Abstract
Xanthomonas campestris pv. campestris (Xcc) is the causal agent of black rot in Brassica vegetables, which can induce the host plant to produce salicylic acid and 4-hydroxybenzoic acid (4-HBA) during infection. Xcc was previously shown to sense and degrade host plant-derived 4-HBA via the sensor PobR and a PobA-dependent pathway. The degradation of 4-HBA is associated with Xcc virulence in cabbage. The present study generated a reporter strain XC1::PpobA-gusA to monitor pobA transcription. 4-HBA-like compounds were screened for their ability to interfere with pobA transcription. Benzoic acid (BA) was found to efficiently decrease pobA transcription in a dose-dependent manner. Xcc neither produced nor degraded BA; however, the exogenous addition of BA to the 4-HBA-containing Xcc culture significantly decreased the 4-HBA degradation rate. Furthermore, addition of BA into the Xcc culture did not significantly affect the transcription of pobA or pobR; however, addition of BA into the 4-HBA-containing culture significantly decreased the transcription of both genes. Isothermal titration calorimetry and an electrophoretic mobility shift assay revealed that BA binds to PobR with a moderate affinity, which interfered with the binding of 4-HBA/PobR complex to the pobA promoter and thereby inhibiting pobA transcription and 4-HBA degradation. The endogenous BA level of the infected cabbage leaves increased in response to Xcc infection. In the presence of BA, the virulence of Xcc on cabbage decreased significantly. Taken together, these results suggest that cabbage utilizes BA to interfere with 4-HBA degradation, thereby reducing Xcc virulence. Thus, BA has the potential to be developed as a bactericide against Xcc infection.
Similar content being viewed by others
Background
The Xanthomonas genus is one of the most ubiquitous groups of plant-associated bacterial pathogens. Members of this genus have been shown to infect a wide range of plant species, including many of agricultural interest, e.g., rice, wheat, cotton, oil-rape, banana, cassava, citrus, and mango (Leyns et al. 1984; Hayward 1993). Among them, Xanthomonas campestris pv. campestris (Xcc) is the causal agent of black rot, which may be the most important disease of crucifers worldwide. Xcc generally enters the plant through hydathodes on the leaf margins or the wounds. The typical symptom of black rot is the formation of V-shaped, chlorotic yellow lesions, and darkened veins that result from bacterial movement in the vascular system (Vicente and Holub 2013). Because of its importance in agriculture and the deep understanding of virulence and plant-pathogen interactions (Büttner and Bonas 2010; Zhou et al. 2017; Timilsina et al. 2020), Xcc is considered to be one of the top 10 plant pathogenic bacteria (Mansfield et al. 2012) and is an ideal model pathogen for research toward solutions in disease control (Timilsina et al. 2020).
In nature, 4-hydroxybenzoic acid (4-HBA) is a common plant phenolic acid released into the soil and waterbodies from plant leaf litter through foliar leachates and lignin decomposition (Macías et al. 2020; Nutautaitė 2022). 4-HBA can also be produced from fossil fuel consumption and the wide use of the preservative paraben (Simoneit 2002; Fuchs et al. 2011; Petric et al. 2021). 4-HBA is notoriously stable, but it does not persist in the environment due to microorganism catabolism. Aerobic catabolism of 4-HBA in bacteria often yields protocatechuic acid (PCA), which is catalyzed by a flavin-dependent monooxygenase (4-HBA-3-hydroxylase). Subsequently, PCA is cleaved through the meta-cleavage pathway (catalyzed by 4,5-dioxygenase) or the ortho-cleavage pathways (catalyzed by 3,4-dioxygenase), which is eventually funneled into the Krebs cycle (Kamimura and Masai 2014; Zhang et al. 2018; Tsagogiannis et al. 2024). Notably, 4-HBA catabolism in Xcc was reported to follow the ortho-cleavage pathway (Chen et al. 2020).
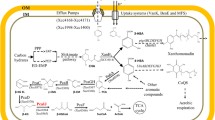
4-HBA is an essential metabolite in Xcc (Fig. 1). Xcc has evolved several mechanisms to maintain 4-HBA homeostasis. First, Xcc uses multiple pathways to biosynthesize 4-HBA. It can efficiently uptake 4-HBA from the surrounding environment (He et al. 2011; Chen et al. 2020) and can de novo synthesize 4-HBA using the precursor chorismite, the end product of the shikimate pathway, via a bifunctional chorismate-lyase XanB2 (Zhou et al. 2013). Xcc can also sense and degrade 4-hydroxycinnamic acid (4-HCA), an important plant phenolic acid, into 4-HBA via the hca gene family-encoded proteins (Chen et al. 2022). Second, Xcc can utilize 4-HBA as a precursor to synthesize coenzyme Q8 (CoQ8) via enzymes encoded by ubi genes (Zhou et al. 2019). Third, Xcc can efflux 4-HBA from cells (Zhou et al. 2013). Finally, Xcc can sense 4-HBA via the sensor regulator PobR and initiate pobA transcription to convert 4-HBA into PCA, which can be further degraded via the β-ketoadipate pathway and eventually formed acetyl-CoA and succinyl-CoA (Chen et al. 2020; Fig. 1). Among these pathways, the 4-HBA degradation pathway has been shown to be a key contributor to 4-HBA homeostasis in Xcc (Chen et al. 2020).
4-HBA is an essential metabolite in the phytopathogen Xanthomonas campestris pv. campestris. In Xcc, a unique chorismatase, XanB2, catalyzes shikimic pathway-derived chorismate into 4-HBA. Xcc can take up 4-HBA. Xcc also takes up 4-HCA and converts 4-HCA into 4-HBA via the hcaLHD cluster-encoding proteins. Xcc uses 4-HBA as a substrate to synthesize coenzyme Q8 (CoQ8) via the ubiABCDEFGHIJX cluster-encoding proteins. In presence of a high level of 4-HBA, Xcc can degrade 4-HBA via the pathway involving PobA-PcaABCDEF. PEP, phosphoenolpyruvic acid; E4P, erythrose 4-phosphate; 4-HBD, 4-hydroxybenzaldehyde; 4-HCA, 4-hydroxycinnamic acid; OHB, 3-octaprenyl-4-hydroxybenzoate; PCA, protocatechuic acid
Our previous study showed that the 4-HBA level in the leaf tissues of cabbage and Chinese radish was significantly induced upon Xcc infection (Chen et al. 2020). The transcription of the pobA-dependent 4-HBA degradation pathway is induced during Xcc infection inside the host plant and is required for full virulence on Chinese cabbage (Chen et al. 2020). In this study, we further revealed that the 4-HBA-like compound benzoic acid (BA) interfered with 4-HBA-induced pobA transcription and 4-HBA degradation by competitively binding to PobR in Xcc. In response to Xcc infection, the BA level at the infection site of cabbage was significantly induced. In the presence of BA, Xcc virulence on cabbage was significantly reduced. These findings suggest that host plants probably use BA as a defense compound, interfering with the 4-HBA degradation activity of Xcc and thereby reducing its virulence.
Results
Screening for compounds that interfere with pobA transcription
Our previous study showed that pobA, a key gene required for 4-HBA degradation in Xcc, was significantly induced in the presence of 4-HBA via the 4-HBA receptor PobR (Chen et al. 2020). In this study, to monitor pobA transcription in XC1, a pobA promoter-gusA fusion reporter strain, XC1::PpobA-gusA, was constructed and β-Glucuronidase (GUS) activity of the reporter strain was determined to indicate the pobA transcriptional level (Fig. 2a). In the presence of 0.01–1.0 mM 4-HBA, PpobA-dependent GUS activity was specifically induced in a dose-dependent manner (Fig. 2b). Further quantitative reverse transcription PCR (qRT-PCR) analysis verified the 4-HBA-dependent pobA transcription (Fig. 2c).
Construction and verification of the reporter strain XC1::PpobA-gusA to monitor the transcriptional levels of pobA. a Schematic diagram of the reporter strain for monitoring pobA transcription. b The pobA promoter PpobA-dependent β-glucuronidase (GUS) activity in XYS liquid medium or XYSG agar plates supplemented with 0.001–1.0 mM 4-hydroxybenzoic acid (4-HBA). c The quantitative reverse transcription-PCR (qRT-PCR) analysis of the relative expression level of pobA in the presence of 0.001–0.5 mM 4-HBA. The mean values of three technical repeats are shown with the standard deviation. Statistically significant differences are indicated by one asterisk (p ≤ 0.05), two asterisks (p ≤ 0.01), or three asterisks (p ≤ 0.001)
To screen for putative compounds interfering with 4-HBA-dependent pobA induction, thirteen 4-HBA-like compounds were independently added to the 4-HBA-containing XYS culture of XC1::PpobA-gusA at a final concentration ranging from 0.1 to 0.4 mM (Fig. 3a). After growth for 24 h, the GUS activity of the XC1::PpobA-gusA culture was determined. Two compounds, BA and 4-HCA, were identified to significantly affect 4-HBA-induced GUS activity of the reporter strain in a dose-dependent manner, other compounds had no such effect at all three concentrations (Fig. 3a). These results indicate that addition of BA or 4-HCA significantly affects 4-HBA-dependent pobA transcription in XC1.
Effects of 4-HBA-like analogues on PpobA-dependent GUS activity in presence of 4-HBA. a GUS activities of the XC1::PpobA-gusA cultures in presence of 0.2 mM 4-HBA and 4-HBA analogues (0.1–0.4 mM). b Left, colonies of strain XC1::PpobA-gusA on agar plates supplemented with 4-HBA and BA; Right, quantitative reverse transcription-PCR (qRT-PCR) analysis of the relative expression level of pobA in 4-HBA treated XC1 in the presence of 0.1–0.2 mM BA. c Relative β-glucuronidase (GUS) activities of the XC1::PpobA-gusA cultures in the presence of five combinations of low levels of 4-HBA and BA . 4-HCA, 4-hydroxycinnamic acid; NaSA, sodium salicylate; SA, 2-hydroxybenzoic acid; 3-HBA, 3-hydroxybenzoic acid; 3M4HBA, 4-hydroxy-3-methoxybenzoic acid; 3M4HBD, 4-hydroxy-3-methoxybenzaldehyde; 3,4-DHBA, protocatechuic acid; 3,4,5-THBA, 3,4,5-trihydroxybenzoic acid; 3MBA, 3-methoxybenzoic acid; CA, cinnamic acid; 3-HCA, 3-hydroxycinnamic acid; 3,4-DHCA, caffeic acid; BA, benzoic acid. The mean values of three technical repeats are shown with the standard deviation. Statistically significant differences are indicated by one asterisk (p ≤ 0.05) or two asterisks (p ≤ 0.01)
BA has an inhibitory effect on 4-HBA-induced pobA transcription
As shown in Fig. 3a, in the presence of 4-HBA, the addition of 0.1–0.4 mM BA inhibited 4-HBA-induced GUS activity in a dose-dependent manner. Similarly, the addition of BA to the 4-HBA-containing XYS agar plate also significantly decreased the density of GUS-dependent blue color of reporter strain XC1::PpobA-gusA (Fig. 3b). Further qRT-PCR analysis revealed that the pobA transcriptional level in the 4-HBA-treated XC1 was significantly decreased in the presence of 0.1–0.2 mM BA (Fig. 3b). Furthermore, five sets of 4-HBA and BA combinations at lower concentrations (4–80 μM) were used to confirm the inhibitory effect of BA on 4-HBA-induced pobA expression (Fig. 3c). Even in the presence of 4 μM 4-HBA, the addition of 2 μM BA still reduced the 4-HBA-dependent GUS activity. These results further confirmed the interference effects of BA on 4-HBA-dependent pobA expression.
BA was not detected in XC1 that was grown in XYS or NYG medium (Additional file 1: Figure S1), suggesting that Xcc does not produce BA. In the absence of 4-HBA, the addition of BA to the XYS cultures for reporter strain XC1::PpobA-gusA had no significant effect on 4-HBA-dependent GUS activity (Additional file 1: Figure S2a). The addition of BA to XYS agar plates also failed to induce the 4-HBA-dependent blue color in reporter strain XC1::PpobA-gusA (Additional file 1: Figure S2b). These results suggest that BA specifically interferes with 4-HBA-induced pobA transcription.
4-HCA induces pobA transcription via its degradation product 4-HBA
Previously, our results showed that Xcc could convert 4-HCA into 4-HBA via the proteins encoded by the hca cluster, where HcaL was the first enzyme involved in this conversion (Chen et al. 2022; Additional file 1: Figure S3a). To determine how 4-HCA induces PpobA-dependent GUS activity in reporter strain XC1::PpobA-gusA, we generated a reporter strain ΔhcaL::PpobA-gusA that are unable to convert 4-HCA into 4-HBA. In the presence of 4-HBA, the addition of 4-HCA (0.1 and 0.5 mM, respectively) failed to induce GUS activity in the reporter strain ΔhcaL::PpobA-gusA (Additional file 1: Figure S3b). However, in absence of 4-HCA, the addition of 4-HBA to the same reporter strain strongly induced GUS activity (Additional file 1: Figure S3b). These results indicate that 4-HCA induces PpobA-dependent GUS activity through its degradation product 4-HBA in reporter strain XC1::PpobA-gusA.
BA interferes with 4-HBA degradation in Xcc
BA has been shown to be catabolized by a few microorganisms aerobically or anaerobically (Carmona et al. 2009; Díaz et al. 2013). The genes encoding the reported BA degradation enzymes were not identified in the genome of Xcc strain XC1. Consistently, XC1 did not exhibit the ability to degrade BA as BA levels in the XYS culture of XC1 remained constant after incubation at 28°C for 36 h (Fig. 4a). The addition of 0.1–0.5 mM BA to the XC1 culture did not significantly affect bacterial growth (Fig. 4b); however, when BA (0.1–0.5 mM) was added to the XC1 culture in XYS medium supplemented with 0.5 mM 4-HBA, the 4-HBA degradation rate was decreased in a dose-dependent manner (Fig. 4c). These results suggest that BA can effectively interfere with 4-HBA degradation in Xcc.
BA interferes with 4-HBA degradation in Xcc. a Ability of Xcc in degrading BA over time. b Growth time course of XC1 in XYS medium supplemented with 0.5 mM 4-HBA and 0.1–0.5 mM BA. c Time course of 4-HBA degradation in XC1 cultures supplemented with 0.5 mM 4-HBA and 0.1–0.5 mM BA. The mean values of three technical repeats are shown
Furthermore, the interfering effects of the BA-like compounds salicylic acid (SA) and 3-hydroxybenzoic acid (3-HBA) on 4-HBA degradation were also determined. The results showed that the addition of SA and 3-HBA (0.1–0.5 mM) into the XC1::PpobA-gusA culture did not significantly interfere with 4-HBA degradation (Additional file 1: Figure S4). These results suggest that BA specifically interferes with 4-HBA degradation.
BA interferes with 4-HBA-induced pobR expression
Previously, we showed that XC1 sensed 4-HBA via the AraC family transcriptional factor PobR to positively regulate the expression of pobA via a direct interaction with promoter PpobA to initiate 4-HBA degradation (Chen et al. 2020; Fig. 5a). In this study, using the previously constructed reporter strain XC1::PpobR-gusA, we showed that the addition of BA had no significant effect on pobR expression (Fig. 5b). However, the addition of 0.2 or 0.4 mM BA to the culture of XC1::PpobR-gusA supplemented with 0.4 mM 4-HBA significantly decreased PpobR-dependent GUS activity (Fig. 5c). These results suggest that BA interferes with 4-HBA degradation by affecting 4-HBA-induced pobR transcription.
BA competes with 4-HBA for pobR transcription. a The pobR/pobA cluster in XC1. b The addition of BA (0.1–0.4 mM) did not affect the GUS activities of reporter strain XC1::PpobR-gusA. c Addition of 0.2–0.4 mM BA to the XC1::PpobR-gusA cultures significantly decreased pobR promoter-dependent GUS activities in presence of 0.2 mM 4-HBA. XC1::gusA was used as a negative control. The mean values of three technical repeats are shown. Statistically significant differences are indicated by one asterisk (p ≤ 0.05) or two asterisks (p ≤ 0.01)
BA interferes with the binding of the 4-HBA/PobR complex to the pobA promoter PpobA
Our previous results showed that 4-HBA binds to its sensor PobR to form a 4-HBA/PobR dimer complex, and the complex further binds to pobA promoter PpobA to activate pobA transcription (Chen et al. 2020). These findings led us to hypothesize that BA might compete with 4-HBA for PobR binding to interfere with 4-HBA degradation. To test this hypothesis, we first examined the interactions between BA and the purified PobR using an isothermal titration calorimetry (ITC) assay. Our results showed that BA had a medium level of binding activity to the PobR dimer, with a Kd of 15.22 μM (Fig. 6a, b). A further electrophoretic mobility shift assay (EMSA) confirmed that the addition of 0.2–3.2 mM BA to the reaction mixture significantly decreased the binding activity of the Cy5-labeled probe Cy5-PpobA and 4-HBA/PobR complex (Fig. 6c). In contrast, in presence of BA/PobR complex and Cy5-labeled probe Cy5-PpobA, no band shift was observed (Additional file 1: Figure S5). These results suggest that BA interferes with 4-HBA degradation, probably by competing with 4-HBA for PobR binding.
BA interferes with 4-HBA degradation by competing with 4-HBA for PobR binding. a SDS-PAGE analysis of purified PobR. b ITC analysis of the binding ability of BA to PobR. c Addition of BA disturbed the binding of 4-HBA/PobR complex to the promoter probe PpobA. EMSA was performed using 233-bp Cy5-labeled DNA probe PpobA in the presence of 100 ng PobR, 0.8 mM 4-HBA, and 0.2–3.2 mM BA
Xcc infection induces cabbage to produce more BA around the infection site
Thus far, the data regarding the inference of BA on 4-HBA catabolism were derived from an in vitro study in which BA was exogenously added to Xcc culture. As Xcc is a xylem-dwelling phytopathogen and its 4-HBA degradation capacity contributes to its virulence, it is important to determine the endogenous BA levels in the host plants and investigate whether the BA levels changes in the host plant after Xcc infection.
To this end, mature leaves of cabbage (Jingfeng-1) were collected, and BA was extracted following the method described by Chen et al. previously (2020). BA levels in the leaf extracts were detected and quantitatively analyzed using ultra-high performance liquid chromatography coupled with triple quadrupole tandem mass spectrometry (UHPLC-QQQ-MS/MS) (Additional file 1: Figure S6). UHPLC-QQQ-MS/MS detected BA in the cabbage leaf extracts (Fig. 7a), revealing that the endogenous concentration of BA was 4.73 pmol/mg (FW) (Fig. 7b).
Relative BA concentration in cabbage leaf tissues. a Detection of BA using UHPLC-QQQ-MS/MS. b The relative BA level in the leaf tissues of non-infected and Xcc-infected cabbage. The mean values of three technical repeats are shown. Statistically significant differences are indicated by one asterisk (p ≤ 0.05)
Furthermore, the BA level was determined around the Xcc infection site in cabbage leaves. The BA level in the Xcc-infected leaves at 6 days post-inoculation (dpi) was 11.89 pmol/mg (FW), which was significantly higher than that in the control leaves (Fig. 7b). These results suggest that local BA biosynthesis in cabbage leaf tissues is induced by Xcc infection.
Virulence assays of XC1 in the presence of BA
In this study, to further verify the interfering effect of BA on 4-HBA degradation, the XC1 strain in the absence and presence of 100 μM BA was further analyzed for virulence in cabbage using the leaf-clipping method. The average lesion length in absence of BA in cabbage was 23.6 mm at 12 dpi, which was approximately 20.3% longer than that in presence of BA (18.8 mm; Fig. 8). As a negative control, the average lesion length of strain ΔpobR was 12.2 mm in cabbage. These results indicate that BA treatment reduces XC1’s virulence on host plants.
Virulence of Xcc strain XC1 on cabbage in absence or presence of BA. a The lesions of the leaves infected by XC1, pobR deletion mutant ΔpobR, or XC1 in the presence of 100 μM BA at 12 days post inoculation (dpi). b The lesion lengths of the leaves infected by XC1, pobR deletion mutant ΔpobR, and XC1 in the presence of 100 μM BA. The average lesion length values with standard deviation are shown. Statistically significant differences are indicated by one asterisk (p ≤ 0.05) or two asterisks (p ≤ 0.01)
Discussion
Plant-pathogen interactions are complex processes, where the pathogen- and plant-derived molecules are often involved. In response to pathogen attack, Brassica plants rapidly synthesize and accumulate a range of broad-spectrum antimicrobial phenolics, such as salicylic acid and 4-HBA, in the areas of pathogen infection (Islam et al. 2019; Chen et al. 2020; Song et al. 2022). These antimicrobial phenolics act to enhance defense activity of plants, disrupt metabolism in pathogens, or prevent pathogen reproduction (Tan et al. 2004). Our previous findings revealed that the phytopathogen Xcc has evolved a pobA/pobR locus to sense and rapidly degrade 4-HBA, which allows it to successfully colonize cruciferous host plants (Chen et al. 2020). The present study further showed that a 4-HBA analog, BA, could interfere with 4-HBA-induced pobA transcription by binding to 4-HBA sensor protein PobR and subsequently disrupting 4-HBA degradation in Xcc. Xcc does not produce BA and cannot degrade BA in culture. Following Xcc infection, cabbage synthesizes more BA. Thus, cabbage likely uses BA to mitigate the virulence of Xcc by interfering with its ability to degrade 4-HBA. These findings demonstrated a new defense strategy used by the host plants to prevent Xcc infection.
Both BA and 4-HBA are building blocks or important structural elements for numerous primary and specialized metabolites, such as hormones, cofactors, defense compounds, and attractants for pollinators and seed dispersers in plants (Widhalm and Dudareva 2015; Marchiosi et al. 2020). Plants have evolved several pathways to synthesize BA and 4-HBA (Qualley et al. 2012; Widhalm and Dudareva 2015). 4-HBA biosynthesis was shown to be induced in the leaf tissues of cabbage and radish following Xcc infection (Chen et al. 2020). The present study demonstrated that BA biosynthesis in the leaf tissue around Xcc infection site was significantly activated (Fig. 7). Hence, the host plants probably utilize BA and 4-HBA as an important defense strategy to prevent Xcc infection.
To establish successful infection, Xcc has to overcome the host plants’ defense system. How Xcc recognizes BA and 4-HBA to coordinate 4-HBA degradation in Xanthomonas is a fundamental question. In natural environment, when BA and 4-HBA are present in the culture, a phenomenon termed carbon catabolite repression (CCR) has been reported in the metabolism process of BA and 4-HBA. For example, in Pseudomonas putida isolate PRS2000, BA is consumed prior to 4-HBA when they are provided simultaneously, and the expression of pcaK, pobA, and pcaGH, which encode the 4-HBA transporter, 4-HBA hydroxylase, and protocatechuate 3,4-dioxygenase, respectively, was decreased (Nichols and Harwood 1995). Further investigation revealed that the transcription regulator BenR not only controlled the expression of BA degradation genes but also dominated the BA-mediated repression of pcaK (Cowles et al. 2000). An observation similar to the effect of BA on 4-HBA degradation and pcaK expression has been reported in Acinetobacter sp. strain ADP1, where the transcription activators in BA degradation process (BenM and CatM) may directly bind to the pcaU promoter region, resulting in the deactivation of pcaK and decreased PcaK-mediated uptake of 4-HBA (Clark et al. 2002; Brzostowicz et al. 2003). However, the metabolization of the BA/4-HBA mixture by the environmental bacterium, Cupriavidus necator JMP134, and the marine bacteria Sagittula stellata and Ruegeria pomeroyi challenged the paradigm of sequential aromatic catabolism reported above (Donoso et al. 2011; Gulvik and Buchan 2013). In the present study, the above-mentioned CCR mechanism that regulates BA and 4-HBA utilization was not observed in XC1, as BA could not be utilized by the pathogen. Interestingly, a CCR-like phenomenon in the case that 4-HBA degradation was disrupted in the presence of BA was observed, when both BA and 4-HBA were present in XC1 culture. This CCR-like phenomenon was echoed by a new mechanism involving competitive binding between BA and the 4-HBA sensor protein PobR in Xcc. Further structural resolution of the 4-HBA/PobR/DNA complex would uncover this new mechanism.
This study showed that the BA concentrations in non-infected and XC1-infected cabbage were 4.75 and 11.89 pmol/mg FW, respectively (Fig. 7b), which is consistent with the BA concentration detected in blueberry, fava bean, tangerine, lemon, and orange by Aresta and Zambonin (2016). The present study also revealed that the interfering effect of BA on 4-HBA degradation occurred at the concentrations ranging from 4 to 80 μM (Fig. 3c). These findings led us to hypothesize that the host plants might synthesize BA to interfere with the 4-HBA-dependent defense and nutrient scavenge of the invading Xcc, thereby reducing Xcc virulence. To verify this hypothesis, the BA-deficient host plant mutants are required for further virulence assay.
BA, a widely distributed common aromatic carboxylic acid in plants, has been described as an antimicrobial and allelopathic compound in root exudates of various plant species, such as tobacco, barley, and lettuce (Liu et al. 2015; Shaposhnikov et al. 2020; Windisch et al. 2021). It is extensively applied as preservatives and flavoring agents in food, medicine, and cosmetic industries, and is a supplemental element in the field of animal husbandry (Rychen et al. 2018). In recent years, the application potential of exogenous BA for plant disease management has also been explored. BA has shown the antibacterial and antifungal activities against Alternaria solani, X. axonopodis pv. phaseoli, and Bipolaris oryzae (Shabana et al. 2008; Nehela et al. 2021; Abo-Elyousr et al. 2022), which can elevate the total phenol and H2O2 content in rice leaves (Rishad et al. 2021), enhance the polyphenol oxidase activity and lignin content in oil palm (Surendran et al. 2018), and promote the plant growth, antioxidant defense machinery, and SA-mediated defense response in tomato (Nehela et al. 2021). These findings expand its potential application in preventing basal stem rot infection of oil palm, blast disease of rice, tomato early blight, and common blight of beans. The present study further revealed that in presence of BA, Xcc displayed reduced virulence in cabbage (Fig. 8), indicating its potential use as a bactericide. Nevertheless, the physiological and biochemical mechanisms behind the protective role deserve further study, and whether BA can be used to prevent Xcc infection in agriculture should be investigated in the future.
Conclusion
The present study showed that the phytopathogen Xcc infection induces cabbage to produce more BA around the infection site. The cabbage utilizes BA to depress pobA transcription and 4-HBA degradation by competitively binding to PobR, thereby reducing Xcc virulence. Thus, BA has the potential to be developed as a bactericide against Xcc infection.
Methods
Bacterial strains and growth conditions
The bacterial strains and plasmids used in this study are listed in Additional file 2: Table S1 and Table S2. Wild-type Xcc strain XC1 and its derivatives were grown at 28°C in NYG media (5 g/L peptone, 3 g/L yeast extract, and 10 g/L glycerol, pH 7.0), NA media (5 g/L peptone, 3 g/L beef extract, 10 g/L sucrose, and 1 g/L yeast extract), XOLN media (0.7 g/L K2HPO4, 0.2 g/L KH2PO4, 0.1 g/L MgCl2·6H2O, 1 g/L (NH4)2SO4, 0.01 g/L FeSO4·7H2O, 0.001 g/L MnCl2·4H2O, 0.625 g/L yeast extract, and 0.625 g/L tryptone, pH 7.15) or XYS media (XOLN media supplemented with 5 g/L sucrose). Escherichia coli strains were grown at 37°C in LB media (5 g/L tryptone, 10 g/L yeast extract, 5 g/L NaCl, pH 7.0). Commercial antibiotics including rifampicin (Rif), kanamycin (Km), ampicillin (Amp), and gentamicin (Gm) were purchased from Sigma-Aldrich and used at the following concentrations when required: 25 μg/mL Rif, 50 μg/mL Km, 100 μg/mL Amp, and 20 μg/mL Gm. Bacterial growth was determined by measuring the optical density at a wavelength of 600 nm.
Gene deletion
Xcc in-frame deletion mutants were generated following the method described by He et al. (2006). Briefly, the upstream and downstream regions (~ 500 bp) of the target gene were amplified and cloned into the suicide vector pK18mobsacB using one-step cloning Kit (C113, Vazyme Biotech, China). The primers used in this study are listed in Additional file 2: Table S3. The constructed plasmid was introduced into XC1 through mating. The resultant colony was then plated on NA agar plates with 25 μg/mL Rif and 5% (w/v) sucrose to screen mutant strains. The gene deletion mutant was verified by PCR and subsequent DNA sequencing.
Construction of gusA-dependent transcriptional reporter strains and the GUS activity assay
The reporter strains were constructed following the procedures described by Chen et al. (2020). Briefly, the ~ 500 bp DNA fragment upstream of the gene translation initiation codon was amplified by PCR and cloned into the multiple cloning site of plasmid pMD18T-T0T1-gusA. The primers used in this study are listed in Additional file 2: Tables S3. The resultant promoter-gusA fusion fragment was further cloned into the pUC18T-mini-Tn7T-Gm plasmid. With the assistance of the Tn7 transposon, the whole sequence of T0T1-promoter-gusA was integrated into the Xcc genome by electroporation and thus generated transcriptional reporter strains (Jittawuttipoka et al. 2009). To detect GUS activity, these transcriptional reporter strains were grown in XYS medium at 28°C. Quantitative and qualitative GUS activity assays were performed using MUG (4-methylumbelliferyl β-D-glucuronide) or x-Gluc (5-bromo-4-chloro-3-indolyl-β-D-glucuronide) as substrates, respectively according to the previously described procedures (Chen et al. 2020).
Total RNA extraction and qRT-PCR for transcription analysis
The total RNA of Xcc strains was isolated using the FastPure Cell/Tissue Total RNA Isolation Kit V2 (Vazyme Biotech, Nanjing, China) according to the manufacturer’s instructions. The PrimeScript reverse transcription Reagent kit with gDNA Eraser (Takara, Japan) was used to remove the genomic DNA and to generate cDNA. Quantitative PCR was performed on a StepOne Plus real time PCR platform (Thermo Fisher Scientific, USA) with SYBR Premix Ex Taq (Takara, Japan). The atpD gene was used as reference to normalize all samples and replicates. The relative quantification of each transcript was calculated by the 2−ΔΔCT method. The primers used were listed in Additional file 2: Table S3.
Extraction and HPLC analysis for 4-HBA and BA in Xcc cultures
The methods for 4-HBA or BA extraction and high-performance liquid chromatography (HPLC) analysis were previously described by Chen et al. (2020). Briefly, 0.5 mL of cell cultures were collected, and 6 M HCl was added to the culture to adjust the pH to 3. Two volumes of ethyl acetate were then added to the culture to extract 4-HBA or BA. The upper phase was collected by centrifugation and then was concentrated by rotary evaporation. The dry crude extracts were dissolved in methanol and centrifuged to remove insoluble substances. Five microliters of the supernatant were loaded for HPLC analysis with a Zorbax Eclipse XDB C18 column (4.6 × 150 mm, 5 μm, Agilent). 0.1% acetic acid water and 0.1% acetic acid acetonitrile (85/15, v/v) were used to separate 4-HBA and BA with a flow rate of 1 mL/min.
BA extraction from leaf tissues and UHPLC-QQQ-MS/MS analysis for BA
To extract the aromatic compounds in cabbage (Jingfeng-1) leaf tissues, fresh leaves (2-month-old) were collected and BA was extracted and analyzed following the method developed by Pan et al. (2010). Briefly, 1 g of leaf tissues was extracted with 10 mL of the extraction buffer (H2O: isopropanol: HCl=2:1:0.002, v/v) for 30 min, followed by a second round of extraction with 20 mL of dichloromethane. Centrifugation was then conducted at 10,000 × g for 10 min and the organic phase was collected and dried by rotary evaporation. Dry crude extracts were dissolved in 40% methanol and 20 μL was subjected to UHPLC-QQQ-MS/MS analysis with an Agilent Zorbax Eclipse XDB C18 column (4.6 × 150 mm, 5 μm). Fractions were eluted with methanol and water (40/60, v/v) containing 0.1% formic acid at a flow rate of 0.4 mL/min.
Protein purification and isothermal titration calorimetry (ITC) assays
PobR protein purification and ITC assays were performed following the protocol described by Chen et al. (2020). Briefly, ITC assays were performed on a MicroCal iTC200 system (GE Healthcare, USA). The PobR protein and BA or 4-HBA were freshly prepared and dissolved in an HEPES buffer. In a binding experiment, PobR was loaded into the sample cell and titrated against BA or 4-HBA loaded in the injection syringe. The titration ratio was 8:1 (400 μM: 50 μM). Titrations were carried out at 18°C with a stirring speed of 750 rpm. Data were analyzed using the Origin 7.0 software package provided by the manufacturer.
Electrophoretic mobility shift assays
EMSA was performed according to the methods described by Chen et al. (2020). Briefly, the 233 bp DNA probe was amplified by PCR using the primer set Cy5-PpobA-F/R (Additional file 2: Table S3). Cy5-labeled PpobA (10 ng) was incubated with PobR in EMSA buffer, and 4-HBA or BA was added to the reaction and incubated at 25°C for 40 min. After incubation, the mixture was electrophoresed at 4°C on a 4.5% native polyacrylamide gel in 0.5 × Tris-borate EDTA (TBE) buffer for 45 min at 125 V. The fluorescence intensity of the gels was scanned using Amersham Typhoon RGB (GE Healthcare).
Virulence assays on cabbage
Cabbage (Brassica oleracea) cultivar ‘Jingfeng-1’ was grown in a growth chamber (Yanghui RDN-500B, Ningbo, China) at 25°C and 75% humidity with a photoperiod of 16 h (8000 Lx). The strain XC1 was grown in XYS liquid medium in absence or presence of 100 μM BA for 12 h. The collected cell pellets were resuspended in PBS buffer at a final OD600 of 0.1. The virulence assays on 2-month-old cabbage were conducted using the leaf-clipping method (Chen et al. 2020). The previously generated mutant strain ΔpobR was used as a negative control. For each strain, a total of 12 leaves were inoculated, and the lesion lengths were measured at 12 dpi.
Statistical analysis
All experiments were performed at least three times, unless otherwise stated. An analysis of variance (ANOVA) was performed for the experimental datasets using JMP software version 5.0 (SAS Institute Inc., Cary, NC). Significant effects of treatment were determined by the F value (p = 0.05). When a significant F test was obtained, separation of means was accomplished by Fisher’s protected LSD (least significant difference) test at p < 0.05.
Availability of data and materials
Not applicable.
Abbreviations
- 3,4,5-THBA:
-
3,4,5-trihydroxybenzoic acid
- 3,4-DHBA:
-
Protocatechuic acid
- 3,4-DHCA:
-
Caffeic acid
- 3-HBA:
-
3-hydroxybenzoic acid
- 3-HCA:
-
3-hydroxycinnamic acid
- 3M4HBA:
-
4-hydroxy-3-methoxybenzoic acid
- 3M4HBD:
-
4-hydroxy-3-methoxybenzaldehyde
- 3-MBA:
-
3-methoxybenzoic acid
- 4-HBA:
-
4-hydroxybenzoic acid
- 4-HBD:
-
4-hydroxybenzaldehyde
- BA:
-
Benzoic acid
- CA:
-
Cinnamic acid
- CCR:
-
Carbon catabolite repression
- CoQ8:
-
Coenzyme Q8
- E4P:
-
Erythrose 4-phosphate
- GUS:
-
β-glucuronidase
- MUG:
-
4-methylumbelliferyl β-D-glucuronide
- NaSA:
-
Sodium salicylate
- OHB:
-
3-octaprenyl-4-hydroxybenzoate
- PCA:
-
Protocatechuic acid
- PEP:
-
Phosphoenolpyruvic acid
- SA:
-
Salicylic acid
- UHPLC-QQQ-MS/MS:
-
Ultra-high performance liquid chromatography coupled with triple quadrupole tandem mass spectrometry
- Xcc :
-
Xanthomonas campestris pv. campestris
- x-Gluc:
-
5-bromo-4-chloro-3-indolyl-β-D-glucuronide
References
Abo-Elyousr KAM, Imran M, Almasoudi NM, Ali EF, Hassan S, Sallam NMA, et al. Controlling of Xanthomonas axonopodis pv. phaseoli by induction of phenolic compounds in bean plants using salicylic and benzoic acids. Plant Pathol. 2022;104:947–57.
Aresta A, Zambonin C. Simultaneous determination of salicylic, 3-methyl salicylic, 4-methyl salicylic, acetylsalicylic and benzoic acids in fruit, vegetables and derived beverages by SPME-LC-UV/DAD. J Pharm Biomed Anal. 2016;121:63–8.
Brzostowicz PC, Reams AB, Clark TJ, Neidle EL. Transcriptional cross-regulation of the catechol and protocatechuate branches of the beta-ketoadipate pathway contributes to carbon source-dependent expression of the Acinetobacter sp. strain ADP1 pobA gene. Appl Environ Microbiol. 2003;69(3):1598–606.
Büttner D, Bonas U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol Rev. 2010;34(2):107–33.
Carmona M, Zamarro MT, Blázquez B, Durante-Rodríguez G, Juárez JF, Valderrama JA, et al. Anaerobic catabolism of aromatic compounds: a genetic and genomic view. Microbiol Mol Biol Rev. 2009;73(1):71–133.
Chen B, Li RF, Zhou L, Qiu JH, Song K, Tang JL, He YW. The phytopathogen Xanthomonas campestris utilizes the divergently transcribed pobA/pobR locus for 4-hydroxybenzoic acid recognition and degradation to promote virulence. Mol Microbiol. 2020;114(5):870–86.
Chen B, Li RF, Zhou L, Song K, Poplawsky AR, He YW. The phytopathogen Xanthomonas campestris scavenges hydroxycinnamic acids in planta via the hca cluster to increase virulence on its host plant. Phytopathol Res. 2022;4(1):1–16.
Clark TJ, Momany C, Neidle EL. The benPK operon, proposed to play a role in transport, is part of a regulon for benzoate catabolism in Acinetobacter sp. strain ADP1. Microbiology. 2002;148(4):1213–23.
Cowles CE, Nichols NN, Harwood CS. BenR, a XylS homologue, regulates three different pathways of aromatic acid degradation in Pseudomonas putida. J Bacteriol. 2000;182(22):6339–46.
Díaz E, Jiménez JI, Nogales J. Aerobic degradation of aromatic compounds. Curr Opin Biotechnol. 2013;24(3):431–42.
Donoso RA, Pérez-Pantoja D, González B. Strict and direct transcriptional repression of the pobA gene by benzoate avoids 4-hydroxybenzoate degradation in the pollutant degrader bacterium Cupriavidus necator JMP134. Environ Microbiol. 2011;13(6):1590–600.
Fuchs G, Boll M, Heider J. Microbial degradation of aromatic compounds - from one strategy to four. Nat Rev Microbiol. 2011;9:803–16.
Gulvik CA, Buchan A. Simultaneous catabolism of plant-derived aromatic compounds results in enhanced growth for members of the Roseobacter lineage. Appl Environ Microbiol. 2013;79(12):3716–23.
Hayward AC. The host of Xanthomonas. In: Swings JG, Civerolo EL, editors. Xanthomonas. London: Chapman & Hall; 1993. p. 51–4.
He YW, Xu M, Lin K, Ng YJ, Wen CM, Wang LH, et al. Genome scale analysis of diffusible signal factor regulon in Xanthomonas campestris pv. campestris: identification of novel cell-cell communication-dependent genes and functions. Mol Microbiol. 2006;59(2):610–22.
He YW, Wu J, Zhou L, Yang F, He YQ, Jiang BL, et al. Xanthomonas campestris diffusible factor is 3-hydroxybenzoic acid and is associated with xanthomonadin biosynthesis, cell viability, antioxidant activity, and systemic invasion. Mol Plant Microbe Interact. 2011;24(8):948–57.
Islam MT, Lee BR, La VH, Lee H, Jung WJ, Bae DW, et al. p-Coumaric acid induces jasmonic acid-mediated phenolic accumulation and resistance to black rot disease in Brassica napus. Physiol Mol Plant Pathol. 2019;106:270–5.
Jittawuttipoka T, Buranajitpakorn S, Fuangthong M, Schweizer HP, Vattanaviboon P, Mongkolsuk S. Mini-Tn7 vectors as genetic tools for gene cloning at a single copy number in an industrially important and phytopathogenic bacteria. Xanthomonas spp FEMS Microbiol Lett. 2009;298(1):111–7.
Kamimura N, Masai E. The protocatechuate 4, 5-cleavage pathway: overview and new findings. Biodegradative bacteria: how bacteria degrade, survive, adapt, and evolve. 2014:207–26.
Leyns F, De Cleene M, Swings JG, De Ley J. The host range of the genus Xanthomonas. Bot Rev. 1984;50:308–56.
Liu Y, Li X, Cai K, Cai L, Lu N, Shi J. Identification of benzoic acid and 3-phenylpropanoic acid in tobacco root exudates and their role in the growth of rhizosphere microorganisms. Appl Soil Ecol. 2015;93:78–87.
Macías FA, Durán AG, Molinillo JMG. Allelopathy: The chemical language of plants. Prog Chem Org Nat Prod. 2020;112:1–84.
Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol. 2012;13(6):614–29.
Marchiosi R, dos Santos WD, Constantin RP, de Lima RB, Soares AR, Finger-Teixeira A, et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem Rev. 2020;19:865–906.
Nehela Y, Taha NA, Elzaawely AA, Xuan TD, Amin MA, Ahmed ME, et al. Benzoic acid and its hydroxylated derivatives suppress early blight of tomato (Alternaria solani) via the induction of salicylic acid biosynthesis and enzymatic and nonenzymatic antioxidant defense machinery. J Fungi (Basel). 2021;7(8):663.
Nichols NN, Harwood CS. Repression of 4-hydroxybenzoate transport and degradation by benzoate: a new layer of regulatory control in the Pseudomonas putida beta-ketoadipate pathway. J Bacteriol. 1995;177(24):7033–40.
Nutautaitė M, Racevičiūtė-Stupelienė A, Bliznikas S, Jonuškienė I, Karosienė J, Koreivienė J, et al. Evaluation of phenolic compounds and pigments in freshwater Cladophora glomerata biomass from various lithuanian rivers as a potential future raw material for biotechnology. Water. 2022;14(7):1138.
Pan X, Welti R, Wang X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat Protoc. 2010;5(6):986–92.
Petric Z, Ružić J, Žuntar I. The controversies of parabens - an overview nowadays. Acta Pharm. 2021;71(1):17–32.
Qualley AV, Widhalm JR, Adebesin F, Kish CM, Dudareva N. Completion of the core β-oxidative pathway of benzoic acid biosynthesis in plants. Proc Natl Acad Sci U S A. 2012;109(40):16383–8.
Rishad MB, Sultana A, Chakraborty S, Humaira RK, Khokon MAR. Effect of foliar application of salicylic acid, chitosan and benzoic acid in elevating total phenol and H2O2 content in rice leaves to modulate resistance against blast disease. Bangladesh J Plant Pathol. 2021;37(1&2):7–14.
Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, et al. Safety and efficacy of benzoic acid for pigs and poultry. EFSA J. 2018;16(3):e05210.
Shabana YM, Abdel-Fattah GM, Ismail AE, Rashad YM. Control of brown spot pathogen of rice (Bipolaris oryzae) using some phenolic antioxidants. Braz J Microbiol. 2008;39(3):438–44.
Shaposhnikov AI, Shakhnazarova VY, Vishnevskaya NA, Borodinaa EV, Strunnikova OK. Aromatic carboxylic acids in barley-root exudates and their influence on the growth of Fusarium culmorum and Pseudomonas fluorescens. Appl Biochem Microbiol. 2020;56:344–51.
Simoneit BRT. Biomass burning-a review of organic tracers for smoke from incomplete combustion. Appl Geochem. 2002;17(3):129–62.
Song K, Chen B, Cui Y, Zhou L, Chan KG, Zhang HY, et al. The plant defense signal salicylic acid activates the RpfB-dependent quorum sensing signal turnover via altering the culture and cytoplasmic pH in the phytopathogen Xanthomonas campestris. mBio. 2022;13(2):e03644-21.
Surendran A, Siddiqui Y, Manickam S, Ali A. Role of benzoic and salicylic acids in the immunization of oil palm seedlings-challenged by Ganoderma boninense. Ind Crops Prod. 2018;122:358–65.
Tan J, Bednarek P, Liu J, Schneider B, Svatos A, Hahlbrock K. Universally occurring phenylpropanoid and species-specific indolic metabolites in infected and uninfected Arabidopsis thaliana roots and leaves. Phytochemistry. 2004;65(6):691–9.
Timilsina S, Potnis N, Newberry EA, Liyanapathiranage P, Iruegas-Bocardo F, White FF, et al. Xanthomonas diversity, virulence and plant-pathogen interactions. Nat Rev Microbiol. 2020;18(8):415–27.
Tsagogiannis E, Asimakoula S, Drainas AP, Marinakos O, Boti VI, Kosma IS, et al. Elucidation of 4-hydroxybenzoic acid catabolic pathways in Pseudarthrobacter phenanthrenivorans Sphe3. Int J Mol Sci. 2024;10;25(2):843.
Vicente JG, Holub EB. Xanthomonas campestris pv. campestris (cause of black rot of crucifers) in the genomic era is still a worldwide threat to brassica crops. Mol Plant Pathol. 2013;14(1):2–18.
Widhalm JR, Dudareva N. A familiar ring to it: biosynthesis of plant benzoic acids. Mol Plant. 2015;8(1):83–97.
Windisch S, Walter A, Moradtalab N, Walker F, Höglinger B, El-Hasan A, et al. Role of benzoic acid and lettucenin A in the defense response of lettuce against soil-borne pathogens. Plants (Basel). 2021;10(11):2336.
Zhang R, Lord DM, Bajaj R, Peti W, Page R, Sello JK. A peculiar IclR family transcription factor regulates para-hydroxybenzoate catabolism in Streptomyces coelicolor. Nucleic Acids Res. 2018;46(3):1501–12.
Zhou L, Wang JY, Wu J, Wang J, Poplawsky A, Lin S, et al. The diffusible factor synthase XanB2 is a bifunctional chorismatase that links the shikimate pathway to ubiquinone and xanthomonadins biosynthetic pathways. Mol Microbiol. 2013;87(1):80–93.
Zhou L, Zhang LH, Cámara M, He YW. The DSF Family of quorum sensing signals: diversity, biosynthesis, and turnover. Trends Microbiol. 2017;25(4):293–303.
Zhou L, Li M, Wang XY, Liu H, Sun S, Chen H, et al. Biosynthesis of Coenzyme Q in the phytopathogen Xanthomonas campestris via a yeast-like pathway. Mol Plant Microbe Interact. 2019;32(2):217–26.
Acknowledgements
We thank Instrument Sharing and Technical Service Platform of School of Life Sciences and Biotechnology at Shanghai Jiao Tong University for technical help with ITC and UHPLC-QQQ-MS/MS.
Funding
This work was financially supported by research grants from the National Natural Science Foundation of China (31972231, 32172355).
Author information
Authors and Affiliations
Contributions
YH and BC conceived the experimental design, interpreted the results, and wrote the manuscript. LZ and CT participated in the interpretation of the results and writing of the manuscript. BC and KS performed the experiments.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Supplementary Information
Additional file 1: Figure S1
. No detectable BA was identified in the NYG or XYS culture of XC1. Figure S2. BA alone has no inductive effect on the transcription of pobA. Figure S3. 4-HCA induces pobA transcription via the central metabolite 4-HBA. Figure S4. Addition of SA or 3-HBA has no interference effect on 4-HBA degradation. Figure S5. BA alone has no interference effect on the binding of PobR to the probe PpobA. Figure S6.Quantitative assays for BA.
Additional file 2: Table S1.
Bacterial strains used in this study. Table S2. Plasmids used in this study. Table S3. Oligonucleotide primers used in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, B., Zhou, L., Song, K. et al. Host plant-derived benzoic acid interferes with 4-hydroxybenzoic acid degradation in the phytopathogen Xanthomonas campestris by competitively binding to PobR. Phytopathol Res 6, 40 (2024). https://doi.org/10.1186/s42483-024-00259-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42483-024-00259-4