Abstract
Background
In 2021, an EULAR task force published a definition of difficult-to-treat rheumatoid arthritis (D2T RA). Our current knowledge of D2T RA with the EULAR definition is based on European and Asian cohorts, and no North American cohort has yet to be published. The aim of this study was to compare D2T RA patients to non-D2T RA who are good responders to advanced therapy, and to describe their evolution in an university health center patient cohort.
Methods
This is a retrospective single centre study of the medical records of all adults with RA on at least one biologic or target synthetic DMARD (b/tsDMARD). D2T RA group was defined according to the EULAR definition of D2T RA. The non-D2T RA group was defined as a b/tsDMARD good responder who had low-disease activity or remission for at least one year on 1 or 2 b/tsDMARD mechanism of action. We compared the patients’ comorbidities, and history of b/tsDMARD use. Descriptive statistics and proportions were calculated. Kaplan-Meier analysis with log-rank test was used to estimate and compare median survival.
Results
Among the 417 patients, 101 (24%) were D2T RA and 316 (76%) were non-D2T RA. D2T RA group was slightly younger (63 ± 9 years versus 65 ± 12 years, p = 0.045), more likely to have concomitant non-inflammatory pain (28% versus 8%, p < 0.0001) and to discontinue at least one b/tsDMARD due to intolerance (39% versus 10%, p < 0.0001). In the D2T RA group, JAK inhibitors were associated with longer drug continuation when used as the third b/tsDMARD. Fewer patients were using corticosteroid at their most recent follow-up in this Canadian cohort compared to others (16% versus from 29 to 74%).
Conclusion
Concomitant non-inflammatory pain was more prevalent in D2T RA patients compared to b/tsDMARD good responder non-D2T RA patients. Steroid-sparing strategies is possible even in D2T RA patients. Future prospective research may compare JAK inhibitors with other mechanisms of action in D2T RA.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that affects 0.5–1% of the world population [1, 2]. The treat-to-target approach in RA, in which treatment is regularly adapted until a desired low disease activity state is achieved, has been greatly influenced by the introduction of new biologic and target synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) [3]. However, 8–20% of patients fail to reach treat-to-target objectives despite recent therapeutic improvements [4, 5]. The persistence of high disease activity leads to a greater burden of disease [6] and an estimated 18,000 euros (equivalent to 20,195 US dollar) per year in average cost increase [7]. Some cohort studies [8,9,10] explored factors predicting failure to multiple different b/tsDMARDs, but they lacked a common definition of treatment-refractory RA. In 2021, a EULAR Task Force [11,12,13] defined “difficult-to-treat RA” (D2T RA) [14] and they later published points to consider in the management of patients with D2T RA [15].
Understanding the determinants of D2T RA is key to tailoring its management. The current hypotheses for D2T RA include: (1) Presence of other causes of pain (e.g.: chronic pain syndromes, osteoarthritis, damage) [6, 16, 17], (2) Drug tolerance issues related to comorbidities or more frequent adverse events (e.g.: lung disease, infections) [18,19,20], (3) Socioeconomic challenges (e.g.: drug cost, poor coping skills, unrealistic patient expectations) [21] and (4) True multidrug resistance. Our current knowledge of D2T RA with the EULAR definition is based on European and Asian cohorts, and no North American cohort has yet to be published. Rheumatology practice varies between countries and continents due to differences in guidelines [22, 23] and prescription habits [24, 25].
The primary objective of this study was to identify characteristics of patients with D2T RA (b/tsDMARD poor responders) compared to b/tsDMARD good responders, and to describe their evolution in an university health center patient cohort. The secondary objective was to describe the type of b/tsDMARD associated with the longest duration on treatment after a patient was classified as D2T RA.
Methods
Study design and patient selection
This is a retrospective single centre cohort study at our university hospital. This study was approved by our institution Ethics Committee (IRB number 2020–5081). After obtaining authorization from the Director of Professional Services of our hospital, we reviewed the electronic medical records of all adults with RA that met the ACR/EULAR 2010 classification criteria [26] and had been seen in our outpatient clinic. All participants had to have been treated with at least one biologic or target synthetic DMARD (b/tsDMARD) to be included. Patients who were never treated with a b/tsDMARD were excluded, because the aim of this study was compare D2T RA patients (poor responders to b/tsDMARDs) to b/tsDMARD good responders. Patients were excluded from the analysis if (1) they only have been treated with conventional synthetic DMARDs (csDMARDs), (2) they had a concomitant rheumatic disease that could cause chronic small joint polyarthritis (e.g.: systemic lupus erythematosus and RA overlap, psoriatic arthritis, inflammatory bowel disease), or (3) their file contained limited information (e.g.: patients who are not seen at least annually at our outpatient clinic. To better study true failure of a mechanism of action, a b/tsDMARD was excluded from our analysis if it was discontinued due to intolerance.
Definitions and outcome variables
To apply the EULAR D2T RA definition retrospectively, patients must have failed ≥ 2 b/tsDMARDs with different mechanisms of action. The decision of introducing or changing a b/tsDMARD was made by the patient’s rheumatologist, in compliance with ACR and EULAR RA guidelines. To ensure that all patients in the D2T RA group had signs of active or progressive disease to fulfill the second EULAR D2T RA criteria, we documented the swollen joint count [28], tender joint count [28], inflammatory markers (erythrocyte sedimentation rate/C-reactive protein), the presence of erosions on X-rays and the health assessment questionnaire (HAQ) score at their most recent visit and at any time a b/tsDMARD was discontinued. A patient entered the D2T RA group after failing their second b/tsDMARD mechanism of action. The non-D2T RA group was defined as low disease activity or remission for at least one year on 1 or 2 b/tsDMARD mechanisms of action. Both groups were mutually exclusive.
Data collection was done by three investigators between April 2020 and September 2021: two core internal medicine residents in their second or third year of training (WQ, AR), and one research assistant (LR). All files reviewed by the research assistant (n = 17/420 files) were subsequently verified by a resident (WQ). The patient’s demographic information and comorbidities were collected from the patient’s hospitalization summaries and medical records (all rheumatology records and consultation records in other medical specialties) since 2015. Based on a predetermined standardized data collection form, we collected information on patient’s demographics (age at their most recent rheumatology follow-up, biological sex) and their medical/psychological comorbidities: hypertension, diabetes, dyslipidemia, chronic kidney failure, liver disease, concomitant non-inflammatory pain, anxiety disorders, and mood disorders. The presence and titer of rheumatoid factor (RF) and anti-citrullinated protein antibody (anti-CCP) were recorded from the earliest information available. ESR/CRP were documented categorically (elevated or normal, based on the laboratory’s reference values) and numerically. Erosions on X-rays and rheumatoid nodules were document categorically (present or absent). Concomitant of use of conventional synthetic DMARDs (csDMARDs), non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids were documented categorically (presence or absence). For both groups, we documented the chronological order of use of b/tsDMARDs according to their mechanism of action.
Concomitant non-inflammatory pain was defined as the diagnosis of fibromyalgia, complex general pain syndrome or chronic pain syndrome by the patient’s rheumatologist. This outcome was considered positive only if the disease activity was well controlled according to the rheumatologist and the patient still had symptoms causing a reduction in their quality of life, at any given time since 2015.
When a b/tsDMARD was discontinued for an intolerance, the type of intolerance was classified as follows: an allergic reaction, management of comorbidities, and side effects. An allergic reaction was defined as urticaria, angioedema, hypotension, or bronchospasm. The management of comorbidities was defined as (1) the drug became contra-indicated because of another health condition (e.g.: cancer and chemotherapy), or (2) the exacerbation of a preexisting comorbidity due to the drug (e.g.: lung infections in a patient with chronic lung disease). Side effects were defined as (1) a non-allergic effect attributed to the medicament, or (2) a severe infection in a patient without an identified predisposing comorbidity (ex: immunodeficiency, chronic lung disorder). Drug access issues or non-adherence were also recorded. Drug access issues were defined as the patient could no longer have access to the drug due to systemic factors (e.g.: insurer refused to pay for the drug, medication withdrawn from the Canadian market). Non-adherence was defined as the clinical diagnosis of non-adherence by the rheumatologist.
Statistical analysis
The patient’s demographics and characteristics were reported using descriptive statistics (mean and standard deviation or median with interquartile ranges) for continuous variables, and relative frequencies (%) for categorical variables. Univariate logistic regression models with odds ratios (OR) estimations were used to assess the characteristics (categorical and continuous) which were associated and could predict the D2T RA status. Characteristics chosen as candidate predictors were selected based on prior (clinical and literature) knowledge of their association with the D2T RA status. Kaplan-Meier analysis with log-rank test was used to estimate and compare median survival between the types of b/tsDMARD. The missing values for some variables and the non-collection of some possible confounders were limitations to perform multivariable analysis, thus we chose Kaplan-Meier bivariate analysis instead of a multivariate Cox regression for survival analysis. The analyses were performed (SM) using SAS 9.4 software. Missing data were managed as missing. A P value < 0.05 was considered statistically significant.
Results
Patient characteristics
After reviewing records from 2090 patients with a chronic inflammatory arthritis, we enrolled 417 RA patients on b/tsDMARDs (Fig. 1): 101 (24%) were D2T RA and 316 (76%) were non-D2T RA (Table 1).
Patients were predominantly women (D2T 80% versus non-D2T 75%, p = 0.31). The mean age at inclusion was slightly younger in the D2T RA group (D2T RA 63 ± 9 years, and non-D2T RA 65 ± 12 years, p = 0.04). The mean duration of disease since RA diagnosis was similar (D2T RA 16 ± 12 years and non-D2T RA 18 ± 14 years, p = 0.25). The time of follow-up since inclusion in the D2T RA group was 5 ± 3 years, and it was 9 ± 5 years in the non-D2T RA group. D2T RA patients had a median disease duration of 37 months (IQR = 20–69 months) before meeting the EULAR 2021 D2T RA definition. They received a median of 4 b/tsDMARDs (IQR = 3–6) with a median of 3 different mechanisms of action (IQR = 3–4). The non-D2T RA group received a median of 1 b/tsDMARD (IQR = 1–2) with a median of 1 mechanism of action (IQR = 1–1).
At RA diagnosis, D2T RA patients were less likely to have an elevated inflammatory marker as defined by ESR (D2T RA 38% versus non-D2T RA 51%, p = 0.03) and CRP (D2T RA 38% versus non-D2T RA 54%, p = 0.03). Concomitant non-inflammatory pain was more prevalent in our D2T RA group (D2T RA 28% versus non-D2T RA 8%, p < 0.0001) (Table 1). D2T RA patients were also more likely to discontinue at least one b/tsDMARD due to intolerance (D2T RA 39% versus non-D2T RA 10%, p < 0.0001). However, there were no differences between the two groups in the types of intolerance: side effects, allergic reaction, or due to comorbidities (Table 1).
How b/tsDMARD were used
In the D2T RA group, the first b/tsDMARD mechanism of action patients received in their pre-D2T RA period were TNF inhibitors (75/101, 74%) and CTLA-4-Ig (17/101, 17%) (Fig. 2), with a median duration of treatment of 11 months (IQR = 7–33) and 8 months (IQR = 6–11), respectively (Supplementary Fig. S1). For their second b/tsDMARD in the pre-D2T RA period, the most frequent mechanism of action was still a TNF inhibitor (52/101, 51%) and CTLA-4-Ig (31/101, 31%), with a median duration of treatment of 7 months (IQR = 5–17) and 12 months (IQR = 5–21 months), respectively (Supplementary Fig. S2).
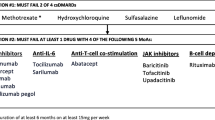
Chronological order of use of b/tsDMARDs according to their mechanism of action in D2T RA. Note This figure contains information on the D2T RA group only. TNF inhibitor tumor necrosis factor inhibitor (adalimumab, etanercept, golimumab; certolizumab, infliximab), IL-6 antagonist interleukin-6 antagonist (tocilizumab, sarilumab), CD20 inhibitor (rituximab), JAK inhibitor janus kinase inhibitor (tofacitinib, baricitinib, upadacitinib), CTLA-4 Immunoglobulin cytotoxic T lymphocyte-associated antigen-4-immunoglobulin (abatacept), Others (includes Il-1 inhibitor, clinical trial b/tsDMARDs)
The majority of D2T RA patients failed two mechanisms of action after using 2 b/tsDMARDs (n = 58/101, 58%) (Supplementary Table S1). D2T RA patients had signs of active disease when a b/tsDMARD was discontinued (Supplementary Table S2). For patients who met the D2T RA criteria and used their third b/tsDMARD, the most frequently used mechanism of action was a JAK inhibitor (22/59, 37%) followed by a TNF inhibitor (19/59, 32%) (Fig. 3). For the fourth b/tsDMARD, there was a more diverse mixture of mechanisms of action: TNF inhibitors (18/63, 29%), IL-6 antagonist (15/63, 24%), JAK inhibitors (13/63, 21%), CD20 inhibitor (11/63, 17%), and CTLA-4-Ig (5/63, 8%).
Continuation probability of the third b/tsDMARD up to 36 Months in D2T RA patients. Note This information is for follow-up after D2T criteria were fulfilled. TNF inhibitor tumor necrosis factor inhibitor (adalimumab, etanercept, golimumab; certolizumab, infliximab); TNF inhibitor (naïve) describes patients who are using TNF inhibitor for the first time; TNF inhibitor (exposed) describes patients who have previously used a TNF inhibitor in the past, JAK inhibitor janus kinase inhibitor (tofacitinib, baricitinib, upadacitinib), CTLA-4 Ig cytotoxic T lymphocyte-associated antigen-4-immunoglobulin (abatacept); Other mechanisms of action: IL-6 antagonist interleukin-6 antagonist (tocilizumab, sarilumab), CD20 inhibitor (rituximab)
There were many chronological orders in which b/tsDMARDs were used (Supplementary Table S3). The most frequently used chronological order (n = 10) was a TNF inhibitor (first b/tsDMARD), followed by CTLA-4Ig (second b/tsDMARD), and then a JAK inhibitor (third b/tsDMARD). All other chronological orders of use accounted for 4% or less of all the possibilities.
After a patient becomes D2T RA, Jak inhibitors had a better median survival at 36 months compared to the other mechanisms of action when it was used as the third b/tsDMARD in the D2T RA group (Fig. 3). At their last treatment, 96% (n = 97/101) of D2T RA patients were still being treated with a b/tsDMARD (Table 1). More D2T RA patients than non-D2T RA patients were using chronic corticosteroids (D2T RA 16% versus non-D2T RA 5%, p = 0.0002) and slightly fewer were using csDMARDs (D2T RA 67% versus non-D2T RA 79%, p = 0.022).
Discussion
Our study shows that in this cohort from an university hospital, D2T RA patients were more likely to have concomitant non-inflammatory pain and to discontinue medication due to intolerance when compared to b/tsDMARD good responders (non-D2T RA). Once a patient meets the D2T RA definition, JAK inhibitors had a longer survival than other b/tsDMARD mechanisms of action when used as the third b/tsDMARD. Fewer patients were managed with glucocorticoids at their most recent follow-up.
The reasons behind D2T RA are heterogeneous. Before switching or adding immunosuppressive medication, it may be useful to identify other causes of pain that can contribute to the patient’s symptoms [15]. The results of our study support the EULAR point to consider that non-inflammatory pain should be assessed in D2T RA patients before switching b/tsDMARDs. Other examples of comorbidities that may influence the clinical assessment of RA disease activity include osteoarthritis, damage from previous inflammation, obesity and depression [15]. The use of ultrasound may help distinguish active inflammatory disease from damage in D2T RA patients [4, 27, 28]. Strategies to manage these other causes of pain include setting realistic goals through shared-decision making, educating the patient on the multiple aetiologies of pain [15, 29] and including non-pharmacological interventions (e.g.: exercise, self-management interventions, psychological) [15]. Then, poorer drug tolerance is another reason for D2T RA [30]. Our D2T RA patients were more likely to discontinue their b/tsDMARD due to an intolerance than non-D2T RA patients. Although we didn’t find a difference in terms of intolerance due to infections or comorbidities, Takanashi et al. published on elderly Japanese patients with a higher comorbidity index [31]. These patients in Takanashi’s cohort were more likely to have D2T RA and infectious complications.
For the patient who has true multidrug resistance, JAK inhibitors may have more favorable outcomes when compared to other mechanisms of action for D2T RA patients [32,33,34]. The results from our study support their findings in that JAK inhibitors may be considered in D2T RA patients. The better outcomes on JAK inhibitors may be explained by the inhibition of a wider range of cytokines involved in the pathogenesis of RA and its analgesic effect [35, 36]. However, the widespread use of JAK inhibitors may be limited due to safety concerns [37,38,39]. Considering that some D2T RA patients have a higher comorbidity index [6, 31], only well-selected subgroups may benefit from this treatment option. Another therapeutic consideration is determining which patient needs chronic low-dose corticosteroids. In our cohort, the proportion of D2T RA patients on chronic corticosteroids was lower than in cohorts from other countries (16% versus from 29 to 74%) [5, 6, 18, 40]. We believe that steroid-sparing strategies may be considered even when a patient has a D2T RA. In our country, the use of chronic corticosteroids is less frequent than in other countries [24, 25], and this steroid-sparing treatment strategies may be facilitated by our universal healthcare coverage. It allows us to switch drugs when necessary, until the most effective b/tsDMARD is found.
Comparison to other D2T RA cohorts
Comparing our cohort of D2T RA patients to other published cohorts (Table 2) needs to take into consideration the different inclusion criteria of our control group. In other studies, the non-D2T RA group included both csDMARD good responders and b/tsDMARD good responders, whereas our study only included b/tsDMARD good responders. The exclusion of csDMARD good responders can explain why this selected cohort has a high prevalence of D2T RA patients (24%).
This Canadian cohort had lower percentage of rheumatoid factor positive (51% versus from 75 to 87%) and anti-CCP positive (42% versus from 73 to 87%) patients compared to other cohort of D2T RA patients. Seronegativity may have clinical implications in difficult-to-treat RA. First, re-assessing the diagnosis may be important when a seronegative RA patient fails to respond to treatment, as other seronegative inflammatory arthritis can present similarly to RA and meet its classification criteria. Key features of these diseases may not appear over time when the patient is using a b/tsDMARD, which further complicates diagnosis. In our study, we excluded all patients with known concomitant rheumatic diseases that could cause chronic small joint polyarthritis (e.g.: systemic lupus erythematosus and RA overlap, skin psoriasis, inflammatory bowel disease). Also, some seronegative RA patients may have less inflammatory disease [41], and concomitant non-inflammatory pain can possibly explain their partial response to b/tsDMARD. Compared to other cohorts, we report a similar prevalence of concomitant non-inflammatory pain (28% versus 20–38%) in our D2T RA patients. Comorbidities had a similar impact on management in patients of our cohort compared to the cohort of Takanashi et al. (9% versus 10%), but less than the cohort of Roodenrijs et al. (9% versus 69%).
There are several limitations to our study. First, we were unable to retrospectively assess our patient’s socioeconomic status, coping strategies and expectations with regards to treatment. These factors may be important determinants in perceived treatment failure and may be covariates of non-inflammatory pain. Second, this study is subject to selection and information biases due to its retrospective and single-center nature. One challenge was to retrospectively apply the third EULAR criteria for D2T RA (management being perceived as problematic by the rheumatologist or patient), because there is subjectivity in the interpretation of the written medical records and patients were not contacted for this retrospective study [42, 43]. Third, we did not contact the patient’s pharmacy to adequately assess adherence. Fourth, a time-cohort bias could have given an advantage to RA treatments recently introduced, such as JAK inhibitors. A strength to our study is that we are the first North American cohort study using the EULAR D2T RA criteria. A second strength is that we are the first D2T RA study in our knowledge to use b/tsDMARD good responders as a control group. This gives us insight into factors that can make a RA patient difficult-to-treat after they are started on b/tsDMARDs.
Future studies could assess whether the use of ultrasound can help distinguish inflammatory disease activity from other causes of pain and if it can reduce possible unnecessary drug switch. Prospective study is also required to determine if one b/tsDMARD mechanism of action is superior to another in multidrug resistant RA.
Conclusion
Compared to b/tsDMARD good responders, concomitant non-inflammatory pain seemed more prevalent in D2T RA patients from our cohort. Steroid-sparing strategies should be tried even in D2T RA patients. Future prospective research is needed to determine the predictive factors and the best therapeutic strategies to prevent a patient with RA from progressing to D2T RA, which may include the use of ultrasound to differentiate between active inflammatory activity and non-inflammatory pain. The role of JAK inhibitors versus other mechanisms of action in this group of D2T RA patients should also be prospectively explored.
Data availability
The datasets supporting the conclusions of this article are available from the corresponding author on reasonable request.
References
Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. 2021;41(5):863–77.
Canada, AAo. The impact of arthritis in Canada: today and over the next 30 years. 2011. http://www.ergoresearchcom/wp-content/uploads/2012/04/Impact%2520on%2520arthrisis%2520in%2520Canada_Today%2520and%2520over%2520the%2520next%252030%2520yearspdf.
Smolen JS. Treat to target in Rheumatology: a historical account on occasion of the 10th anniversary. Rheum Dis Clin North Am. 2019;45(4):477–85.
de Hair MJH, Jacobs JWG, Schoneveld JLM, van Laar JM. Difficult-to-treat rheumatoid arthritis: an area of unmet clinical need. Rheumatology (Oxford). 2018;57(7):1135–44.
Takanashi S, Kaneko Y, Takeuchi T. Characteristics of patients with difficult-to-treat rheumatoid arthritis in clinical practice. Rheumatology (Oxford). 2021;60(11):5247–56.
Roodenrijs NMT, van der Goes MC, Welsing PMJ, Tekstra J, Lafeber F, Jacobs JWG, et al. Difficult-to-treat rheumatoid arthritis: contributing factors and burden of disease. Rheumatology (Oxford). 2021;60(8):3778–88.
Roodenrijs NMT, Welsing PMJ, van der Goes MC, Tekstra J, Lafeber F, Jacobs JWG, et al. Health care utilisation and economic burden of difficult-to-treat rheumatoid arthritis: a cost-of-illness study. Rheumatology (Oxford). 2021;60(10):4681–4690. https://doi.org/10.1093/rheumatology/keab078
Kearsley-Fleet L, Davies R, De Cock D, Watson KD, Lunt M, Buch MH, et al. Biologic refractory disease in rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis. 2018;77(10):1405–12.
Novella-Navarro M, Plasencia C, Tornero C, Navarro-Compan V, Cabrera-Alarcon JL, Peiteado-Lopez D, et al. Clinical predictors of multiple failure to biological therapy in patients with rheumatoid arthritis. Arthritis Res Ther. 2020;22(1):284.
Ochi S, Mizoguchi F, Nakano K, Tanaka Y. Difficult-to-treat rheumatoid arthritis with respect to responsiveness to biologic/targeted synthetic DMARDs: a retrospective cohort study from the FIRST registry. Clin Exp Rheumatol. 2022;40(1):86–96.
Roodenrijs NMT, Hamar A, Kedves M, Nagy G, van Laar JM, van der Heijde D, et al. Pharmacological and non-pharmacological therapeutic strategies in difficult-to-treat rheumatoid arthritis: a systematic literature review informing the EULAR recommendations for the management of difficult-to-treat rheumatoid arthritis. RMD open. 2021;7(1):e001512.
Roodenrijs NMT, Kedves M, Hamar A, Nagy G, van Laar JM, van der Heijde D, et al. Diagnostic issues in difficult-to-treat rheumatoid arthritis: a systematic literature review informing the EULAR recommendations for the management of difficult-to-treat rheumatoid arthritis. RMD open. 2021;7(1):e001511.
Roodenrijs NMT, de Hair MJH, van der Goes MC, Jacobs JWG, Welsing PMJ, van der Heijde D, et al. Characteristics of difficult-to-treat rheumatoid arthritis: results of an international survey. Ann Rheum Dis. 2018;77(12):1705–9.
Nagy G, Roodenrijs NMT, Welsing PM, Kedves M, Hamar A, van der Goes MC, et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2021;80(1):31–5.
Nagy G, Roodenrijs NMT, Welsing PMJ, Kedves M, Hamar A, van der Goes MC, et al. EULAR points to consider for the management of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2022;81(1):20–33.
Leon L, Madrid-Garcia A, Lopez-Viejo P, Gonzalez-Alvaro I, Novella-Navarro M, Freites Nunez D, et al. Difficult-to-treat rheumatoid arthritis (D2T RA): clinical issues at early stages of disease. RMD open. 2023;9(1):e002842.
Tan Y, Buch MH. ‘Difficult to treat’ rheumatoid arthritis: current position and considerations for next steps. RMD open. 2022;8(2):e002387.
Watanabe R, Hashimoto M, Murata K, Murakami K, Tanaka M, Ohmura K, et al. Prevalence and predictive factors of difficult-to-treat rheumatoid arthritis: the KURAMA cohort. Immunological Med. 2021:1–10.
Dey M, Nagy G, Nikiphorou E. Comorbidities and extra-articular manifestations in difficult-to-treat rheumatoid arthritis: different sides of the same coin? Rheumatology (Oxford). 2023;62(5):1773–9.
Conran C, Kolfenbach J, Kuhn K, Striebich C, Moreland L. A review of difficult-to-treat rheumatoid arthritis: definition, clinical presentation, and management. Curr Rheumatol Rep. 2023;25(12):285–94.
Roodenrijs NMT, van der Goes MC, Welsing PMJ, van Oorschot EPC, Nikiphorou E, Nijhof NC, et al. Non-adherence in difficult-to-treat rheumatoid arthritis from the perspectives of patients and rheumatologists: a concept mapping study. Rheumatology (Oxford). 2021;60(11):5105–16.
Fraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2021;73(7):924–39.
Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99.
Haraoui B, Jovaisas A, Bensen WG, Faraawi R, Kelsall J, Dixit S, et al. Use of corticosteroids in patients with rheumatoid arthritis treated with infliximab: treatment implications based on a real-world Canadian population. RMD open. 2015;1(1):e000078.
Andersen KM, Schieir O, Valois MF, Bartlett SJ, Bessette L, Boire G, et al. A bridge too far? Real-world practice patterns of early glucocorticoid use in the Canadian early arthritis cohort. ACR Open Rheumatol. 2022;4(1):57–64.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81.
Michitsuji T, Fukui S, Morimoto S, Endo Y, Nishino A, Nishihata S, et al. Clinical and ultrasound features of difficult-to-treat rheumatoid arthritis: a multicenter RA ultrasound cohort study. Scand J Rheumatol. 2024;53(2):123–9.
Garcia-Salinas R, Sanchez-Prado E, Mareco J, Ronald P, Ruta S, Gomez R, et al. Difficult to treat rheumatoid arthritis in a comprehensive evaluation program: frequency according to different objective evaluations. Rheumatol Int. 2023;43(10):1821–8.
Zangi HA, Ndosi M, Adams J, Andersen L, Bode C, Bostrom C, et al. EULAR recommendations for patient education for people with inflammatory arthritis. Ann Rheum Dis. 2015;74(6):954–62.
Novella-Navarro M, Ruiz-Esquide V, Torres-Ortiz G, Chacur CA, Tornero C, Fernandez-Fernandez E, et al. A paradigm of difficult-to-treat rheumatoid arthritis: subtypes and early identification. Clin Exp Rheumatol. 2023;41(5):1114–9.
Takanashi S, Kaneko Y, Takeuchi T. Elderly patients with comorbidities in the definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2021;80(11):1491–3.
Ochi S, Sonomoto K, Nakayamada S, Tanaka Y. Preferable outcome of Janus kinase inhibitors for a group of difficult-to-treat rheumatoid arthritis patients: from the FIRST Registry. Arthritis Res Ther. 2022;24(1):61.
Watanabe R, Okano T, Gon T, Yoshida N, Fukumoto K, Yamada S, et al. Difficult-to-treat rheumatoid arthritis: current concept and unsolved problems. Front Med (Lausanne). 2022;9:1049875.
Hecquet S, Combier A, Steelandt A, Pons M, Wendling D, Molto A, et al. Characteristics of patients with difficult-to-treat rheumatoid arthritis in a French single-centre hospital. Rheumatology (Oxford). 2023;62(12):3866–74.
Simon LS, Taylor PC, Choy EH, Sebba A, Quebe A, Knopp KL, et al. The Jak/STAT pathway: a focus on pain in rheumatoid arthritis. Semin Arthritis Rheum. 2021;51(1):278–84.
Harrington R, Al Nokhatha SA, Conway R. JAK inhibitors in rheumatoid arthritis: an evidence-based review on the emerging Clinical Data. J Inflamm Res. 2020;13:519–31.
Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med. 2022;386(4):316–26.
US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. 2022. https://www.fdagov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death. Accessed 31 Jan 2022.
European Medicines Agency, Xeljanz. 2022. https://www.emaeuropaeu/en/medicines/human/referrals/xeljanz#:~:text=On%2014%20November%20EMA%20concluded,high%20risk%20of%20blood%20clots. Accessed 31 Jan 2022.
Giollo A, Zen M, Larosa M, Astorri D, Salvato M, Calligaro A, et al. Early characterization of difficult-to-treat rheumatoid arthritis by suboptimal initial management: a multicentre cohort study. Rheumatology (Oxford). 2023;62(6):2083–9.
Carbonell-Bobadilla N, Soto-Fajardo C, Amezcua-Guerra LM, Batres-Marroquin AB, Vargas T, Hernandez-Diazcouder A, et al. Patients with seronegative rheumatoid arthritis have a different phenotype than seropositive patients: a clinical and ultrasound study. Front Med (Lausanne). 2022;9:978351.
Roodenrijs NMT, Welsing PMJ, van der Goes MC, Jacobs JW, van der Heijde D, van Laar JM, et al. Response to: ‘Correspondence on ‘EULAR definition of difficult-to-treat rheumatoid arthritis’’ by Novella-Navarro. Ann Rheum Dis. 2023; 82(3):e56.
Novella-Navarro M, Plasencia-Rodriguez C, Tornero C, Navarro-Compan V, Cabrera-Alarcon JL, Peiteado D, et al. Correspondence on: ‘EULAR definition of difficult-to-treat rheumatoid arthritis’. Ann Rheum Dis. 2023;82(3):e55.
Acknowledgements
Not applicable.
Funding
Dr. Michou was supported by a career award from the Fonds de recherche du Québec-Santé (FRQ-S). Dr. Fortin is supported by a Tier 1 Canada Research Chair on Systemic Autoimmune Rheumatic Diseases. This project was funded by the Centre Hospitalier Universitaire de Québec Foundation.
Author information
Authors and Affiliations
Contributions
Study design: WQ, AR, LB, PRF, JPB, LM. Patient recruitment and acquisition of data: WQ, AR, LR. Analysis and interpretation of data: WQ, AR, NS, LB, PRF, JPB, LM. Revision of manuscript content: WQ, AR, NS, LR, LB, PRF, JPB, LM. Approving final version of manuscript: WQ, AR, NS, LR, LB, PRF, JPB, LM. LM takes responsibility for the integrity of the data analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the CHU de Québec-Université Laval Ethics Committee (IRB number 2020–5081). Authorization from the Director of Professional Services of the CHU de Québec-Université Laval was obtained prior to reviewing the patients’ electronic medical record.
Consent for publication
Not applicable.
Competing interests
Dr Louis Bessette has served on Speaker’s bureau from Amgen, BMS, Janssen, UCB, AbbVie, Pfizer, Merck, Lilly, Novartis, Sanofi, Sandoz, Fresenius Kabi, Teva; has served as consultant. Amgen, BMS, Janssen, UCB, AbbVie, Pfizer, Merck, Lilly, Novartis, Sanofi, Sandoz, Gilead, Fresenius Kabi, Teva; has received research support from Amgen, BMS, Janssen, UCB, AbbVie, Pfizer, Merck, Celgene, Sanofi, Lilly, Novartis, Gilead. Dr Jacques P Brown has received research support from Mereo BioPharma, Radius Health, and Servier; has served as a consultant for Amgen, Gilead, Paladin, Pfizer, Servier and Ultragenyx; and has served on speakers’ bureau for Amgen, all outside of the scope of this manuscript. Dr Paul R Fortin has served as consultant on Lupus Advisory Boards of AstraZeneca, GSK and AbbVie. Dr Laëtitia Michou has received honoraria for a conference from Roche, Janssen, Abbvie, Amgen; has served as consultant on Advisory Boards of Pfizer, Roche, Amgen. The other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qi, W., Robert, A., Singbo, N. et al. Characteristics of patients with difficult-to-treat rheumatoid arthritis: a descriptive retrospective cohort study. Adv Rheumatol 64, 55 (2024). https://doi.org/10.1186/s42358-024-00396-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-024-00396-6