Abstract
Based on patient computerized tomography data, we segmented a region containing an intracranial hematoma using the threshold method and reconstructed the 3D hematoma model. To improve the efficiency and accuracy of identifying puncture points, a point-cloud search arithmetic method for modified adaptive weighted particle swarm optimization is proposed and used for optimal external axis extraction. According to the characteristics of the multitube drainage tube and the clinical needs of puncture for intracranial hematoma removal, the proposed algorithm can provide an optimal route for a drainage tube for the hematoma, the precise position of the puncture point, and preoperative planning information, which have considerable instructional significance for clinicians.
Similar content being viewed by others
Introduction
Trauma can cause blood vessels to burst in the brain or between the skull and brain tissue. Subsequently, an intracranial hematoma may form from blood pooling up in the brain or between the skull and brain, which compresses the brain tissue. An intracranial hematoma is a common but serious secondary damage mode of craniocerebral injury. The incidence of closed craniocerebral injury is approximately 10%, while the incidence of severe craniocerebral injury is approximately 40%–50%. The complications of intracranial hematomas include their effect on cerebral blood flow, cerebral hernia, cerebral edema, and Cushing’s reaction, which can seriously damage brain tissue, and some of these damage modes are irreversible and life-threatening [1,2,3,4]. Puncture and ablation of the intracranial hematoma is a widely used medical method. With the increasingly busy modern life, heavy work stress, mental pressure, and lack of physical labor, the incidence rates show that the age of onset of this condition is decreasing. Once it occurs, the disease places a burden on individuals and families, so all countries and the international community have attached considerable importance to it [2]. The advantages of puncture operation include effective application, high security, quick recovery, short operating time, and relatively few postoperative complications [5,6,7]. At present, the methods of positioning such as the stereotactic technique [8] and neuronavigation technique [9] mainly involve a freehand technique for insertion of a drainage tube, which is based on fixed anatomical landmarks, does not consider individual variations, and often exhibits insufficient precision. Hence, a time-efficient and low-cost technique to localize the hematoma puncture point and to provide path planning will be beneficial, especially when highly sophisticated and expensive navigation systems cannot be made available in developing regions. In the present study, according to a dataset of brain computerized tomography (CT) images, we reconstructed the 3D model of a patient’s brain by using a 3D Slicer software [10,11,12,13,14] and extracted the area of intracranial hematoma as well. To quickly find the best puncture point in theory, an algorithm based on k-means clustering [15] was proposed to optimize the search space, eliminate redundant computation, effectively sort out the search space, and reduce the possibility of particles falling into a local extremum [16]. Then, the algorithm is improved from the viewpoint of the search mode, and the point cloud search algorithm of adaptive weighted particle swarm optimization [17,18,19] is proposed, which greatly reduces the calculation time and the number of iterations of the algorithm. In the experimental environment, the algorithm proposed in this study can greatly improve the search efficiency of this method in obtaining the global optimal solution. Compared with the direct optimization algorithm [20], the average maximum acceleration ratio is 1288.67%. According to the characteristics of the multitube drainage tube [21] and the clinical needs of the puncture process for intracranial hematoma removal, the bifurcation location algorithm provides the precise position of the puncture point, the optimum location of probes, and the best route under ideal conditions. In this study, the 3D model is effectively combined with the multitube drainage tube; furthermore, a preoperative simulation is proposed, which can provide significant guidance and value in formulating the puncture operation plan and decreasing the risk of blindness.
Segmentation of the intracranial hematoma
In clinical medicine, for patients with an intracranial hematoma, most hospitals conduct CT, hematoma location, puncture marking, followed by other processes [5, 6]. We used the 3D Slicer software to implement three-dimensional reconstruction based on brain CT data. This approach can greatly reduce the treatment time and improve efficiency. 3D Slicer software can reconstruct anatomical parts such as the hematoma, blood vessels, skull, and nerve bundles. In this way, doctors can avoid important parts such as blood vessels and nerve bundles when they mark the puncture points and accurately and efficiently puncture the hematoma, thus reducing treatment time [12,13,14]. The 3D Slicer program is a software platform for the analysis (including registration and interactive segmentation) and visualization (including volume rendering) of medical images and for research in image guided therapy. The platform originated in an MSc thesis program in 1998 and is jointly sponsored by the Surgery Plan Laboratory at Brigham and Women’s Hospital and the Artificial Intelligence Laboratory at the Massachusetts Institute of Technology [10,11,12].
Hematoma modeling
After the CT data have been preprocessed, three-dimensional reconstruction of the brain can be realized to obtain a three-dimensional image. In 3D Slicer, the region of interest (ROI) function is used to extract the hematoma area in the image, and the Segmentations module is selected to model the hematoma, providing models in the STL and OBJ formats to extract the “optimal external central axis” needed in this study.
Hematoma location locking
First, the Paint function in Slicer is applied to draw the hematoma, and then, the Fast Marching Method is used to draw the models of the intracerebral hematoma within a three-dimensional sphere, which is of great help in the follow-up work. Finally, the three-dimensional sphere is initialized by the Fast Marching function. Because the mask area around the hematoma is very large after initialization, the mask is narrowed by adjusting the slider for Segment volume, and the position of the hematoma is locked (Fig. 1).
Applying the threshold method to segment the hematoma
We select the Threshold function, in which the threshold is set to 50–100, set the selected intensity range to an editable intensity for the mask, and then, convert the masked area into a 3D model. For multiple small masks that appear in CT images, we use the Smoothing function to make the hematoma easier to segment by removing protrusions and filling holes to make the segmentation boundaries smoother. After smoothing, we use the Islands function to segment the hematoma from the numerous masks. We select the Keep selected island and click on the hematoma to segment it (Fig. 2). For specific modeling steps and methods, please refer to ref. [12].
Methods
Basic concept and definition
As an effective tool to describe the morphological characteristics of the geometry, the center axis has many advantages; for example, it can describe the geometry information inside the model and at any position of the boundary [22,23,24]. Furthermore, it provides a method to intuitively describe the geometric characteristics of a complex model and represent the topological relation of the model. The definition of the external central axis is as follows: Let Ω be a model in n-dimensional Euclidean space Rn, and assume it is homeomorphic to a 3D closed sphere; we define Ω as an n − D model. The external center axis of Ω is the set of the centers of the maximum circumscribed spheres [25]. Inspired by the concept of the external center axis, we found the optimal external center axis suitable for this study. The maximum Euclidean distance in the hematoma point cloud is determined, and the line segment joining the two points is defined as the optimal external center axis.
Algorithm of optimal external central axis extraction
The optimal external central axis can be obtained using a direct optimization algorithm [20]. However, a large amount of computation is required because the time complexity increases at the N2 level with an increasing quantity of point clouds. In the case of a large-scale geometric model, the approach to obtain the central axis is excessively time consuming. Therefore, to reduce the computation redundancy, a point cloud clustering algorithm based on k-means is proposed to optimize the search space [15, 16]. The algorithm presents strong adaptive ability, robustness, and low computation requirements. However, it does not consider the search method, and thus, it is not a suitable method for extracting the axis. Based on the above analysis, we propose the point cloud search arithmetic method for modified adaptive weighted particle swarm optimization [17,18,19, 26,27,28,29,30]. The algorithm reduces computational redundancy and greatly enhances the efficiency of searching axes in complex geometries. Our proposed algorithm is written in MATLAB (The MathWorks, Inc., MA).
Algorithm flow
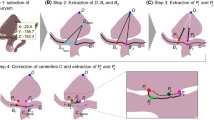
The flow of the point cloud search arithmetic method for modified adaptive weighted particle swarm optimization based on k-means clustering is as follows:
Step 1. First, define the data set D in 3D Euclidean space and randomly select n items (n is the number of selected clusters) as centroids Ci(i = 1, 2, ⋯, n), where each centroid is the clustering center of a category. Then, calculate the Euclidean distance between Ci and Dj(1, 2, ⋯, k) in D. For example, if Dj is the nearest to Ci, it is classified as cluster i.
Step 2. Through the first step, the data set D is initially divided into k classes. Calculate the mean value of each dimension of all data items in each cluster. A new centroid is formed and updated to the new centroid.
Step 3. Repeat steps 1 and 2 to obtain the new centroid until the centroid of each class no longer changes.
Step 4. Randomly initialize the particles in the solution space.
Step 5. Each particle updates its velocity and position by tracking its own and the group’s optimal solution. The velocity updating formula of particle i in the dth dimension is as follows:
The position updating formula is
Repeat step 5 and incrementally iterate until the optimal position of the given threshold is found.
Note: \( {v}_{id}^k \) is the d − th dimensional component of the k − th iterative particle i flight velocity vector; \( {x}_{id}^k \) is the d − th dimensional component of the position vector; c1c2 is the maximum learning step parameter; r1r2 is the random function in the range of [0 − 1]; and ω is the inertia weight of the search, which has a considerable impact on the search scope and method.
Step 6. Compute the mean distance between d1, d2, ⋯, dn: \( {D}_{EXP}=\frac{2\ast \sum {D}_{ij}}{k\ast \left(k-1\right)} \), where \( {D}_{ij}=\sqrt{{\left({x}_i-{x}_j\right)}^2+{\left({y}_i-{y}_j\right)}^2+{\left({z}_i-{z}_j\right)}^2} \) denotes the distance between two clustering centers.
Step 7. Calculate and obtain the difference between Dij and DEXP: Sij = Dij − DEXP, where ωij denotes the ratio of Sij to ∑Sij as the generating weight of the small block ωij: \( {\omega}_{ij}=\frac{S_{ij}}{\sum {S}_{ij}} \).
Step 8. Dynamically adjust the inertial weight of particles according to the NAIW method proposed by Li et al. [30].
where ωmax and ωmin are 0.9 and 0.4, respectively [20], Δxi is the distance between the ith particles and the extreme position of the group, and k is the iteration coefficient. The NAIW method balances the weight of the particles under different circumstances, and it has advantages in convergence and robustness. Figure 3 is the flow diagram of the algorithm.
Optimal path planning
According to clinical demand and geometry symmetry, the straight line, where the optimal external axis is, is the path of the drainage tube into the brain. The multitube drainage tube can be divided into two subtubes and three subtube drainage tubes. The purpose of this study is to provide the corresponding optimal path planning for a specific multitube drainage tube [21]. The Table 1 is the explanation of symbols.
The known conditions can be determined by M 1 and M 2
According to the intersection coordinates M1(x1, y1, z1) and M2(x2, y2, z2), we obtain the parameter equation of the center axis as follows:
\( \Big\{{\displaystyle \begin{array}{c}x={x}_1+\left({x}_2-{x}_1\right)\ t\\ {}y={y}_1+\left({y}_2-{y}_1\right)\ t\\ {}z={z}_1+\left({z}_2-{z}_1\right)\ t\end{array}},\mathrm{where}\ 0\le t\le 1 \) (6)
The midpoint coordinates of M1M2 are \( {M}_{1/2}=\left(\frac{x_1+{x}_2}{2},\frac{y_1+{y}_2}{2},\frac{z_1+{z}_2}{2}\right) \).
The distance from M1 to M2 is \( {d}_{12}=\sqrt{{\left({x}_2-{x}_1\right)}^2+{\left({y}_2-{y}_1\right)}^2+{\left({z}_2-{z}_1\right)}^2} \).
The plane equation of M3M4 is
The distance from M3 to M4 is \( {d}_{34}=\sqrt{{\left({x}_4-{x}_3\right)}^2+{\left({y}_4-{y}_3\right)}^2+{\left({z}_4-{z}_3\right)}^2} \).
A comparison of the distance between d12 and \( {d}_{B_i} \) can offer directional evidence for puncture surgery.
The flow diagram of the bifurcation location algorithm of the drainage tube is as shown in Fig. 4.
Optimal path planning of two subtube drainage tubes
According to the clinical demand, the best position of the drainage tube in the hematoma is the midpoint between the two holes, coinciding with the midpoint of the central axis, that is, \( {M}_{1/2}=\left(\frac{x_1+{x}_2}{2},\frac{y_1+{y}_2}{2},\frac{z_1+{z}_2}{2}\right) \), and the path of the main drainage tube coincides with the optimal external center axis.
Calculating the coordinates of B 1 and B 2
Substituting t1, t2 into Eq. (6), we obtain the coordinates of \( {B}_1\left({x}_{B_1},{y}_{B_1},{z}_{B_1}\right) \) and \( {B}_2\left({x}_{B_2},{y}_{B_2},{z}_{B_2}\right) \).
Calculating the direction vector of the subtube
To find two line segments through M3 with a fixed angle between this axis and the center axis, let the intersection points between the line segment and the axis be M31, M32; these points should meet the following limits:
Substituting t31, t32 into Eq. (6), we obtain the coordinates of M31, M32; the direction vector of subtubes are \( \overrightarrow{M_{31}{M}_3}=\left({m}_{31},{n}_{31},{k}_{31}\right) \) and \( \overrightarrow{M_3{M}_{32}}=\left({m}_{32},{n}_{32},{k}_{32}\right) \), and the parameter equation of the two subtubes is as follows:
Scenario one:
\( \left\{{\displaystyle \begin{array}{c}x={x}_{B_1}+{m}_{31}l\\ {}y={y}_{B_1}+{m}_{31}l\\ {}z={z}_{B_1}+{m}_{31}l\end{array}},\right\{{\displaystyle \begin{array}{c}x={x}_{B_2}+{m}_{32}l\\ {}y={y}_{B_2}+{m}_{32}l\\ {}z={z}_{B_2}+{m}_{32}l\end{array}} \) (12)
Scenario two:
\( \left\{{\displaystyle \begin{array}{c}x={x}_{B_1}+{m}_{32}l\\ {}y={y}_{B_1}+{m}_{32}l\\ {}z={z}_{B_1}+{m}_{32}l\end{array}},\right\{{\displaystyle \begin{array}{c}x={x}_{B_2}+{m}_{31}l\\ {}y={y}_{B_2}+{m}_{31}l\\ {}z={z}_{B_2}+{m}_{31}l\end{array}},\left(\mathrm{where}\ l\ge 0\right) \) (13)
The intersection points between the four rays and the boundary of the hematoma are B11, B21, B12, and B22.
In the first scenario, the coordinates of the probe tip of the subtube are as follows:
\( {B}_1^F=\left(\frac{x_{B_1}+{x}_{B_{11}}}{2},\frac{y_{B_1}+{y}_{B_{11}}}{2},\frac{z_{B_1}+{z}_{B_{11}}}{2}\right),{B}_2^F=\left(\frac{x_{B_2}+{x}_{B_{21}}}{2},\frac{y_{B_2}+{y}_{B_{21}}}{2},\frac{z_{B_2}+{z}_{B_{21}}}{2}\right) \) (15)
In the second scenario, the coordinates of the probe tip of the subtube are.
\( {B}_1^F=\left(\frac{x_{B_1}+{x}_{B_{12}}}{2},\frac{y_{B_1}+{y}_{B_{12}}}{2},\frac{z_{B_1}+{z}_{B_{12}}}{2}\right),{B}_2^F=\left(\frac{x_{B_2}+{x}_{B_{22}}}{2},\frac{y_{B_2}+{y}_{B_{22}}}{2},\frac{z_{B_2}+{z}_{B_{22}}}{2}\right) \) (16)
The optimal path planning of three subtube drainage tubes
According to the clinical demand, the best position of the drainage tube in the hematoma is the midpoint of the second hole, coinciding with the midpoint of the central axis, i.e., \( {M}_{1/2}=\left(\frac{x_1+{x}_2}{2},\frac{y_1+{y}_2}{2},\frac{z_1+{z}_2}{2}\right) \), with the path of the main drainage tube coinciding with the optimal external center axis.
Calculating the coordinate of B 1, B 2, and B 3
Substituting t11, t12 and t13 into Eq. (6), we obtain the coordinates \( {B}_1\left({x}_{B_1},{y}_{B_1},{z}_{B_1}\right) \), \( {B}_2\left({x}_{B_2},{y}_{B_2},{z}_{B_2}\right) \), and \( {B}_3\left({x}_{B_3},{y}_{B_3},{z}_{B_3}\right) \).
Calculating the direction vector of the subtube
To find a line segment through M3 with a fixed angle between the axis M1M2 and the center axis, let the intersection points between the line segment and the axis be M31(x31, y31, z31); they should satisfy the following limits:
We obtain \( \overrightarrow{M_{31}{M}_3}=\left({x}_3-{x}_{31},{y}_3-{y}_{31},{z}_3-{z}_{31}\right)=\left({m}_1,{n}_1,{k}_1\right) \).
Because the angle between two subtubes and the angle between the subtube and the main tube are fixed values, we can calculate the other two direction vectors of the subtubes based on the geometrical relationship.
The parametric equation of the three subtubes is as follows.
There are three cases for the direction vector of each subtube; let the coordinates of the subtube and the boundary of the hematoma be B11, B12, B13; B21, B22, B23; B31, B32, B33.
When we find the maximum value \( {d}_{B_i} \), the coordinates of the bifurcation subtube probe in hematoma can be obtained as \( \frac{B_1{B}_{1i}}{2} \), \( \frac{B_2{B}_{2j}}{2} \), and \( \frac{B_3{B}_{3k}}{2} \).
Results and discussion
According to the CT data, the hematoma area was segmented, and an independent three-dimensional diagram was obtained, as shown in Fig. 5.
The STL format of a three-dimensional point cloud is obtained by hematoma modeling. Figure 6 shows the three-dimensional point cloud diagram in the hematoma area, and Fig. 7 shows the triangulation diagram of this area [24].
In this case, the point cloud search arithmetic method for modified adaptive weighted particle swarm optimization to search the hematoma region uses 81,226 point-chains. The coordinates of the intersection between the central axis and the boundary of the hematoma are M1(x1, y1, z1) and M2(x2, y2, z2). The optimal external central axis of the hematoma is shown in Fig. 8. The intersection points of the central axis and the boundary of the hematoma are M1(28.555, 9.623, 7.123) and M2(43.384, 21.295, 48.887).
Therefore, the midpoint coordinates of the center axis M1M2 are M1/2(35.970, 5.836, 28.010), and the distance from M1 to M2 is d12 = ∣ M1M2 ∣ ≈ 54.0377. Figure 8 is optimal external central axis of the hematoma.
The route planning for the puncture operation is as follows:
Because \( \frac{d_{B_3}-{d}_{B_2}}{2}+{d}_{B_2}=35<{d}_{12}\approx 54.0377 \), it is suggested that doctors choose three subtube drainage tubes when performing puncture operations. From the coordinate of the center axis, the plane equation through M3M4 can be obtained as
According to the point cloud search arithmetic method for modified adaptive weighted particle swarm optimization, we obtain the coordinates as M3(26.201.451, 18.457) and M4(36.353, −7.797, 37.816), and the distance from M3 to M4 is d34 = 38.134751.
Through the above algorithm process, the coordinates of the three bifurcation points can be calculated
The three direction vectors of the subtube are as follows:
There are three cases for the direction vector of each subtube; let the coordinates of the subtube and the boundary of the hematoma be B1, 1, B1, 2, B1, 3; B2, 1, B2, 2, B2, 3; B3, 1, B3, 2, B3, 3. The maximum value from the bifurcation point to the boundary of the hematoma is
The coordinates of the bifurcation probe to reach the hematoma area are
Figure 9 is a schematic diagram of the path planning process.
In this study, 3D Slicer software was used to reconstruct a three-dimensional model of an intracranial hematoma, and an improved algorithm based on clustering and adaptive particle swarm optimization to extract the optimal external central axis of the hematoma was proposed. According to the characteristics of the drainage tube and the specific morphology of the intracranial hematoma, optimal path planning under ideal conditions was performed, and the preoperative simulation was provided, which will be useful in clinical medicine.
The bifurcation localization algorithm of the drainage tube proposed in this study assumes an ideal state. It is necessary to consider the distribution of peripheral nerves and blood vessels and the specific location of the hematoma in the brain to apply this method to clinical medicine. The focus of subsequent research work will be to optimize the algorithm by considering the above factors, to implement the diffusion model and simulation of the drug injection, to plan the optimal surgical project, and to provide a dynamic simulation before operation.
Availability of data and materials
The datasets used and/or analysed during the current study are not publicly available due to personal privacy but are available from the corresponding author on reasonable request.
Supplementary Materials: The following are available online at www.mdpi.com/xxx/s1, Figures, Tables .
Abbreviations
- CT:
-
Computerized tomography
- ROI:
-
Region of interest
References
Fiorella D, Zuckerman SL, Khan IS, KumarN G, Mocco J (2015) Intracerebral hemorrhage: a common and devastating disease in need of better treatment. World Neurosurg 84(4):1136–1141. https://doi.org/10.1016/j.wneu.2015.05.063
Qureshi AI, Tuhrim S, Broderick JP, Batjer H, Hondo H, Hanley DF (2001) Spontaneous intracerebral hemorrhage. N Engl J Med 344(19):1450–1460. https://doi.org/10.1056/NEJM200105103441907
Xiao FR, Chiang IJ, Wong JM, Tsai YH, Huang KC, Liao CC (2011) Automatic measurement of midline shift on deformed brains using multiresolution binary level set method and Hough transform. Comput Biol Med 41(9):756–762. https://doi.org/10.1016/j.compbiomed.2011.06.011
Samadani U, Rohde V (2009) A review of stereotaxy and lysis for intracranial hemorrhage. Neurosurg Rev 32(1):15–22. https://doi.org/10.1007/s10143-008-0175-z
Wang GQ, Li SQ, Huang YH, Zhang WW, Ruan WW, Qin JZ et al (2014) Can minimally invasive puncture and drainage for hypertensive spontaneous basal ganglia intracerebral hemorrhage improve patient outcome: a prospective non-randomized comparative study. Mil Med Res 1:10. https://doi.org/10.1186/2054-9369-1-10
Zhou HG, Zhang Y, Liu L, Han X, Tao YH, Tang YP et al (2011) A prospective controlled study: minimally invasive stereotactic puncture therapy versus conventional craniotomy in the treatment of acute intracerebral hemorrhage. BMC Neurol 11:76. https://doi.org/10.1186/1471-2377-11-76
Delcourt C, Anderson C (2012) Acute intracerebral haemorrhage: grounds for optimism in management. J Clin Neurosci 19(12):1622–1626
Backlund EO, von Holst H (1978) Controlled subtotal evacuation of intracerebral haematomas by stereotactic technique. Surg Neurol 9(2):99–101. https://doi.org/10.1016/j.jocn.2012.05.018
Yan YF, Ru DW, Du JR, Shen X, Wang ES, Yao HB (2015) The clinical efficacy of neuronavigation-assisted minimally invasive operation on hypertensive basal ganglia hemorrhage. Eur Rev Med Pharmacol Sci 19(14):2614–2620
Cao YF (2019) Introduction of 3D slicer. https://www.slicercn.com/?page_id=485. Accessed 10 Apr 2019
Pinter C, Lasso A, Pieper S, Plesniak W, Kikinis R, Miller J (2019) Segment editor. https://slicer.readthedocs.io/en/latest/user_guide/module_segmenteditor.html. Accessed 10 Apr 2019
Zhang XL, Zhang KX, Pan QL, Chang JC (2019) Three-dimensional reconstruction of medical images based on 3D slicer. J Complexity Health Sci 2(1):1–12. https://doi.org/10.21595/chs.2019.20724
Pszczolkowski S, Law ZK, Gallagher RG, Meng DW, Swienton DJ, Morgan PS et al (2019) Automated segmentation of haematoma and perihaematomal oedema in MRI of acute spontaneous intracerebral haemorrhage. Comput Biol Med 106:126–139. https://doi.org/10.1016/j.compbiomed 2019.01.022
Zhang J, Yan CH, Chui CK, Ong SH (2010) Fast segmentation of bone in CT images using 3D adaptive thresholding. Comput Biol Med 40(2):231–236. https://doi.org/10.1016/j.compbiomed.2009.11.020
Wagstaff K, Cardie C, Rogers S, Schrodl S (2001) Constrained k-means clustering with background knowledge. In: Abstracts of the 18th international conference on machine learning. Morgan Kanufman Press, San Francisco
Likas A, Vlassis N, Verbeek JJ (2003) The global k-means clustering algorithm. Pattern Recogn 36(2):451–461. https://doi.org/10.1016/S0031-3203(02)00060-2
Bai QH (2010) Analysis of particle swarm optimization algorithm. Computer Inf Sci 3(1):180–184. https://doi.org/10.5539/cis.v3n1p180
Xing H, Pan XJ (2018) Application of improved particle swarm optimization in system identification. In: Abstracts of 2018 Chinese control and decision conference. IEEE, Shenyang. https://doi.org/10.1109/CCDC.2018.8407336
Su Q, Yang LH, Fu YG, Wu YJ, Gong XT (2014) Parameter training approach based on variable particle swarm optimization for belief rule base. J Comput Appl 34(8):2161–2165
Khong SZ, Nešić D, Manzie C, Tan Y (2013) Multidimensional global extremum seeking via the DIRECT optimisation algorithm. Automatica 49(7):1970–1978. https://doi.org/10.1016/j.automatica.2013.04.006
Cui JZ, Cui Y (2019) The multifunctional drainage tube with multi-tube for intracranial hematoma. CN patent CN208405725U
Siddiqi K, Pizer SM (2008) Medial representations: mathematics, algorithms and applications. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-8658-8
Wang Y, Li J, Chen S (2011) A novel method of extracting 3D blood vessel images axis based on energy constraint equation. J Comput Inf Syst 7(4):1319–1327
Zhong YJ, Chen FL (2018) Computing medial axis transformations of 2D point clouds. Graph Model 97:50–63. https://doi.org/10.1016/j.gmod.2018.03.004
Zhong YJ (2018) Computing medial axis transformations of the geometric model. J Comput Aided Des Comput Graph 30(8):1394–1412. https://doi.org/10.3724/SP.J.1089.2018.16790
Feng CS, Cong S, Feng XY (2007) A new adaptive inertia weight strategy in particle swarm optimization. In: Abstracts of 2007 IEEE congress on evolutionary computation. IEEE, Singapore, pp 25–28. https://doi.org/10.1109/CEC.2007.4425017
Zhang LM, Tang YG, Hua CC, Guan XP (2015) A new particle swarm optimization algorithm with adaptive inertia weight based on Bayesian techniques. Appl Soft Comput 28:138–149. https://doi.org/10.1016/j.asoc.2014.11.018
Li ZQ, Zheng H, Pei CM (2010) Particle swarm optimization algorithm based on adaptive inertia weight. In: Abstracts of the 2010 2nd international conference on signal processing systems. IEEE, Dalian, pp 5–7
Ao YC, Shi YB, Zhang W, Li YJ (2014) Improved particle swarm optimization with adaptive inertia weight. J Univ Electron Sci Technol China 43(6):874–880
Li LS, Zhang XJ (2018) New chaos particle swarm optimization based on adaptive inertia weight. Comput Eng Appl 54(9):139–144
Acknowledgments
Authors thank Tangshan Gongren Hospital for their support of original data.
Funding
This research was funded by the National Science Foundation of China, Nos. 51674121 and 61702184; the Returned Overseas Scholar Funding of Hebei Province, No. C2015005014; the Hebei Key Laboratory of Science and Application, and Tangshan Innovation Team Project, No. 18130209B.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. Conceptualization, JChang, JCui, QP; methodology, QP and WZ; software, WZ and XZ; formal analysis, QP; resources, JChang; data curation, JChang and JCui; writing—original draft preparation, QP; writing—review and editing, QP; visualization, QP and WZ; supervision, JChang and JCui; funding acquisition, JChang.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pan, Q., Zhu, W., Zhang, X. et al. Research on a bifurcation location algorithm of a drainage tube based on 3D medical images. Vis. Comput. Ind. Biomed. Art 3, 2 (2020). https://doi.org/10.1186/s42492-019-0039-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42492-019-0039-0