41, P = 0.031) and control group (3.33% vs 15.63%, χ2 = 6.07, P = 0.017). There was no statistically significant difference in the incidence of LPTS between VPA and control groups (χ2 = 1.15, P = 0.36).
These findings indicated that LEV had a significant preventive effect against LPTS, whereas VPA did not (Table 2).
Discussion
In this study, we found that the cumulative incidence of LPTS in the LEV group was significantly lower than those in the VPA and control groups. The incidence of LPTS in the VPA group was not significantly different from that in the control group. These findings indicated that LEV could prevent LPTS in patients suffering from TBI, whereas VPA could not.
Our results were consistent with those of Pearl et al. published in 2013 [10]. In their study, LEV (55 mg/kg per day bid for 30 days, starting within 8 hours post-brain injury) more effectively prevented LPTS in 20 TBI children aged 6–17 years with high risk factors of developing posttraumatic epilepsy. They found that only 1 of the 20 patients developed LPTS during the two-year follow-up period.
With respect to adverse events, brief dizziness occurred in the LEV group, which disappeared a few days later. Pearl et al. found that the most common severe adverse events were headache, fatigue, drowsiness and irritability, and there was a higher incidence of infection, mood change, or behavior problems among the treatment patients compared to the observation patients. This may be because that the dose of LEV (adult, 1000 mg/day) was better tolerated in the adult patients in our trial than in the pediatric patients (55 mg/kg per day) in the Pearl’ trial.
Our result was also consistent with the the study of Klein et al. in 2012 [9]. In their study, treatment with LEV at 55 mg/kg per day lasted for 30 days, starting within 8 hours post-brain injury. They found that 15.1% (13/86) of the total adults developed epilepsy two years post-brain injury. More specifically, 10.9% (5/46) of the treated adults and 20% (8/40) of the untreated adults developed epilepsy two years post-brain injury (relative risk, 0.47; P = 0.18). However, although LEV tended to prevent LPTS, this effect was not statistically significant [9].
In the trial by Klein et al., LEV treatment was terminated in 3% of the patients in the LEV group due to somnolence. The most common adverse events were fatigue, headache, and somnolence. The mood scores and number of infections did not differ between the LEV group and the control group [9]. In another study, LEV treatment at 2000 mg/day caused somnolence, manifested as reduced awake time during the Maintenance of Wakefulness Test and increased nap duration and episodes [15]. However, a daily dose of 1000 mg caused no changes in the daytime sleepiness in the Multiple Sleep Latency Test [16]. Therefore, LEV dosage of 1000 mg/day was used in our trial for the prevention of LPTS without somnolence. In studies of Pearl et al. [10] and Klein et al. [9], LEV was administered at the dose of 55 mg/kg per day, and both studies reported common adverse events of headache, fatigue, and drowsiness, indicating that the dose of LEV for prevention of LPTS needs to be reduced (< 55 mg/kg per day) in clinical settings [9, 10].
Our results were also consistent with the study conducted by Chaudhry et al. [17], which reported that LEV caused less adverse effects and had a better tolerability. Although the pathogenesis of LPTS is not fully clear, this condition can be prevented during its incubation period after TBI [13].
Laure Peter-Derex et al. conducted a phase 3 PEACH trial in France, in which patients who had a non-traumatic intracerebral hemorrhage received LEV treatment, starting within 24 h after hemorrhage. They found that LEV effectively prevented acute seizures in intracerebral hemorrhage [18].
There are several limitations in this study. First, the blood concentration of LEV and VPA were not measured in the patients. Second, the study was not blinded, which might have caused a bias in analysis. Third, the sample size was relatively small. Fourth, the duration of follow-up was relatively short. Multicenter, randomized, placebo-controlled trials are needed in the future to explore the preventive effects of LEV against LPTS.
Conclusions
In this study, we found that VPA could not prevent LPTS and caused more adverse events. This is consistent with the findings of Temkin et al. and Paqnl et al. [13, 19]. Valproate sodium can exert antiepileptogenic effects at high doses that may be toxic for human use. However, LEV can effectively reduce the cumulative incidence of LPTS in patients with TBI.
Availability of data and materials
Not applicable.
Abbreviations
- ASM:
-
Anti-seizure medication
- EPTS:
-
Early posttraumatic seizure
- GCS:
-
Glasgow coma scale
- LEV:
-
Levetiracetam
- LPTS:
-
Late posttraumatic seizure
- PTS:
-
Post-traumatic seizures
- SBC:
-
Simple brain contusion
- TBI:
-
Traumatic brain injury
- VPA:
-
Valproic acid
References
Lowenstein DH. Epilepsy after head injury: an overview. Epilepsia. 2009;50(Suppl 2):4–9.
Salinsky M, Storzbach D, Goy E, Evrard C. Traumatic brain injury and psychogenic seizures in veterans. J Head Trauma Rehabil. 2015;30(1):E65-70.
D’Ambrosio R, Perucca E. Epilepsy after head injury. Curr Opin Neurol. 2004;17(6):731–5.
Temkin NR. Risk factors for posttraumatic seizures in adults. Epilepsia. 2003;44(s10):18–20.
Herman ST. Epilepsy after brain insult: targeting epileptogenesis. Neurology. 2002;59(9 Suppl 5):S21–6.
Benardo LS. Prevention of epilepsy after head trauma: do we need new drugs or a new approach? Epilepsia. 2003;44(s10):27–33.
Englander J, Bushnik T, Duong TT, Cifu DX, Zafonte R, Wright J, et al. Analyzing risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Arch Phys Med Rehabil. 2003;84(3):365–73.
Asikainen I, Kaste M, Sarna S. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long-term outcome. Epilepsia. 1999;40(5):584–9.
Klein P, Herr D, Pearl PL, Natale J, Levine Z, Nogay C, et al. Results of phase 2 safety and feasibility study of treatment with levetiracetam for prevention of posttraumatic epilepsy. Arch Neurol. 2012;69(10):1290–5.
Pearl PL, McCarter R, McGavin CL, Yu Y, Sandoval F, Trzcinski S, et al. Results of phase II levetiracetam trial following acute head injury in children at risk for posttraumatic epilepsy. Epilepsia. 2013;54(9):e135–7.
Szaflarski JP, Sangha KS, Lindsell CJ, Shutter LA. Prospective, randomized, single-blinded comparative trial of intravenous levetiracetam versus phenytoin for seizure prophylaxis. Neurocrit Care. 2010;12(2):165–72.
Younus SM, Basar S, Gauri SA, Khan AA, Imran M, Abubakar S, et al. Comparison of Phenytoin versus Levetiracetam in Early Seizure Prophylaxis after Traumatic Brain Injury, at a Tertiary Care Hospital in Karachi. Pakistan Asian J Neurosurg. 2018;13(4):1096–100.
Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323(8):497–502.
Barlow Philip. A Practial review of the glasgow coma scale and score. Surgeon. 2012;10:114–9.
Yilmaz H. Comparison of motor activity and sleep in patients with complex partial seizures on levetiracetam treatment and a group of healthy subjects. Behav Neurol. 2007;18:165–70.
Zhou JY, Tang XD, Huang LL, Zhong ZQ, Lei F, Zhou D. The acute effects of levetiracetam on nocturnal sleep and daytime sleepiness in patients with partial epilepsy. J Clin Neurosci. 2012;19(7):956–60.
Chaudhry SA, Jong G, Koren G. The fetal safety of Levetiracetam: a systematic review. Reprod Toxicol. 2014;46:40–5.
Peter-Derex L, Philippeau F, Garnier P, et al. Safety and efficacy of prophylactic levetiracetam for prevention of epileptic seizures in the acute phase of intracerebral haemorrhage (PEACH): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2022;21:781–91.
Pagni CA, Zenga F. Prevention and treatment of post-traumatic epilepsy. Expert Rev Neurother. 2006;6(8):1223–33.
Acknowledgements
No acknowledgement.
Funding
This study was funded by the Scientific Research Fund of China Association Against Epilepsy (No 2012002).
Author information
Authors and Affiliations
Contributions
Yanli Wang participated in data collection and manuscript writing. Yiqi Wang participated in data collection. Huifang Wang and Xiaoping Du analyzed the results. Jie Miao participated in data collection and analyzed the results. James X. Tao was involved in the revision of the clinical trial protocol, and Meizhen Sun designed and supervised this study. All authors approved the final version of the manuscript.
Authors’ information
Yanli Wang came from Shanxi Traditional Chinese Medical Hospital, James X. Tao came from the University of Chicago, and the other authors came from The First Hospital of Shanxi Medical University.
Corresponding authors
Ethics declarations
Ethics approval and informed consent
The study was approved by the the First Hospital of Shanxi Medical University (No. KYLL-2023–030). All participants had given informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Wang, Y., Wang, H. et al. Effect of levetiracetam and valproate on late-onset post-traumatic seizures. Acta Epileptologica 5, 10 (2023). https://doi.org/10.1186/s42494-023-00121-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42494-023-00121-8