Abstract
Background
Embolic stroke of undetermined source (ESUS) accounts for a substantial proportion of ischaemic strokes. A stroke recurrence score has been shown to predict the risk of recurrent stroke in patients with ESUS based on a combination of clinical and imaging features. This study aimed to externally validate the performance of the ESUS recurrence score using data from a randomized controlled trial.
Methods
The validation dataset consisted of eligible stroke patients with available magnetic resonance imaging (MRI) data enrolled in the PreDAFIS sub-study of the MonDAFIS study. The score was calculated using three variables: age (1 point per decade after 35 years), presence of white matter hyperintensities (2 points), and multiterritorial ischaemic stroke (3 points). Patients were assigned to risk groups as described in the original publication. The model was evaluated using standard discrimination and calibration methods.
Results
Of the 1054 patients, 241 (22.9%) were classified as ESUS. Owing to insufficient MRI quality, three patients were excluded, leaving 238 patients (median age 65.5 years [IQR 20.75], 39% female) for analysis. Of these, 30 (13%) patients experienced recurrent ischaemic stroke or transient ischemic attack (TIA) during a follow-up period of 383 patient-years, corresponding to an incidence rate of 7.8 per 100 patient-years (95% CI 5.3–11.2). Patients with an ESUS recurrence score value of ≥ 7 had a 2.46 (hazard ratio (HR), 95% CI 1.02–5.93) times higher risk of stroke recurrence than patients with a score of 0–4. The cumulative probability of stroke recurrence in the low-(0–4), intermediate-(5–6), and high-risk group (≥ 7) was 9%, 13%, and 23%, respectively (log-rank test, χ2 = 4.2, p = 0.1).
Conclusions
This external validation of a published scoring system supports a threshold of ≥ 7 for identifying ESUS patients at high-risk of stroke recurrence. However, further adjustments may be required to improve the model’s performance in independent cohorts. The use of risk scores may be helpful in guiding extended diagnostics and further trials on secondary prevention in patients with ESUS.
Trial registration: Clinical Trials, NCT02204267. Registered 30 July 2014, https://clinicaltrials.gov/ct2/show/NCT02204267.
Similar content being viewed by others
Background
After ischaemic stroke, a comprehensive diagnostic evaluation is crucial for initiating appropriate secondary prevention measures [16]. Current international guidelines recommend the use of an antiplatelet drug in non-AF (atrial fibrillation) patients or direct oral anticoagulation in AF patients [2]. Approximately 15–20% of stroke cases are classified as “cryptogenic”, meaning that the aetiology remains unknown despite a comprehensive diagnostic work-up. Embolic stroke is suspected in a significant percentage of cryptogenic cases even in the absence of a proven cardioembolic source [3, 10, 26].
In 2014, the concept of embolic stroke of undetermined source (ESUS) was introduced to categorise these patients and determine the best secondary treatment in randomised controlled trials [11]. Two major trials were conducted in ESUS patients but failed to demonstrate a benefit of DOAC treatment versus aspirin in secondary stroke prevention [3, 5, 12]. This may stem from the heterogeneous nature of strokes summarised under the ESUS label, which encompasses a wide range of possible causes [20, 21].
To advance our understanding of ESUS and develop better treatment strategies, it seems to be essential to further identify ESUS patients with an elevated risk of recurrent stroke. Furthermore, identifying high-risk ESUS patients can help allocate resources more efficiently, as diagnostic evaluation can be resource intensive in these patients.
An integer-based scoring system incorporating both clinical and imaging factors has been proposed by Ntaios et al. and was shown to be useful in the risk stratification of patients with ESUS. In particular, compared to 403 ESUS patients in the lowest tertile (i.e., score of 0–4), 202 patients in the highest tertile (i.e., score of 7–12) had a 4.7 times higher risk of stroke recurrence [19]. Despite these promising results, this score has not been externally validated yet. To address this, the present analysis aimed to externally validate the score’s performance in an independent cohort of patients with ESUS.
Methods
Validation cohort
We used data from the Prediction of Atrial Fibrillation based on Stroke Lesion Characterisation in the MonDAFIS Study (PreDAFIS) cohort, which is a sub-study of the Impact of Standardized MONitoring for Detection of Atrial Fibrillation in Ischemic Stroke (MonDAFIS) study. The study design and participant information have been described in detail previously [7, 8, 22]. Briefly, the MonDAFIS study was an investigator-initiated, randomised, multicentre study sponsored by the Charité – Universitätsmedizin Berlin and funded by Bayer Vital GmbH Germany [8]. The MonDAFIS cohort comprised patients from 38 certified German stroke units who presented with acute ischaemic stroke or transient ischaemic attack with an existing neurological deficit at admission. Patients without known AF at admission were eligible for inclusion and were followed up at 6, 12, and 24 months after enrolment [7]. 3465 patients were allocated to either receive systematic Holter-ECG monitoring (up to 7 days in-hospital) in addition to standard diagnostic care (intervention group), or to receive standard care alone (control group).
The PreDAFIS substudy was initiated to further investigate the role of MRI in identifying patients at high risk of AF and predicting outcomes in these patients. Patients in the MonDAFIS intervention arm with available MRI data were included in the PreDAFIS substudy [22].
Patients in the PreDAFIS cohort were screened according to the ESUS criteria proposed by the Cryptogenic Stroke/ESUS International Working Group [11]. These criteria require that a patient must have a non-lacunar brain infarct with no evidence of extracranial or intracranial atherosclerosis causing ≥ 50% luminal stenosis in the arteries that supply the area of ischaemia, no major-risk cardioembolic source, and no other specific cause of stroke, such as arteritis, dissection, migraine/vasospasm, or drug misuse. Patients classified as having ESUS were eligible for inclusion in the validation dataset.
Score calculation and risk group allocation
The ESUS recurrence score was calculated using three variables, as previously reported: age (1 point every decade after 35 years), existing white matter hyperintensities (WMH) on MRI (2 points), and acute or chronic multiterritorial ischaemic stroke (3 points) [19].
The severity of WMH was assessed by two experienced neurology residents with training in MRI diagnostics. For this purpose, a modified four-grade version of the Fazekas scale was applied to FLAIR or T2 images [6]. A grade of 0 indicated the absence of deep or periventricular WMH. Grade 1 indicates the presence of periventricular caps, pencil-thin lining of the ventricles, or punctate foci in the deep white matter. Grade 2 was defined as the presence of a smooth periventricular halo or convergence of deep white matter foci in subcortical regions. Grade 3 represents severe confluent periventricular WMH that extends into deep subcortical white matter or large confluent areas. Patients with grade 2 or higher were classified as having WMH.
Multiterritorial stroke is defined as multiple ischaemic lesions affecting at least two of the three territories: left anterior, right anterior, or posterior circulation [22]. Acute stroke lesion locations were evaluated using semi-automatically generated segmentation masks and a published brain atlas that defined arterial territories [22, 27]. FLAIR-weighted magnetic resonance images were re-examined to check for multiterritorial lesion distributions in cases with a known history of ischaemic stroke.
An ESUS recurrence score of 0 to 4 points placed patients in the low-risk group, 5 to 6 points in the intermediate-risk group, and more than 7 points in the high-risk group.
Statistical analysis and score validation
The score was validated using discrimination and calibration measures. Discrimination is a metric that compares a model's ability to differentiate between patients who have experienced the event in question and those who have not. Calibration is the accuracy with which a model predicts risk. A well-calibrated model predicts the correct probability of an event at all risk levels [24].
Descriptive analysis
Descriptive statistics are reported as counts, percentages, medians, and interquartile ranges. To analyse differences between groups for categorical or continuous variables, the Mann–Whitney test or chi-square test was applied, as appropriate for the data type. The risk of stroke recurrence between the groups was compared using Kaplan–Meier cumulative risk estimation [24].
Measures of discrimination
Survival curves were generated for the stroke recurrence risk groups using the Kaplan–Meier approach. To quantify the differences between the risk groups, hazard ratios were evaluated using a Cox model [25]. A log-rank test was performed to assess statistical significance. Discrimination was further evaluated by calculating Harrell's index of concordance (C-index) [1, 9].
Assessment of general fit and calibration
Calibration slope analysis was used to assess the accuracy of the score. The calibration slope was estimated by fitting a Cox regression model with the risk score as the predictor variable in a Cox model. Furthermore, to check for differences in the regression coefficients for one or more score variables (age, WMH, and multiterritorial infarcts), we fitted a Cox regression with the calculated score as an offset. The resulting model indicated differences between the regression coefficients of the validation and derivation datasets. A coefficient of zero would indicate an optimal specification in our validation dataset. A joint test (ANOVA) was performed to assess the statistical significance of deviations from zero. In addition, this analysis was performed using the published raw coefficients of the derivation model to calculate the prognostic index [1, 25]. The prognostic index (PI) was defined by
All analyses were conducted using R version 4.2.2 [23].
Results
Baseline patient characteristics
In the PreDAFIS cohort, 241 of 1,054 (22.9%) patients were classified as having ESUS. Three patients were excluded due to insufficient MRI quality. Thus, the validation dataset comprised 238 patients (Fig. 1). Of these, 92 (39%) were female. The median age was 65.5 years (IQR 20.75). The median follow-up time was 721 (IQR 83) days, corresponding to a total follow-up time of 382.5 patient-years. 30 (13%) patients experienced recurrent stroke or TIA. This corresponds to 7.8 (95% CI 5.3–11.2) recurrent strokes per 100 patient-years. Additional baseline characteristics are summarised in Table 1.
Score validation
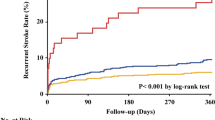
The median score value in the validation cohort was 5 (IQR 4), with 98 (41%) patients assigned to the low-risk group and 70 (29%) patients each assigned to the intermediate- or high-risk group (Fig. 2). The median scores for the low-, intermediate-, and high-risk group were 3 (IQR 2), 6 (IQR 1), and 7 (IQR 1), respectively. The rate of stroke recurrence was 4.8 (2.1–9.5) per 100 patient-years in the low-risk group, 8.0 (3.7–15.2) in the intermediate-risk group, and 12.5 (6.7–21.4) in the high-risk group. The cumulative probability of recurrent stroke was 8.6% (4–15%) in the low-risk group, 13% (6–22%) in the intermediate-risk group, and 23% (12–36%) in the high-risk group (log-rank test: χ2 = 4.2, p = 0.1).
Figure 3 shows the Kaplan–Meier curves for stroke-free survival based on the assigned risk group with a log-rank test of p = 0.12. In Cox regression analysis, high-risk patients had a 2.46 (1.02–5.93, p = 0.046) times increased risk of stroke recurrence compared to low-risk patients. Discrimination between the low- and intermediate-risk groups did not seem to be maintained, as the hazard ratios did not show a statistically significant increase in the Cox model (Table 2). Harrell's C-index was 0.59.
The Cox regression model for the score showed a slope of 0.22 (standard error [SE] 0.08), which was significantly different from 1 (p = 0.007). Similar results were obtained when the slope was calculated using the prognostic index (0.70 [SE 0.26, p = 0.007]). Table 3 presents the results of the Cox model analyses using the individual score items as covariates, with the calculated score or the prognostic index as an offset. Using the integer score as an offset in the first model, the coefficients of the three score variables were statistically significantly different from zero (joint test: χ2 = 120, p < 0.001, Table 3).
However, the joint test was not statistically significant when using the prognostic index as an offset, and the coefficients for WMH and multiterritorial infarcts were not statistically significantly different from zero. A small difference in the Agedecades after 35-variable was detected (beta: − 0.33 [SE 0.1451], p = 0.024, Table 3).
There was a trend of higher risk of stroke recurrence in patients with a score of ≥ 7 compared to those with a score of ≤ 6 (HR 1.95, 0.95–4.02, p = 0.07). In an exploratory analysis, we found that the optimal cut-off was 8 points in our cohort (HR 4.16, 1.85–9.35, p = 0.001, Fig. 4).
Exploratory analysis of hazard ratios of different score cut-offs. The previously suggested threshold of ≥ 7 points showed a trend towards statistical significance. Patients with a score of ≥ 8 points had a 4.16 times increased risk of stroke recurrence (95% CI 1.85–9.35, p = 0.001). HR hazard ratio, CI Confidence interval
Discussion
The aim of this analysis was to provide independent external validation of the performance of a score previously proposed to predict recurrent stroke in patients with ESUS [19]. Our analysis in a cohort from a randomised trial confirmed the reliability of the previously suggested threshold of ≥ 7 points to identify ESUS patients at high risk for recurrent stroke [19]. Patients with a score of ≥ 7 were more than twice as likely to have another stroke than patients with a score ≤ 4 points. In an exploratory analysis, an increase of only one point to a cut-off of 8 points resulted in an even better performance in differentiating between high-risk and low-medium risk patients in our validation cohort. These results support the use of the an ESUS recurrence score in clinical practice for the identification of high-risk ESUS patients, especially given the applicability of the score, as it requires only three variables: age, WMH, and multiterritorial infarct.
One may argue that a C-index of 0.59 is only moderate and, therefore, insufficient for accurately estimating the risk of stroke recurrence in a specific patient. However, the purpose of this score is not to provide an accurate risk estimate. Rather, its aim is to identify an ESUS subgroup that is at high risk for stroke recurrence. A similar approach was adopted with the CHA2DS2-VASc score, which, despite having a similarly moderate C-statistic (0.60) in its derivation cohort [17], is recommended by guidelines for stratifying stroke risk in AF patients [14, 15]. The CHA2DS2-VASc score is not used clinically to determine the exact stroke risk of a particular AF patient but rather to identify a subgroup at low stroke risk. Similarly, the ESUS recurrence score identifies an ESUS subgroup (i.e., patients with a score of ≥ 7) that has a higher risk of stroke recurrence compared to patients with a lower score.
We calculated a wide variety of additional validation measures, including those of general fit and discrimination. A calibration slope of 0.22 suggests poorer discrimination in our cohort than in the derivation cohort. One explanation why the slope may differ from 1 in our cohort is that there are differences in the regression coefficients for the score variables in our validation dataset compared to the derivation dataset. We checked for model misspecifications in two Cox regression models. In the first model, the integer score is incorporated as an offset, that is, the coefficient of the score is set to 1. In such a model, the coefficients of the score variables should be close to zero given a perfect fit. However, in this cohort, we observed that all coefficients were statistically significantly different from zero when analysed using the integer score (Table 3).
During the development of the score in the original cohort [19], it was derived as an integer-based model by dividing each coefficient of the derivation model by the lowest coefficient and rounding to the nearest integer. To eliminate possible rounding errors, we directly used the coefficients from the derivation model and calculated the prognostic index for each patient. In the second model, this prognostic index was added as an offset as in the first model. In our validation dataset, we found no overall evidence of a lack of fit of the prognostic index since the joint test of the covariates was not statistically significant (χ2 = 7.34, p = 0.06). However, the statistically significant difference to zero of the Agedecades after 35-coefficient indicates substantial differences in the association between age and recurrent stroke in the validation and derivation datasets (Table 3). Indeed, there was no statistically significant age difference in patients with or without recurrent stroke in our cohort (Table 1) as opposed to the derivation cohort (median age without and with recurrent stroke in the derivation dataset was 63.7 vs 70.3 years [p < 0.001], [19]).
Some of the differences analysed in these two models might be partly explained by differences in the definitions or measurements of the score variables. Due to its availability in our dataset, WMH was defined using the Fazekas scale with a cut-off of ≥ 2 points in FLAIR or T2 weighted magnetic resonance images. This was slightly different from the definition of WMH used in the original study: WMH was defined as patchy or diffuse areas of hypodensity in computer tomography or hyperintensity in magnetic resonance imaging. Further differences may stem from differences in the clinical characteristics among the cohorts. Finally, the moderate number of patients and recurrent strokes in our cohort resulted in a reduction in statistical power and an increased likelihood of type II errors. Previous proposals for a minimum sample size in external validation studies have suggested at least 100 events; however, these were based on a single simulation study [1, 28].
It is noteworthy that the score’s ability to differentiate between high-risk (≥ 7 points) and low-intermediate-risk patients (≤ 6 points) was only moderate in our derivation cohort (Fig. 4). Using a more conservative cut-off value of ≥ 8 points, the high-risk group had a four times increased risk of stroke recurrence compared to patients with ≤ 7 points. It is likely that this shift in results after changing the cut-off by only one point is due to a statistical uncertainty induced by the relatively small sample size as well. However, the score’s ability to differentiate between high-risk and low-risk patients is arguably sufficient for the intended use cases of the score.
Altogether, we consider our cohort as a suitable dataset for the external validation of this ESUS recurrence risk score. Patients in the MonDAFIS study were followed for 2 years, resulting in a thorough examination of these patients. The baseline characteristics of our cohort were mostly similar to those of the derivation cohort. However, we observed a higher stroke recurrence rate of 7.8 per 100 patient-years (vs 3.7 per 100 patient years in the derivation cohort). This might in part be explained by patients being slightly older and more affected by certain cardiovascular risk factors. On the other hand, ESUS patients in our cohort appeared to be less severely affected by the index stroke, as reflected by a lower NIHSS score at admission of 2 points compared with a NIHSS score of 6 points in the derivation cohort [19].
Two large randomised clinical trials, the NAVIGATE ESUS trial [12] and the RESPECT-ESUS trial [5], were recently conducted to evaluate the efficacy of oral anticoagulants versus aspirin in reducing recurrent strokes in patients with ESUS. Although neither trial showed a significant reduction in recurrent strokes in the anticoagulation arm compared with the aspirin arm, subsequent subgroup studies identified groups that benefited from anticoagulation compared with aspirin, such as patients with left ventricular dysfunction [18], patients with an enlarged left atrial diameter [13], and patients aged ≥ 75 years [4]. As the ESUS recurrence score identifies patients at an increased risk of stroke recurrence, it may be useful for further subgroup analyses. It could be hypothesised that high-risk patients would benefit from anticoagulation therapy as well. Furthermore, including cardiac parameters, such as left ventricular dysfunction or atrial volume, in the score calculation might improve the ability of the score to identify high-risk patients who benefit from oral anticoagulation.
Conclusions
In conclusion, we provide a state-of-the-art, independent, external validation analysis for an easily applicable score that identifies patients with ESUS at a high risk of stroke recurrence. Our findings support the utility of this score in identifying high-risk patients, which may be useful in designing future secondary prevention studies in patients with ESUS. Furthermore, the score might find its way into clinical practice to improve the allocation of resources in the diagnostic work-up of patients with ESUS, especially given its applicability, as it is calculated from only three easily assessed parameters.
Availability of data and materials
Deidentified participant data with corresponding data dictionary of the data underlying the current manuscript will be made available upon reasonable request. Data will be shared to external researchers for scientific non‑commercial purposes after approval of the proposal by the MonDAFIS steering board including a signed data access agreement.
Abbreviations
- AF:
-
Atrial fibrillation
- ANOVA:
-
Analysis of variance
- CHA2DS2-VASc:
-
Congestive heart failure, hypertension, age ≥ 75, diabetes mellitus, stroke/TIA/thromboembolism, vascular disease, age 65–74, sex category
- CI:
-
Confidence interval
- DOAC:
-
Direct oral anticoagulant
- ECG:
-
Electrocardiogram
- ESUS:
-
Embolic stroke of undetermined source
- FLAIR:
-
Fluid-attenuated inversion recovery
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- MonDAFIS:
-
Impact of Standardized MONitoring for Detection of Atrial Fibrillation in Ischemic Stroke
- MRI:
-
Magnetic resonance imaging
- NAVIGATE-ESUS:
-
New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial versus ASA to Prevent Embolism in Embolic Stroke of Undetermined Source
- NIHSS:
-
National Institutes of Health Stroke Scale
- PI:
-
Prognostic Index
- PreDAFIS:
-
Prediction of Atrial Fibrillation based on Stroke Lesion Characterisation in the MonDAFIS Study
- RESPECT-ESUS:
-
Randomized, Double-Blind, Evaluation in Secondary Stroke Prevention Comparing the Efficacy and Safety of the Oral Thrombin Inhibitor Dabigatran Etexilate versus Acetylsalicylic Acid in Patients with Embolic Stroke of Undetermined Source
- SE:
-
Standard error
- TIA:
-
Transient ischemic attack
- WMH:
-
White matter hyperintensities
References
Collins, G. S., de Groot, J. A., Dutton, S., Omar, O., Shanyinde, M., Tajar, A., Voysey, M., Wharton, R., Yu, L.-M., Moons, K. G., & Altman, D. G. (2014). External validation of multivariable prediction models: A systematic review of methodological conduct and reporting. BMC Medical Research Methodology, 14(1), 40. https://doi.org/10.1186/1471-2288-14-40
Dawson, J., Béjot, Y., Christensen, L. M., De Marchis, G. M., Dichgans, M., Hagberg, G., Heldner, M. R., Milionis, H., Li, L., Pezzella, F. R., Taylor Rowan, M., Tiu, C., & Webb, A. (2022). European Stroke Organisation (ESO) guideline on pharmacological interventions for long-term secondary prevention after ischaemic stroke or transient ischaemic attack. European Stroke Journal, 7(3), I–XLI. https://doi.org/10.1177/23969873221100032
Diener, H.-C., Easton, J. D., Hart, R. G., Kasner, S., Kamel, H., & Ntaios, G. (2022). Review and update of the concept of embolic stroke of undetermined source. Nature Reviews Neurology, 18(8), 455–465. https://doi.org/10.1038/s41582-022-00663-4
Diener, H.-C., Sacco, R. L., Easton, J. D., Granger, C. B., Bar, M., Bernstein, R. A., Brainin, M., Brueckmann, M., Cronin, L., Donnan, G., Gdovinová, Z., Grauer, C., Kleine, E., Kleinig, T. J., Lyrer, P., Martins, S., Meyerhoff, J., Milling, T., Pfeilschifter, W., … Schäbitz, W.-R. (2020). Antithrombotic treatment of embolic stroke of undetermined source: RE-SPECT ESUS elderly and renally impaired subgroups. Stroke, 51(6), 1758–1765. https://doi.org/10.1161/STROKEAHA.119.028643
Diener, H.-C., Sacco, R. L., Easton, J. D., Granger, C. B., Bernstein, R. A., Uchiyama, S., Kreuzer, J., Cronin, L., Cotton, D., Grauer, C., Brueckmann, M., Chernyatina, M., Donnan, G., Ferro, J. M., Grond, M., Kallmünzer, B., Krupinski, J., Lee, B.-C., Lemmens, R., … Toyoda, K. (2019). Dabigatran for prevention of stroke after embolic stroke of undetermined source. New England Journal of Medicine, 380(20), 1906–1917. https://doi.org/10.1056/NEJMoa1813959
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., & Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. American Journal of Neuroradiology, 8(3), 421–426.
Haeusler, K. G., Kirchhof, P., Heuschmann, P. U., Laufs, U., Busse, O., Kunze, C., Thomalla, G., Nabavi, D. G., Röther, J., Veltkamp, R., & Endres, M. (2016). Impact of standardized MONitoring for Detection of Atrial Fibrillation in Ischemic Stroke (MonDAFIS): Rationale and design of a prospective randomized multicenter study. American Heart Journal, 172, 19–25. https://doi.org/10.1016/j.ahj.2015.10.010
Haeusler, K. G., Kirchhof, P., Kunze, C., Tütüncü, S., Fiessler, C., Malsch, C., Olma, M. C., Jawad-Ul-Qamar, M., Krämer, M., Wachter, R., Michalski, D., Kraft, A., Rizos, T., Gröschel, K., Thomalla, G., Nabavi, D. G., Röther, J., Laufs, U., Veltkamp, R., … MonDAFIS Investigators. (2021). Systematic monitoring for detection of atrial fibrillation in patients with acute ischaemic stroke (MonDAFIS): A randomised, open-label, multicentre study. The Lancet. Neurology, 20(6), 426–436. https://doi.org/10.1016/S1474-4422(21)00067-3
Harrell, F. E. (2015). Regression modeling strategies: With applications to linear models, logistic and ordinal regression, and survival analysis. Berlin: Springer. https://doi.org/10.1007/978-3-319-19425-7
Hart, R. G., Catanese, L., Perera, K. S., Ntaios, G., & Connolly, S. J. (2017). Embolic stroke of undetermined source: A systematic review and clinical update. Stroke, 48(4), 867–872. https://doi.org/10.1161/STROKEAHA.116.016414
Hart, R. G., Diener, H.-C., Coutts, S. B., Easton, J. D., Granger, C. B., O’Donnell, M. J., Sacco, R. L., & Connolly, S. J. (2014). Embolic strokes of undetermined source: The case for a new clinical construct. The Lancet Neurology, 13(4), 429–438. https://doi.org/10.1016/S1474-4422(13)70310-7
Hart, R. G., Sharma, M., Mundl, H., Kasner, S. E., Bangdiwala, S. I., Berkowitz, S. D., Swaminathan, B., Lavados, P., Wang, Y., Wang, Y., Davalos, A., Shamalov, N., Mikulik, R., Cunha, L., Lindgren, A., Arauz, A., Lang, W., Czlonkowska, A., Eckstein, J., … Connolly, S. J. (2018). Rivaroxaban for stroke prevention after embolic stroke of undetermined source. New England Journal of Medicine, 378(23), 2191–2201. https://doi.org/10.1056/NEJMoa1802686
Healey, J. S., Gladstone, D. J., Swaminathan, B., Eckstein, J., Mundl, H., Epstein, A. E., Haeusler, K. G., Mikulik, R., Kasner, S. E., Toni, D., Arauz, A., Ntaios, G., Hankey, G. J., Perera, K., Pagola, J., Shuaib, A., Lutsep, H., Yang, X., Uchiyama, S., … Connolly, S. J. (2019). Recurrent stroke with rivaroxaban compared with aspirin according to predictors of atrial fibrillation: secondary analysis of the NAVIGATE ESUS randomized clinical trial. JAMA Neurology, 76(7), 764–773. https://doi.org/10.1001/jamaneurol.2019.0617
Hindricks, G., Potpara, T., Dagres, N., Arbelo, E., Bax, J. J., Blomström-Lundqvist, C., Boriani, G., Castella, M., Dan, G.-A., Dilaveris, P. E., Fauchier, L., Filippatos, G., Kalman, J. M., La Meir, M., Lane, D. A., Lebeau, J.-P., Lettino, M., Lip, G. Y. H., Pinto, F. J., … ESC Scientific Document Group. (2021). 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. European Heart Journal, 42(5), 373–498. https://doi.org/10.1093/eurheartj/ehaa612
January, C. T., Wann, L. S., Calkins, H., Chen, L. Y., Cigarroa, J. E., Cleveland, J. C., Ellinor, P. T., Ezekowitz, M. D., Field, M. E., Furie, K. L., Heidenreich, P. A., Murray, K. T., Shea, J. B., Tracy, C. M., & Yancy, C. W. (2019). 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Journal of the American College of Cardiology, 74(1), 104–132. https://doi.org/10.1016/j.jacc.2019.01.011
Jensen, M., & Thomalla, G. (2020). Causes and secondary prevention of acute ischemic stroke in adults. Hämostaseologie, 40(1), 22–30. https://doi.org/10.1055/s-0039-1700502
Lip, G. Y. H., Nieuwlaat, R., Pisters, R., Lane, D. A., & Crijns, H. J. G. M. (2010). Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on Atrial Fibrillation. Chest, 137(2), 263–272. https://doi.org/10.1378/chest.09-1584
Merkler, A. E., Pearce, L. A., Kasner, S. E., Shoamanesh, A., Birnbaum, L. A., Kamel, H., Sheth, K. N., & Sharma, R. (2021). Left ventricular dysfunction among patients with embolic stroke of undetermined source and the effect of rivaroxaban vs aspirin: A subgroup analysis of the NAVIGATE ESUS randomized clinical trial. JAMA Neurology, 78(12), 1454–1460. https://doi.org/10.1001/jamaneurol.2021.3828
Ntaios, G., Georgiopoulos, G., Perlepe, K., Sirimarco, G., Strambo, D., Eskandari, A., Nannoni, S., Vemmou, A., Koroboki, E., Manios, E., Rodríguez-Campello, A., Cuadrado-Godia, E., Roquer, J., Arnao, V., Caso, V., Paciaroni, M., Diez-Tejedor, E., Fuentes, B., Pardo, J. R., … Michel, P. (2019). A tool to identify patients with embolic stroke of undetermined source at high recurrence risk. Neurology, 93(23), e2094–e2104. https://doi.org/10.1212/WNL.0000000000008571
Ntaios, G., Pearce, L. A., Veltkamp, R., Sharma, M., Kasner, S. E., Korompoki, E., Milionis, H., Mundl, H., Berkowitz, S. D., Connolly, S. J., Hart, R. G., & Investigators, N. A. V. I. G. A. T. E. E. S. U. S. (2020). Potential embolic sources and outcomes in embolic stroke of undetermined source in the NAVIGATE-ESUS Trial. Stroke, 51(6), 1797–1804. https://doi.org/10.1161/STROKEAHA.119.028669
Ntaios, G., Perlepe, K., Lambrou, D., Sirimarco, G., Strambo, D., Eskandari, A., Karagkiozi, E., Vemmou, A., Koroboki, E., Manios, E., Makaritsis, K., Vemmos, K., & Michel, P. (2019). Prevalence and overlap of potential embolic sources in patients with embolic stroke of undetermined source. Journal of the American Heart Association, 8(15), e012858. https://doi.org/10.1161/JAHA.119.012858
Pimentel, B. C., Ingwersen, T., Haeusler, K. G., Schlemm, E., Forkert, N. D., Rajashekar, D., Mouches, P., Königsberg, A., Kirchhof, P., Kunze, C., Tütüncü, S., Olma, M. C., Krämer, M., Michalski, D., Kraft, A., Rizos, T., Helberg, T., Ehrlich, S., Nabavi, D. G., … Thomalla, G. (2022). Association of stroke lesion shape with newly detected atrial fibrillation—Results from the MonDAFIS study. European Stroke Journal, 7(3), 230–237. https://doi.org/10.1177/23969873221100895
R Core Team. (2023). R: A Language and Environment for Statistical Computing [Computer software].
Ramspek, C. L., Jager, K. J., Dekker, F. W., Zoccali, C., & van Diepen, M. (2021). External validation of prognostic models: What, why, how, when and where? Clinical Kidney Journal, 14(1), 49–58. https://doi.org/10.1093/ckj/sfaa188
Royston, P., & Altman, D. G. (2013). External validation of a Cox prognostic model: Principles and methods. BMC Medical Research Methodology, 13(1), 33. https://doi.org/10.1186/1471-2288-13-33
Schäbitz, W.-R., Köhrmann, M., Schellinger, P. D., Minnerup, J., & Fisher, M. (2020). embolic stroke of undetermined source: Gateway to a new stroke entity? The American Journal of Medicine, 133(7), 795–801. https://doi.org/10.1016/j.amjmed.2020.03.005
Schirmer, M. D., Giese, A.-K., Fotiadis, P., Etherton, M. R., Cloonan, L., Viswanathan, A., Greenberg, S. M., Wu, O., & Rost, N. S. (2019). Spatial signature of white matter hyperintensities in stroke patients. Frontiers in Neurology. https://doi.org/10.3389/fneur.2019.00208
Vergouwe, Y., Steyerberg, E. W., Eijkemans, M. J. C., & Habbema, J. D. F. (2005). Substantial effective sample sizes were required for external validation studies of predictive logistic regression models. Journal of Clinical Epidemiology, 58(5), 475–483. https://doi.org/10.1016/j.jclinepi.2004.06.017
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL. The MonDAFIS study is an investigator‑initiated, prospective, randomised, multicentre study sponsored by the Charité ‑ Universitätsmedizin Berlin, Germany and supported by an unrestricted research grant from Bayer Vital GmbH, Germany to the Charité ‑ Universitätsmedizin Berlin, Germany.
Author information
Authors and Affiliations
Contributions
GN, ME, KGH and GT contributed to the conception and design of the work. TI, MO, PK, RV, JR, UL, DGN, ME, KGH and GT contributed to data acquisition and patient recruitment. TI, MO, ES, CM, BC and GT contributed to statistical analysis and interpretation. TI and GT drafted the first version of the manuscript. All authors contributed to critical revision of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The MonDAFIS study was approved by the Ethics Committee of participating study sites, first by the Charité Ethics Committee, Berlin, Germany (EA2_033_14). All study patients gave written informed consent.
Consent for publication
Not applicable.
Competing interests
MO, ES, CM, BC, ST and TI report no conflict of interests outside the submitted work. PK receives research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past. PK is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). RV reports grants, personal fees and other from Bayer, grants from Boehringer, grants and personal fees from BMS, grants from Daiichi Sankyo, grants from Medtronic, personal fees from Javelin, grants from Biogen, grants and personal fees from Pfizer, personal fees from Abbott, personal fees from Astra Zeneca, other from Novartis, outside the submitted work, and is an editorial board member of Neurological Research and Practice. RV is an investigator of Imperial BRC and partially funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 754517 (PRESTIGE-AF). JR has received speaker ́s honoraria and consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers-Squibb, Pfizer, Daiichi Sankyo, Alexion and Astra Zeneca. UL reports honoraria/reimbursements for lectures, participation in studies, scientific cooperations (with Saarland University), consulting, travel, support (of colleagues) or support of scientific meetings by Amgen, Bayer, Boehringer-Ingelheim, Daiichi-Sankyo, MSD, Sanofi, Servier outside the submitted work. DGN has received speaker’s and consulting fees from Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol-Myers-Squibb, Pfizer, and Daiichi Sankyo. ME reports grants from Bayer and fees paid to the Charité from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers-Squibb, Covidien, Daiichi Sankyo, Glaxo Smith Kline, Novartis, Pfizer, and Sanofi, and is an editorial board member of Neurological Research and Practice. KGH reports speaker ́s honoraria, consulting fees, lecture honoraria and/or study grants from Abbott, Alexion, Amarin, AstraZeneca, Bayer Healthcare, Sanofi, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Pfizer, Bristol-Myers Squibb, Biotronik, Medtronic, Novartis, Portola, Getemed AG, Premier Research, W.L. Gore and Associates, SUN Pharma and Edwards Lifesciences. GT has received speaker ́s honoraria or consulting fees from Acandis, Bayer Healthcare, Boehringer Ingelheim, Covidien, Bristol‑MyersSquibb, Portola, Stryker and Pfizer, and is a section editor of Neurological Research and Practice.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ingwersen, T., Olma, M.C., Schlemm, E. et al. Independent external validation of a stroke recurrence score in patients with embolic stroke of undetermined source. Neurol. Res. Pract. 5, 51 (2023). https://doi.org/10.1186/s42466-023-00279-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42466-023-00279-z