Abstract
Despite the advances in medical and device therapies for heart failure (HF), sudden cardiac death (SCD) remains a tremendous global burden in patients with HF. Among the risk factors for SCD, HF has the greatest impact. Previous studies focusing on patients with systolic dysfunction have found several predictive factors associated with SCD, leading to the subsequent development of strategies of primary prevention, like placement of implantable cardioverter-defibrillator (ICD) in high-risk patients. Although patients with HF with preserved ejection fraction (HFpEF) were less prone to SCD compared to patients with HF with reduced ejection fraction (HFrEF), patients with HFpEF did account for a significant proportion of all HF patients who encountered SCD. The cutoff value of left ventricular ejection fraction (LVEF) to define the subset of HF did not reach consensus until 2016 when the European Society of Cardiology proposed a new classification system by LVEF. There is a great unmet need in the field of SCD in HFpEF regarding risk stratification and appropriate device therapy with ICD implantation. In this article, we will approach SCD in HFpEF from HFrEF subsets. We also aim at clarifying the mechanisms, risk factors, and prevention of SCD in HFpEF.
Similar content being viewed by others
Backgrounds
The definition of sudden cardiac death (SCD) by World Health Organization (WHO) in 1985 referred to “sudden unexpected death occurring either within 1 h of symptom onset (witnessed), or within 24 h of having been observed alive and symptom-free (un-witnessed).” [1, 2]. The original purpose of this definition was to identify “sudden arrhythmic death (SAD)” rather than SCD of non-arrhythmic causes. However, this definition was not universally applied in all relevant studies over the past three decades. The diversity in the definition of SCD came from clinicians’ subjective predilections [3]. Not until recent years did the San Francisco Postmortem Systematic Investigation conduct a systematic autopsy in patients died of SCD and found only 55.8% of WHO-defined SCD to be SAD after excluding non-arrhythmic causes, i.e., intracranial hemorrhage, pulmonary embolism, cardiac tamponade, acute heart failure (HF), and occult drug overdose [4]. This review will be focused on arrhythmic events-related SCD.
HF increases the risks of SCD compared to the general population. On the other hand, up to 50% of deaths in HF patients presented with a sudden and unexpected pattern [5]. It is noteworthy that the majority of trials investigating SCD in HF enrolled patients with systolic dysfunction [6,7,8,9,10,11,12,13,14]. As the new HF classification was released by the European Society of Cardiology, HF was re-classified into three categories: HF with reduced (HFrEF), mildly reduced (HFmrEF), and preserved ejection fraction (EF, HFpEF) with the corresponding EF of ≤ 40%, 41–49%, and ≥ 50%, respectively [15, 16]. With this new classification, many clinical trials were undertaken to identify risk of SCD in HF patients, particularly in the categories of HFmrEF and HFpEF. A common approach is to examine the traditional well-established risk factors for predicting SCD in HFrEF patients in a new patient group of HFpEF. However, such approaches generated controversial results due to the heterogeneity in HFpEF phenotypes, and the appropriate risk stratification in this population remains unclear. In this review, we will discuss the epidemiology, mechanisms, risk factors, and preventions of SCD in HFpEF patients and try to address some unanswered questions. Patients with cardiomyopathies, i.e., hypertrophic cardiomyopathy or sarcoidosis, have already known to carry a much higher risk of SCD than other populations and will not be included in this review. Patients with inherited arrhythmia but without structural abnormality will not be discussed, either.
Epidemiology of SCD in HF
SCD in the general population
The accurate incidence of SCD in the general population is diverse due to the variations in SCD definition among different trials, the differences in emergency medical service announcement protocols, the differences in autopsy rates, and the discrepancy in the national recording systems [17]. It is estimated that the global incidence rate varies from 20 to over 100 per 100,000 person-years across different populations and countries [18, 19]. Myocardial substrates (i.e., fibrosis or hypertrophy), predisposing factors (i.e., genetics or diabetes), and triggers (i.e., ischemia or electrolyte imbalance) are all important contributors to SCD occurrence. In general population, coronary artery disease is the most common etiology, accounting for over 50% of SCD, while other causes include valvular heart diseases, cardiomyopathy, arrhythmic channelopathy, etc., and the timing of SCD occurrence depending on age and disease onsets [17, 20, 21].
SCD in patients with HFrEF
The incidence of SCD in patients with HFrEF could be identified from clinical trials. In MUSTT trial which enrolled patients with ischemic cardiomyopathy, asymptomatic non-sustained ventricular tachycardia, and a LVEF ≤ 40%, the patients in the medical treatment arm had 32% of SCD incidence rate after 5-year follow-up [22]. In DANISH trial which enrolled patients with non-ischemic cardiomyopathy and a LVEF ≤ 35%, SCD incidence rate was 8.2% in the standard care arm after a median follow-up of 67.6 months [23]. Multiple trials have demonstrated that left ventricular systolic dysfunction is the greatest risk factor for SCD.
SCD in patients with HFpEF
Although patients with HFpEF were less prone to SCD compared to patients with HFrEF, patients with HFpEF did account for a significant proportion of all HF patients who encountered SCD. An observational study in Netherland showed that the incidence of SCD in patients with a LVEF > 50% and a previous HF history was 19%, while the incidence of SCD was 61% in patients with a LVEF > 50% but without HF history [24]. Another study by Stecker et al. assessed 121 SCD patients who had LVEF data before SCD. They found that 48% of these SCD patients have a normal (≥ 55%) LVEF, while 30% and 22% of these SCD patients have a severely reduced (≤ 35%) and mildly to moderately reduced (36–54%) LVEF, respectively [25]. The incidence of SCD in patients with HFpEF remains unknown in these two studies. In the TOPCAT, I-PRESERVE, and CHARM-Preserved trials, SCD consistently accounted for around one fourth of total death in HFpEF patients in these trials [26,27,28]. In the I-PRESERVE trial, the annual SCD rate was 1.35% in patients with HFpEF [27].
Mechanisms of SCD in HF
There are several mechanisms reported to cause SCD in HF. Packer et al. proposed two main pathways that led to SCD in HFrEF, one is the ventricular tachyarrhythmia precipitated by myocardial infarction and catecholamine surge, while the other one is bradyarrhythmia caused by adverse ventricular remodeling-related mechanical failure. The latter mechanism was of particular importance and characterized by “self-organizing criticality” in which the interdependence of each cell exaggerates, resulting in unstable equilibrium and easy collapse [29]. Previous studies in HFpEF population showed that the causes of death were cardiovascular origin in 60–70% patients, with the majority being SCD (20–40%) [30]. However, trying to apply this framework to identify the causes of death in HFpEF patients remains challenging. A systematic review focusing on the mode of death in HF with normal/near-normal LVEF found that specific causes of SCD were poorly defined [31]. More studies are needed to clarify which pathway is more predominant in SCD in HFpEF, so that the corresponding preventive strategies may be more precise and effective.
Mechanisms of SCD in HFrEF
HF was associated with increased action potential duration (APD) and transmural heterogeneity of repolarization, leading to increased arrhythmic events and SCD [5, 32, 33]. This electrical remodeling is the consequence of dysregulation of different ion channels, especially the potassium channels (IK), and the transient outward potassium current (Ito) has been shown to result in electrical dispersion and triggered activity in the failing hearts. Sodium channels (INa) are also responsible for the maintenance of AP plateau and repolarization so that alterations of INa in HF patients may lead to pro-arrhythmic states [5]. Intracellular calcium not only mediates myocardial contraction but also influences electrophysiological balance [34, 35]. Dysregulation of calcium channels is also responsible for excitation–contraction uncoupling and arrhythmogenesis [5].
Myocardial ischemia is the most common etiology of SCD because ischemia leads to APD prolongation and abnormal calcium handling, bringing the cellular membrane potential to a lower threshold for ventricular excitation. Heterogeneous distributed ischemic myocardium also enhanced reentry arrhythmia around the ischemic obstacles [5]. In the Assessment of Treatment with Lisinopril and Survival (ATLAS) study, acute coronary events (i.e., thrombus, ruptured plaque, or myocardial infarction) were commonly observed in patients with SCD in the setting of CAD with HFrEF [36]. Gap junction down regulation in molecular levels might cause impedance mismatch and differential conduction in cellular levels, leading to unidirectional block and reentry formation in pacing-induced HFrEF model [5, 37].
Mechanisms of SCD in HFpEF
Myocardial substrate remodeling, including myocardial scars, left ventricular hypertrophy (LVH), cardiac fibrosis, and collagen deposition, might enhance anisotropic ventricular conduction and facilitate re-entrant ventricular arrhythmia [2]. It is noteworthy that LVH was estimated to have a worldwide prevalence of up to 40% [38]. In the Oregon Sudden Unexpected Death Study (Oregon SUDS), both concentric LVH (odds ratio [OR] 3.20; 95% CI 1.90–5.39; p < 0.001) and eccentric LVH (OR 2.47; 95% CI 1.30–4.66; p = 0.006) were reported to have a statistically significant impact on SCD prediction in multivariate analyses [39]. Despite employing different echocardiographic criteria of LVH among several studies, LVH was consistently shown to be a robust predictor of SCD in HFpEF patients as does the severely decreased LVEF, and could be a risk stratification parameter for SCD in HFpEF patients [40].
Genetic predisposition also contributes to SCD in HF patients. Several genes have been identified to be associated with SCD, although many of them are merely arrhythmic syndromes related. In cardiomyopathy-related HF, MYBPC3 gene for hypertrophic cardiomyopathy (HCM) and PKP2/DSP gene for arrhythmogenic right ventricular cardiomyopathy are reported to be associated with SCD in HF. Nowadays, genome-wide association studies (GWAS) might help to validate the gene variants and expand the novel ones into clinical decision [41,42,43].
As the speculation proposed by Tomaselli GF et al., multi-hit hypothesis including genetic predisposition (first hit), development of HF and abnormalities in conduction and repolarization (second hit), and environmental factors like ischemia (third hit) together contributed to the occurrence of SCD in HF [5].
Risk factors and risk stratification of SCD in HF
Risk stratification of SCD in HFrEF
The most widely accepted predictor for SCD in HF is the reduced LVEF (< 35–40%). With this predictor, clinical studies in this HF subset have generated recommendations of medication and device therapy to reduce SCD incidence in high-risk HF patients. Beyond LVEF grows many fields of study interests in SCD prediction and risk stratification.
Echocardiography is widely used and provides a simple way to estimate the LVEF for SCD risk prediction. Other parameters have been explored to assess the myocardial structures to predict SCD risk in HFpEF and HFmrEF populations. In a meta-analysis, mitral annular calcification, mitral E/A ratio beyond 0.7–1.5, LVH, and left atrial enlargement were significantly associated with increased SCD, indicating that these parameters other than LVEF could also predict SCD risk in HFrEF patients [44].
Myocardial scar assessment is another intuitive approach for risk stratification in HF. Myocardial scar might serve as a conduction obstacle and allow reentrant arrhythmic events to occur. Late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR) offers the quantification of the scar zones in myocardium. However, the total amount of scar in myocardium by LGE quantification is not predictive of scar-related ventricular arrhythmia [45]. Rather, the heterogeneously distributed scar together with low voltage zone shown on electrophysiological mapping was nicely correlated with reentrant ventricular arrhythmia [46, 47]. Although CMR is capable of identifying the total scar burdens and the heterogeneous zones [48], to date CMR assessment still cannot predict if implantable cardioverter-defibrillator (ICD) implantation might reduce the risk of SCD in HF patients [49]. By analyzing a cohort of dilated cardiomyopathy patients (n = 1165) who underwent CMR with LGE, Marco et al. reported that LGE was an independent and strong predictor of the arrhythmic endpoints including appropriate ICD therapies, sustained ventricular tachycardia, cardiac arrest, and sudden death. They further developed a new clinical algorithm which integrated LGE and LVEF and found that this algorithm significantly improved the risk stratification for VA and sudden death, providing relevant implications for ICD allocation in patients with dilated cardiomyopathy [50]. An ESTIMATED score (constructed by the LGE extent > 14%, syncope, atrial flutter/fibrillation, non-sustained ventricular tachycardia, advanced AV block, and age ≤ 20 or > 50 years) was used to predict the risk of SCD in 395 patients with non-ischemic cardiomyopathy [51]. This score showed good calibrations for SCD prediction in the derivation and validation sets in a 3-year follow-up. The authors speculated that this score might help to identify candidates for primary prevention by ICD in patients with non-ischemic cardiomyopathy.
Prolonged T peak-T end intervals (TpTe) on ECG, which suggests an increased dispersion of transmural repolarization, have been reported to predict SCD or ventricular arrhythmias in patients with HCM and patients receiving cardiac resynchronization therapy (CRT) [52, 53]. Consistently, a short TpTe was associated with less ventricular arrhythmia or death in HFrEF (LVEF 23 ± 7%) patients who underwent ICD implantation for primary prevention [54]. Aro et al. further analyzed the ECG on the Oregon SUDS and found 6 electrical risk factors, including heart rate > 75 bpm, QRS transition > V4, LVH, frontal QRS-T angle > 90°, QTc interval > 450/460 ms, and TpTe > 89 ms to be significantly associated with SCD in patients with HFrEF. This electrical risk scores were externally validated in the Atherosclerosis Risk in Communities (ARIC) study and showed that increased electrical abnormalities were associated with higher odds ratio of SCD (≥ 4 ECG markers, odds ratio 26.1 [9.9–68.5], p < 0.001) [55].
Heart rate variability (HRV) and heart rate turbulence (HRT) were parameters to evaluate cardiac autonomic function. Decreased HRV and HRT indicated the loss of vagal tone and have been reported to increase the risks of SCD and non-SCD in patients with MI and left ventricular dysfunction [45, 56]. In a meta-analysis, however, HRV and HRT failed to predict SCD in patients with non-ischemic cardiomyopathy with HFrEF [57].
Risk stratification of SCD in HFpEF
In a prospective multicenter study, 301 patients with non-ST-elevation myocardial infarction (NSTEMI) and 268 patients with ST-elevation myocardial infarction (STEMI) with preserved LVEF were enrolled to receive baseline echocardiographic evaluation. During a median 30 months of follow-up, decreased global longitudinal strain (GLS), increased mechanical dispersion, and increased post-systolic strain index (PSSI) were associated with increased arrhythmias risk in both the NSTEMI and STEMI groups [58]. This finding indicated that regional myocardial motion impairment on echocardiography could be predictors for SCD in patients with ischemic heart disease and HFpEF.
ECG also serves as a simple tool to identify the pro-arrhythmic state. T wave alternans (TWA), defined by beat-to-beat variation in T wave morphology, have been found to be associated with lethal arrhythmia since early twentieth century [3]. TWA has been validated in some clinical trials of ischemic cardiomyopathy with HFrEF to predict SCD [59,60,61]. In a prospective observational study, TWA analyzed after acute myocardial infarction (MI) in patients with HFpEF (an LVEF ≥ 40%) was associated with increased SCD risk [62].
Electrophysiological studies (EPS) with provocative testing (programmed ventricular stimulation) are well investigated in risk stratification of SCD. In MUSTT trial, patients who had ischemic cardiomyopathy and non-sustained VT benefited from ICD implantation if inducible sustained VT was observed during EPS [63]. In another post-MI group with HFpEF, patients with at least one of the following high-risk features: premature ventricular contraction (PVC), non-sustained VT, late potentials, prolonged QTc, increased TWA, abnormal HRT, and reduced HRV were referred for EPS. ICD was implanted in case of inducible malignant ventricular arrhythmias. In the follow-up duration, no SCD occurred in patients without risk factors and in those with a negative result on EPS, with a two-step negative predictive value (NPV) of 100% [64].
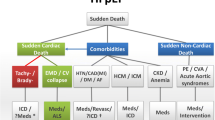
In summary, a low LVEF predicts the SCD risks in HF patients, but risk stratification predictors other than LVEF are attracting increasing interests. Unlike the risk predictors for SCD in patients with ischemic cardiomyopathy and HFrEF, the risk predictors for SCD in non-ischemic HFpEF patients remain inconclusive [65]. We speculated that anatomical remodeling, autonomic dysfunction, electrical remodeling, and genetic characteristics could be equally important and will serve as the 4 pillars to determine the SCD risks in patients with HF (Fig. 1) [45].
Four pillars of sudden cardiac death in HFpEF and relevant mechanisms. Dash lines mean need of more literature to elucidate. Abbreviation: HFpEF (heart failure with preserved ejection fraction); CMR (cardiac magnetic resonance); LGE (late gadolinium enhancement); HCM (hypertrophic cardiomyopathy); ARVC (arrhythmogenic right ventricular cardiomyopathy); GWAS (genome-wide association studies), VT (ventricular tachycardia); VF (ventricular fibrillation); ICD (implantable cardioverter-defibrillator); PEA (pulseless electric activity)
Prevention and Risk reduction in SCD in HFpEF
Since ICD trials enrolling patients with systolic dysfunction (LVEF \(\le \) 35–40%), including both ischemic and non-ischemic causes showed benefit in reducing SCD from ICD primary prevention [12, 13, 23, 63, 66]. SCD prevention by ICD implantation in HFrEF is therefore a well-established treatment. Other backbone drugs or device therapy for HFrEF, including angiotensin-converting enzymes inhibitors (ACEi), beta blockers, mineralocorticoid receptor antagonists (MRA), neprilysin inhibitors, and CRT, also showed a trend toward SCD reduction in subgroups in the randomized controlled trials [29]. Although the new sodium–glucose cotransporter 2 inhibitors (SGLT2i) have received a first-line Class I indication for HFrEF in the latest HF guideline [16], meta-analysis study including 19 RCTs with over 50,000 participants showed no decrease in SCD or ventricular arrhythmia in the SGLT2i arm. [67].
Due to the limited data, prevention of SCD in HFpEF is still inconclusive. Considering heterogeneity in HFpEF and complex co-morbidities in these patients, priority should be placed at treating the disease-causing mechanisms, like ischemia, valvular heart diseases, hypertension, or diabetes, and preventing any precipitating factor such as electrolyte imbalance [30, 68]. We proposed the possible indications for ICD implantation for primary prevention in HFpEF patients with cardiomyopathies (Table 1) [69].
Conclusions
Researches regarding SCD in patients with systolic dysfunction have been well studied. As the new HF classification has been established, and epidemiologic data highlighted the large number of SCD cases in HFpEF, more attentions are attracted to the risk of SCD in this group. When trying to extrapolate the well-established HF management concepts from HFrEF to HFpEF, heterogeneity in HFpEF remains a major challenge in defining an integral framework for risk stratification. Multi-pronged approaches to prevent anatomic remodeling, autonomic dysfunction, electrical remodeling, and identify genetic characteristics are essential. Effective prevention such as ICD implantation could thereafter be implemented only if we can predict SCD in HFpEF precisely [69].
Availability of data and materials
Not applicable.
Abbreviations
- SCD:
-
Sudden cardiac death
- SAD:
-
Sudden arrhythmic death
- HF:
-
Heart failure
- HFrEF:
-
Heart failure with reduced ejection fractions
- HFmrEF:
-
Heart failure with mildly reduced ejection fractions
- HFpEF:
-
Heart failure with preserved ejection fractions
- LVEF:
-
Left ventricular ejection fractions
- AP:
-
Action potential
- LVH:
-
Left ventricular hypertrophy
- CAD:
-
Coronary artery disease
- HCM:
-
Hypertrophic cardiomyopathy
- ARVC:
-
Arrhythmogenic right ventricular cardiomyopathy
- NSTEMI:
-
Non-ST elevation myocardial infarction
- STEMI:
-
ST elevation myocardial infarction
- GLS:
-
Global longitudinal strain
- PSSI:
-
Post-systolic strain index
- CMR:
-
Cardiac magnetic resonance
- LGE:
-
Late gadolinium enhancement
- ICD:
-
Implantable cardioverter-defibrillator
- TWA:
-
T wave alternans
- TpTe:
-
T peak-T end interval
- CRT:
-
Cardiac resynchronization therapy
- MI:
-
Myocardial infarction
- HRV:
-
Heart rate variability
- HRT:
-
Heart rate turbulence
- EPS:
-
Electrophysiologic studies
- VT:
-
Ventricular tachycardia
- PVC:
-
Premature ventricular contraction
- ACEi:
-
Angiotensin-converting enzymes inhibitors
- MRA:
-
Mineralocorticoid receptor antagonists
- SGLT2i:
-
Sodium–glucose cotransporter 2 inhibitors
References
Sudden cardiac death. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1985;726:5–25
Dimos A, Xanthopoulos A, Papamichalis M, Bourazana A, Tavoularis D, Skoularigis J, Triposkiadis F. Sudden arrhythmic death at the higher end of the heart failure spectrum. Angiology. 2020;71:389–96.
Saour B, Smith B, Yancy CW. Heart failure and sudden cardiac death. Card Electrophysiol Clin. 2017;9:709–23.
Tseng ZH, Olgin JE, Vittinghoff E, Ursell PC, Kim AS, Sporer K, Yeh C, Colburn B, Clark NM, Khan R, Hart AP, Moffatt E. Prospective countywide surveillance and autopsy characterization of sudden cardiac death: POST SCD study. Circulation. 2018;137:2689–700.
Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95:754–63.
Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. 1995;273:1450–6.
Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann-Zalan I, DeMets DL, Carvedilol Prospective Randomized Cumulative Survival Study G. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106:2194–9.
Effect of metoprolol CR/XL in chronic heart failure. Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001–7.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17.
Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, Group E-HS. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Investigators P-H and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004.
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML, Multicenter Automatic Defibrillator Implantation Trial III. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83.
Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH, Sudden Cardiac Death in Heart Failure Trial I. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37.
Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM, Comparison of Medical Therapy P, Defibrillation in Heart Failure I. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESCSD. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine SA, Group ESCSD. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726.
Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116:1887–906.
Wong CX, Brown A, Lau DH, Chugh SS, Albert CM, Kalman JM, Sanders P. Epidemiology of sudden cardiac death: global and regional perspectives. Heart Lung Circ. 2019;28:6–14.
Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, Mariani R, Gunson K, Jui J. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51:213–28.
Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125:1043–52.
Lane RE, Cowie MR, Chow AW. Prediction and prevention of sudden cardiac death in heart failure. Heart. 2005;91:674–80.
Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–90.
Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjaer H, Brandes A, Thogersen AM, Gustafsson F, Egstrup K, Videbaek R, Hassager C, Svendsen JH, Hofsten DE, Torp-Pedersen C, Pehrson S, Investigators D. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–30.
Gorgels APM, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJJ. Out-of-hospital cardiac arrest-the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24:1204–9.
Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161–6.
Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, Investigators T. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92.
Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, Lopez-Sendon J, Teerlink JR, White M, McMurray JJ, Komajda M, McKelvie R, Ptaszynska A, Hetzel SJ, Massie BM, Carson PE, Investigators IP. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010;121:1393–405.
Solomon SD, Wang D, Finn P, Skali H, Zornoff L, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Pocock S, Pfeffer MA. Effect of candesartan on cause-specific mortality in heart failure patients: the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2004;110:2180–3.
Packer M. What causes sudden death in patients with chronic heart failure and a reduced ejection fraction? Eur Heart J. 2020;41:1757–63.
Manolis AS, Manolis AA, Manolis TA, Melita H. Sudden death in heart failure with preserved ejection fraction and beyond: an elusive target. Heart Fail Rev. 2019;24:847–66.
Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, Butler J. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017;69:556–69.
Akar FG, Rosenbaum DS. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ Res. 2003;93:638–45.
Tse G. Mechanisms of cardiac arrhythmias. J Arrhythm. 2016;32:75–81.
Bers DM. Calcium and cardiac rhythms: physiological and pathophysiological. Circ Res. 2002;90:14–7.
Morad M, Maylie J. Calcium and cardiac electrophysiology. Some experimental considerations. Chest. 1980;78:166–73.
Uretsky BF, Thygesen K, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Packer M, Poole-Wilson PA, Ryden L. Acute coronary findings at autopsy in heart failure patients with sudden death: results from the assessment of treatment with lisinopril and survival (ATLAS) trial. Circulation. 2000;102:611–6.
Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, DiSilvestre D, Tunin RS, Kass DA, Tomaselli GF. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H1223–30.
Cuspidi C, Sala C, Negri F, Mancia G, Morganti A, Italian Society of H. Prevalence of left-ventricular hypertrophy in hypertension: an updated review of echocardiographic studies. J Hum Hypertens. 2012;26:343–9.
Aro AL, Reinier K, Phan D, Teodorescu C, Uy-Evanado A, Nichols GA, Gunson K, Jui J, Chugh SS. Left-ventricular geometry and risk of sudden cardiac arrest in patients with preserved or moderately reduced left-ventricular ejection fraction. Europace. 2017;19:1146–52.
Stevens SM, Reinier K, Chugh SS. Increased left ventricular mass as a predictor of sudden cardiac death: is it time to put it to the test? Circ Arrhythm Electrophysiol. 2013;6:212–7.
Musunuru K, Hershberger RE, Day SM, Klinedinst NJ, Landstrom AP, Parikh VN, Prakash S, Semsarian C, Sturm AC, American Heart Association Council on G, Precision M, Council on Arteriosclerosis T, Vascular B, Council on C, Stroke N and Council on Clinical C. Genetic Testing for Inherited Cardiovascular Diseases: A Scientific Statement From the American Heart Association. Circ Genom Precis Med. 2020;13:e000067.
Fatkin D, Calkins H, Elliott P, James CA, Peters S, Kovacic JC. Contemporary and future approaches to precision medicine in inherited cardiomyopathies: JACC focus seminar 3/5. J Am Coll Cardiol. 2021;77:2551–72.
Osman J, Tan SC, Lee PY, Low TY, Jamal R. Sudden Cardiac Death (SCD) - risk stratification and prediction with molecular biomarkers. J Biomed Sci. 2019;26:39.
Konety SH, Koene RJ, Norby FL, Wilsdon T, Alonso A, Siscovick D, Sotoodehnia N, Gottdiener J, Fox ER, Chen LY, Adabag S and Folsom AR. Echocardiographic Predictors of Sudden Cardiac Death: The Atherosclerosis Risk in Communities Study and Cardiovascular Health Study. Circ Cardiovasc Imaging. 2016;9.
Deyell MW, Krahn AD, Goldberger JJ. Sudden cardiac death risk stratification. Circ Res. 2015;116:1907–18.
Stevenson WG, Friedman PL, Sager PT, Saxon LA, Kocovic D, Harada T, Wiener I, Khan H. Exploring postinfarction reentrant ventricular tachycardia with entrainment mapping. J Am Coll Cardiol. 1997;29:1180–9.
Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, Gerstenblith G, Weiss RG, Marban E, Tomaselli GF, Lima JA, Wu KC. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–14.
Wu KC, Gerstenblith G, Guallar E, Marine JE, Dalal D, Cheng A, Marban E, Lima JA, Tomaselli GF, Weiss RG. Combined cardiac magnetic resonance imaging and C-reactive protein levels identify a cohort at low risk for defibrillator firings and death. Circ Cardiovasc Imaging. 2012;5:178–86.
Kadish AH, Bello D, Finn JP, Bonow RO, Schaechter A, Subacius H, Albert C, Daubert JP, Fonseca CG, Goldberger JJ. Rationale and design for the defibrillators to reduce risk by magnetic resonance imaging evaluation (DETERMINE) trial. J Cardiovasc Electrophysiol. 2009;20:982–7.
Di Marco A, Brown PF, Bradley J, Nucifora G, Claver E, de Frutos F, Dallaglio PD, Comin-Colet J, Anguera I, Miller CA, Schmitt M. Improved risk stratification for ventricular arrhythmias and sudden death in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2021;77:2890–905.
Li X, Fan X, Li S, Sun W, Shivkumar K, Zhao S, Lu M, Yao Y. A novel risk stratification score for sudden cardiac death prediction in middle-aged, nonischemic dilated cardiomyopathy patients: the ESTIMATED score. Can J Cardiol. 2020;36:1121–9.
Shimizu M, Ino H, Okeie K, Yamaguchi M, Nagata M, Hayashi K, Itoh H, Iwaki T, Oe K, Konno T, Mabuchi H. T-peak to T-end interval may be a better predictor of high-risk patients with hypertrophic cardiomyopathy associated with a cardiac troponin I mutation than QT dispersion. Clin Cardiol. 2002;25:335–9.
Barbhaiya C, Po JR, Hanon S, Schweitzer P. Tpeak—Tend and Tpeak—Tend /QT ratio as markers of ventricular arrhythmia risk in cardiac resynchronization therapy patients. Pacing Clin Electrophysiol. 2013;36:103–8.
Rosenthal TM, Stahls PF 3rd, Abi Samra FM, Bernard ML, Khatib S, Polin GM, Xue JQ, Morin DP. T-peak to T-end interval for prediction of ventricular tachyarrhythmia and mortality in a primary prevention population with systolic cardiomyopathy. Heart Rhythm. 2015;12:1789–97.
Aro AL, Reinier K, Rusinaru C, Uy-Evanado A, Darouian N, Phan D, Mack WJ, Jui J, Soliman EZ, Tereshchenko LG, Chugh SS. Electrical risk score beyond the left ventricular ejection fraction: prediction of sudden cardiac death in the Oregon Sudden Unexpected Death Study and the Atherosclerosis Risk in Communities Study. Eur Heart J. 2017;38:3017–25.
Lombardi F, Stein PK. Origin of heart rate variability and turbulence: an appraisal of autonomic modulation of cardiovascular function. Front Physiol. 2011;2:95.
Goldberger JJ, Subacius H, Patel T, Cunnane R, Kadish AH. Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2014;63:1879–89.
Haugaa KH, Grenne BL, Eek CH, Ersboll M, Valeur N, Svendsen JH, Florian A, Sjoli B, Brunvand H, Kober L, Voigt JU, Desmet W, Smiseth OA, Edvardsen T. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc Imaging. 2013;6:841–50.
Bloomfield DM, Steinman RC, Namerow PB, Parides M, Davidenko J, Kaufman ES, Shinn T, Curtis A, Fontaine J, Holmes D, Russo A, Tang C, Bigger JT Jr. Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: a solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation. 2004;110:1885–9.
Chow T, Kereiakes DJ, Onufer J, Woelfel A, Gursoy S, Peterson BJ, Brown ML, Pu W, Benditt DG, Investigators MT. Does microvolt T-wave alternans testing predict ventricular tachyarrhythmias in patients with ischemic cardiomyopathy and prophylactic defibrillators? The MASTER (Microvolt T Wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patients) trial. J Am Coll Cardiol. 2008;52:1607–15.
Gold MR, Ip JH, Costantini O, Poole JE, McNulty S, Mark DB, Lee KL, Bardy GH. Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: primary results from the T-wave alternans sudden cardiac death in heart failure trial substudy. Circulation. 2008;118:2022–8.
Ikeda T, Yoshino H, Sugi K, Tanno K, Shimizu H, Watanabe J, Kasamaki Y, Yoshida A, Kato T. Predictive value of microvolt T-wave alternans for sudden cardiac death in patients with preserved cardiac function after acute myocardial infarction: results of a collaborative cohort study. J Am Coll Cardiol. 2006;48:2268–74.
Buxton AE, Lee KL, DiCarlo L, Gold MR, Greer GS, Prystowsky EN, O’Toole MF, Tang A, Fisher JD, Coromilas J, Talajic M, Hafley G. Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. Multicenter unsustained tachycardia trial investigators. N Engl J Med. 2000;342:1937–45.
Gatzoulis KA, Tsiachris D, Arsenos P, Antoniou CK, Dilaveris P, Sideris S, Kanoupakis E, Simantirakis E, Korantzopoulos P, Goudevenos I, Flevari P, Iliodromitis E, Sideris A, Vassilikos V, Fragakis N, Trachanas K, Vernardos M, Konstantinou I, Tsimos K, Xenogiannis I, Vlachos K, Saplaouras A, Triantafyllou K, Kallikazaros I, Tousoulis D. Arrhythmic risk stratification in post-myocardial infarction patients with preserved ejection fraction: the PRESERVE EF study. Eur Heart J. 2019;40:2940–9.
Adabag S, Rector TS, Anand IS, McMurray JJ, Zile M, Komajda M, McKelvie RS, Massie B, Carson PE. A prediction model for sudden cardiac death in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2014;16:1175–82.
Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N Engl J Med. 1996;335:1933–40.
Sfairopoulos D, Zhang N, Wang Y, Chen Z, Letsas KP, Tse G, Li G, Lip GYH, Liu T and Korantzopoulos P. Association between sodium-glucose cotransporter-2 inhibitors and risk of sudden cardiac death or ventricular arrhythmias: a meta-analysis of randomized controlled trials. Europace. 2021.
Pannone L, Falasconi G, Cianfanelli L, Baldetti L, Moroni F, Spoladore R and Vergara P. Sudden cardiac death in patients with heart disease and preserved systolic function: current options for risk stratification. J Clin Med. 2021;10.
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018;138:e272–391.
Acknowledgements
None.
Funding
This study was supported by grants from Taichung Veterans General Hospital, Taiwan (TCVGH-1091004C, TCVGH-1103105C, TCVGH-1103101D).
Author information
Authors and Affiliations
Contributions
SJ Wu drafted the manuscript; YC Hsieh revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Yes.
Competing interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, SJ., Hsieh, YC. Sudden cardiac death in heart failure with preserved ejection fraction: an updated review. Int J Arrhythm 23, 7 (2022). https://doi.org/10.1186/s42444-021-00059-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42444-021-00059-3