Abstract
Background
Most of the few studies that have established reference ranges for serum uric acid (SUA) have not taken into account factors which may interfere with its levels and followed rigorous laboratory quality standards. The aim of this study was to establish reference ranges for SUA and determine the prevalence of hyperuricemia.
Method
Cross-sectional study including 15,100 participants (all sample) aged 35 to 74 years from baseline data of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), a multicentric cohort of volunteer civil servants from five universities and one research institute located in different regions of Brazil. It was established a reference sample excluding participants with factors that directly influence SUA levels: glomerular filtration rate lower than 60 ml/min, excessive alcohol intake, use of diuretics, aspirin, estrogen or urate-lowering therapy. SUA was measured using the uricase method and following rigorous international quality standards. Reference ranges were defined as values between percentiles 2.5 (P2.5) and 97.5 (P97.5) of SUA distribution in the reference sample, stratified by sex. Hyperuricemia was defined as SUA ≥ 7 mg/100 ml in the all sample.
Results
The reference sample was composed of 10,340 individuals (55.3% women, median age 50 years). Reference ranges (P2.5 to P97.5) for SUA were: 4.0 to 9.2 mg/100 ml for men and 2.8 to 6.9 mg/100 ml for women. Sex was a major determinant for SUA levels (median [IQR], mg/100 ml: 6.1 [5.3–7.0] for men versus 4.5 [3.9–5.3] for women, p < 0.001). Higher levels of SUA were found in patients with higher BMI. Higher age had (a modest) influence only for women. The prevalence of hyperuricemia for all sample (N = 15,100) was 31.9% (95% confidence interval [CI] 30.8–33.0%) in men and 4.8% (95% CI 4.3–5.3%) in women.
Conclusion
SUA reference ranges were 4.0 to 9.2 mg/100 ml for men and 2.8 to 6.9 mg/100 ml for women. Prevalence of hyperuricemia was 31.9% in men and 4.8% in women. Updated SUA reference ranges and prevalence of hyperuricemia are higher nowadays and might be used to guide laboratories and the screening for diseases related to SUA.
Similar content being viewed by others
Background
High serum uric acid (SUA) is a well-known risk factor for gout and kidney stones [1] and an increasing number of studies links SUA as a risk factor for hypertension, cardiovascular disease, chronic kidney disease, insulin resistance, diabetes and cancer [2,3,4,5,6]. On the other hand, low levels of SUA have been recently related to Alzheimer and Parkinson diseases, chronic obstructive pulmonary disease [7] and even cardiovascular mortality [8,9,10]. Moreover, there is data evidencing the relation of SUA to fasting plasma glucose levels [11] as well as reduction in kidney function [12] in an U-shape curve fashion, indicating that both lower and higher values are associated with the outcome.
Many previous studies which have established SUA reference ranges in different populations were performed more than 40 years ago, when nutritional and clinical scenarios were different from nowadays [13,14,15,16,17,18,19,20], while SUA levels have been increasing in the general population in the last three decades at least [21, 22]. One of the most important limitations of previous studies is the use of the phosphotungistic method for measuring SUA [13, 14, 23, 24]. The uricase method is currently recognized as the gold standard for SUA levels determination [25], which was not available until the 1970s.
Several studies did not use validated international recommendations [26] for collection, transportation, processing and analysis of blood samples that are important for achieving good standards in laboratorial measures. In addition, factors known to modulate SUA levels such as alcohol consumption, chronic kidney disease and use of medications (e.g. diuretics) were most not excluded from study samples [23, 27]. More recent studies have also established SUA reference ranges for general population, however, without strict control for SUA determinant factors [28,29,30,31].
Production of reviewed data about distribution and SUA reference ranges is relevant to identify groups who may have higher risk to related diseases [32], and there is no universal reference range for SUA to date, as determined for fasting plasma glucose [33] or cholesterol [34], for example. Furthermore, laboratories around the world often create their own reference values based on local populations in pilot studies or use values indicated by laboratory kits. Prevalence of hyperuricemia might also be higher than previous reports due to the higher obesity prevalence in population [35]. Therefore, the aim of this study was to establish reference ranges for SUA for men and women in a large sample following rigorous laboratory quality standards and excluding factors which may directly interfere with SUA levels, as well as to establish the prevalence of hyperuricemia.
Methods
Study design
The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) is a cohort study of 15,105 civil servants from five universities (Federal Universities of Bahia, Espírito Santo, Minas Gerais, and Rio Grande do Sul and the University of São Paulo) and one research institute (Oswaldo Cruz Foundation) located in different regions of Brazil [36,37,38]. All active or retired employees from the 6 institutions aged 35–74 years were eligible for the cohort. Exclusion criteria were current or recent pregnancy, intention to quit working at the institution in the near future, severe cognitive or communication impairment, and, if retired, residence outside of a study center’s corresponding metropolitan area. Sample size estimation was based on the main study outcomes—diabetes and myocardial infarction [36]. The first examination was carried out from 2008 through 2010 (baseline data). The ELSA-Brasil protocol was approved at each of the six study centers by the local Institutional Review Board addressing research in human participants. All participants provided signed informed consent.
This study is a cross-sectional analysis from ELSA-Brasil baseline data and sample size was composed of all participants with valid SUA levels at baseline, which is not sufficient to represent the entire population in Brazil, although it is very large and multicentric.
Each participant was interviewed at the workplace and again during a visit in the research center. Trained staff conducted interviews and examination following strict quality control procedures [38] and only data related to this study will be described in this section.
Definitions: all sample, reference sample, reference range and hyperuricemia
All participants from the ELSA-Brasil cohort included in the analyses we called all sample, and we established a reference sample after exclusion of participants with known factors that directly affect SUA levels: use of aspirin, thiazides, urate-lowering drugs, estrogen replacement therapy, excessive alcohol intake (> 140 g/week for women and > 210 g/week for men) [39] and estimated glomerular filtration rate < 60 ml/min/1.73 m2.
The reference range was defined as the central values between the 2.5th and 97.5th percentiles of SUA distribution in the reference sample for men and women, as established by Clinical and Laboratory Standards Institute (CLSI) [40]. Hyperuricemia was defined for SUA higher than or equal 7 mg/100 ml [41] for all sample for men and women.
We determined SUA according to the sociodemographic characteristics, habits, menopause and its distribution according to body mass index (BMI) and age in all and reference sample for men and women.
SUA and other variables
Procedures for collection of biological samples in ELSA-Brasil were standardized to assure uniformity at all investigation centres and followed the recommendations of the Brazilian Society of Clinical Pathology/Laboratory Medicine for blood collection [42]. Blood samples were drawn after 12-h fasting. Up to 30 min after the end of blood collection, all tubes were centrifuged under refrigeration for 15 min. Blood samples were stored in cryotubes at – 80 °C to the date of transport and processing, at most one month. Analysis of samples was centered at the laboratory of the University Hospital at University of São Paulo. SUA was measured by uricase method (automated colorimetric enzymatic) [43] by the ADVIA 1200 Siemens® equipment [36, 42]. This method is the recommended by Clinical and Laboratory Standards Institute [26] and the American College of Rheumatology and European League Against Rheumatism guideline [25].
Age, sex, education level (categorized as < 9, 9 to 11 and > 11 years of formal education), monthly family income (≤ US$1245, US$1246–3319, and ≥ US$3320), ethnicity according to the Brazilian census classification (black, brown or mixed, white, other—Asian, indigenous) [36], smoking status (never, past or current), excessive alcohol intake (> 140 g/week for women and > 210 g/week for men) [39] and menopause were self-reported in questionnaires at baseline. All participants were asked about their use of prescribed and nonprescribed drugs in the previous 2 weeks. BMI was calculated as weight divided by height in meters squared (kg/m2) [36, 37]. Physical activity was classified as poor, intermediate, or ideal according to the American Heart Association ideal cardiovascular health score [44]. Glomerular filtration rate was calculated using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [37, 45] and chronic kidney disease (CKD) was defined as a glomerular filtration rate < 60 ml/min [46].
Statistical analysis
Continuous variables are expressed as mean and standard deviation or median and interquartile range (IQR) and compared using analysis of variance (ANOVA) or Mann–Whitney U-test as appropriate after assessing normality assumptions. Categorical variables are expressed as proportions and compared using the chi-square or Fisher exact test. We calculated 95% confidence intervals (95% CI) for the frequency of hyperuricemia, whenever applicable. The Komolgoroff–Smirnov test was used for normality assessment. Significance level was set at 0.05.
Results
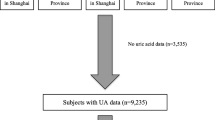
From the 15,105 participants from the ELSA-Brasil cohort, 5 individuals (< 0.1%) were excluded because they did not have a valid SUA level determination at study baseline. The all sample represents the 15,100 participants included in the analyses and the reference sample comprises 10,340 individuals after exclusion of participants with known factors that directly affect SUA levels: 873 (5.8%) individuals using aspirin, 1797 (11.9%) using thiazides, 90 (0.6%) using urate-lowering drugs, 539 (3.6%) under estrogen replacement therapy, 900 (6.0%) reporting excessive alcohol intake, 528 (3.5%) with estimated glomerular filtration rate < 60 ml/min/1.73 m2 and 33 (0.2%) with missing information in any of these criteria.
The all sample (N = 15,100) had a median age of 51 [IQR 45–58] years, mean BMI 27.0 (SD 4.7) Kg/m2 and consisted of 8216 (54.4%) women (Table 1). Median SUA was 6.3 [IQR 5.5–7.3] mg/100 ml for men and 4.7 [IQR 4.0–5.5] mg/100 ml for women. The reference sample (N = 10,340) had a median age of 50 [IQR 44–56] years, mean BMI 26.5 (SD 4.5) Kg/m2 and consisted of 5716 women (55.3%) (Table 1). Median SUA was 6.1 [IQR 5.3–7.0] mg/100 ml for men and 4.5 [IQR 3.9–5.3] mg/100 ml for women. Distribution of SUA were not normal, shifted to the right, for all and reference sample (Fig. 1).
Reference range for SUA (P2.5 to P97.5) was defined for reference sample: 4.0 to 9.2 mg/100 ml for men and 2.8 to 6.9 mg/100 ml for women (Tables 2, 3). Considering only individuals with BMI < 25 kg/m2, the SUA reference ranges would be 3.7 to 8.5 mg/100 ml for men and 2.7 to 6.2 mg/100 ml for women (Table 2). Prevalence of hyperuricemia for all sample was 31.9% (95% CI: 30.8–33.0%) in men and 4.8% (95% CI: 4.3–5.3%) in women and in reference sample was 25.3% (95% CI: 24.1–26.6%) in men and 2.2% (95% CI: 1.9–2.7%) in women (Table 1).
Sex determined an important difference in the median values of SUA: 1.6 mg/100 ml higher in men in both reference and all sample. Higher BMI determined higher SUA levels for both men and women (p for trend < 0.001 for both sexes) in both reference and all sample (Table 2). For men there was no difference in SUA of reference sample among different age groups (categorized by 5 years) in reference sample (p for trend = 0.088), but age determined slightly higher SUA (p for trend = 0.038) in all sample. Older age determined higher SUA levels in reference and all sample for women (p for trend < 0.001 for both samples) (Table 3).
SUA levels according to other sociodemographic and clinical characteristics for reference and all sample are shown in Table 4. There was no difference in SUA levels according to ethnicity in men, but withe women had lower SUA levels (p = 0.01 in reference sample and p < 0.001 in all sample). SUA is slightly higher in individuals with lower educational level in women in both reference and all sample (p for trend < 0.001). Monthly mean family income influences the SUA levels only for women in all sample. Former smokers have higher SUA than current and never smokers in reference (p = 0.009 for men and p < 0.001 for women) and in all sample (p < 0.001 for men and woman). Menopause is associated with higher SUA levels for reference (4.7 [IQR 4.0–5.4] vs. 4.3 [IQR 3.8–5.3] mg/100 ml, p < 0.001) and all sample (4.9 [IQR 4.1–5.8] vs. 4.4 [IQR 3.8–5.1] mg/100 ml, p < 0.001). More frequent physical activity is associated with lower SUA in reference (p for trend = 0.01 for men and < 0.001 for women) and all sample (p for trend < 0.001 for men and women).
Discussion
This study determined the SUA reference ranges of a large and multicentric sample in a population defined as reference with exclusion of known conditions which affect SUA levels, using uricase method and following rigorous laboratory quality standards. It also determined the distribution of SUA regarding sex, age and BMI, the influence of sociodemographic characteristics, smoking and menopause on SUA along with the prevalence of hyperuricemia.
SUA reference range values in the present study are higher than those found previously. In a German study of the 1970s [23], it was found SUA levels from 3.4 to 7.0 mg/100 ml for men and 2.4 to 5.7 mg/100 ml for women, however, authors did not use the uricase method to determine the SUA levels. In an Indian study using uricase method to measure SUA [27], the reference range in 1470 subjects was slightly lower than those found in our study: 3.5 to 8.7 mg/100 ml in men and 2.5 to 6.9 mg/100 ml in women. Other studies included only participants with BMI between 18–25 kg/m2 and found 3.5–8.2 and 2.7–6.9 mg/100 ml for men, and 2.7–6.5 and 2.1–5.9 mg/100 ml for women [28, 30]. These results may reflect different populations studied, and exclusion criteria used.
Hyperuricemia is classically defined as SUA ≥ 7 mg/100 ml, considering the risk for incident gout [41, 47]. We found a high prevalence of hyperuricemia in men, even considering our reference sample. Nevertheless, the current gout classification criteria highlights SUA ranges and not a cut-off for hyperuricemia, indicating progressive disease risk for higher SUA values [25]. We also showed the prevalence of hyperuricemia considering the 6 mg/100 ml cut-off, considering the recent literature valuing it to the cardiovascular risk [3]. The association between SUA and hypertension, cardiovascular diseases, metabolic syndrome, diabetes, heart failure and CKD seems to be correlated with SUA levels either. Thus, the updated SUA values distribution in population receives special importance for quantifying the individuals at risk for these conditions [32].
SUA levels distribution (sex-specific) is not Gaussian. Other studies [14, 24, 27] also suggested a non-normal distribution, although they did not inform whether SUA levels distribution is parametric or not after applying a statistical test.
Sex was a major determinant for the SUA levels: in this study, the difference in median values for men and women was 1.6 mg/100 ml, higher in men. Other authors [13,14,15,16,17,18,19, 24, 27] found narrower SUA differences between sexes (0.8 to 1.6 mg/dl). In fact, our study’s (all sample) SUA levels are approximately 2.0 mg/100 ml higher in men and 1.5 mg/100 ml in women when compared with those past studies [13, 14, 16, 18, 20, 21, 23, 48]. Most studies of SUA levels distribution in large populations date from the 1960s and 1970s [13, 14, 16]. Since then, there has been a progressive SUA levels increasing [21, 24, 27], probably associated with current higher BMI values [35] in population, as well as changes in diet and alcohol consumption increase [49, 50]. Thus, SUA distribution curves needed to be updated to correctly reflect the nutritional and clinical profile of individuals.
SUA levels were directly related to BMI as observed by other authors [14, 21, 27]. There was a difference of 1.4 mg/100 ml in men and 1.1 mg/dl in women between the median SUA values in the highest and lowest strata of BMI (< 25 vs. > 35 kg/m2). Regarding age, we found similar SUA levels across age strata in men. However, older women showed slightly higher SUA levels. These results were similar to previous studies [13, 14, 21, 27] and menopause may be associated to this increase as suggested by Stockl et al. [51]. In fact, the SUA levels variations related to age and menopause are narrow and may have no clinical impact.
There was no relationship between SUA and self-reported ethnicity in our study as in another Brazilian population-based study [52] (except for women, with no clinical relevance values), which might be explained by the frequent miscegenation. However, we can find description in the literature of higher SUA levels and gout prevalence in Maoris, a specific population from New Zealand [53], and in African Americans [54,55,56], both related to the greater frequency of acquired risk factors, including obesity, physical inactivity, hypertension, diabetes, CKD, high seafood intake, elevated blood levels of lead, and use of antihypertensive medications [54].
There was a relationship of lower SUA with higher family income only for women in all sample. A former retrospective study has also found lower SUA levels in higher income groups [57]. Regarding smoking, other authors [48, 58] found that men who smoke have lower SUA levels, in spite of a study showing the opposite [59]. Frequent physical activity was associated with lower SUA levels, as also reported previously by Chen and Kawamoto [60, 61]. Fragala et al. [62] found this association only for males.
This study has some limitations. First, it is not confirmatory in determining the association between SUA and variables such as BMI and age due to its cross-sectional design. Second, the original cohort was not designed to establish the SUA reference range and prevalence of hyperuricemia and therefore may not represent the entire Brazilian population, although the sample is large and multicentric. Finally, we could not demonstrate the levels of SUA for individuals outside ELSA-Brasil age range (35 to 74 years).
Conclusions
Our manuscript proposes SUA reference ranges for men and women. We found high prevalence of hyperuricemia in men, even in a reference sample, after exclusion of conditions which influence SUA levels. Sex and BMI were major determinants for SUA. Updated SUA reference ranges and prevalence of hyperuricemia are higher nowadays and might be used to guide laboratories and screen diseases related to SUA.
Availability of data and materials
The datasets used and analysed during the current study are available from authors ISS and IMB on reasonable request.
Abbreviations
- SUA:
-
Serum uric acid
- ELSA-Brasil:
-
Brazilian Longitudinal Study of Adult Health
- CSLI:
-
Clinical and Laboratory Standards Institute
- BMI:
-
Body mass index
- CKD-EPI:
-
Chronic Kidney Disease Epidemiology Collaboration
- CKD:
-
Chronic kidney disease
- IQR:
-
Interquartile range
- ANOVA:
-
Analysis of variance
References
Shiozawa A, Szabo SM, Bolzani A, Cheung A, Choi HK. Serum uric acid and the risk of incident and recurrent gout: a systematic review. J Rheumatol. 2017;44(3):388–96.
Gaffo AL, Jacobs DR, Sijtsma F, Lewis CE, Mikuls TR, Saag KG. Serum urate association with hypertension in young adults: analysis from the Coronary Artery Risk Development in Young Adults cohort. Ann Rheum Dis. 2013;72(8):1321–7.
Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. 2000;283(18):2404–10.
Choi HK, Ford ES. Haemoglobin A1c, fasting glucose, serum C-peptide and insulin resistance in relation to serum uric acid levels—the Third National Health and Nutrition Examination Survey. Rheumatology (Oxford). 2008;47(5):713–7.
Cea Soriano L, Rothenbacher D, Choi HK, García Rodríguez LA. Contemporary epidemiology of gout in the UK general population. Arthritis Res Ther. 2011;13(2):R39.
Cho SK, Chang Y, Kim I, Ryu S. U-shaped association between serum uric acid level and risk of mortality: a cohort study. Arthritis Rheumatol. 2018;70(7):1122–32.
Sarangi R, Varadhan N, Bahinipati J, Dhinakaran A, Anandaraj, Ravichandran K. Serum uric acid in chronic obstructive pulmonary disease: a hospital based case control study. J Clin Diagn Res. 2017;11(9):BC09-BC13.
Lu N, Dubreuil M, Zhang Y, Neogi T, Rai SK, Ascherio A, et al. Gout and the risk of Alzheimer’s disease: a population-based, BMI-matched cohort study. Ann Rheum Dis. 2016;75(3):547–51.
Zhang W, Iso H, Murakami Y, Miura K, Nagai M, Sugiyama D, et al. Serum uric acid and mortality form cardiovascular disease: EPOCH-JAPAN Study. J Atheroscler Thromb. 2016;23(12):1365–6.
Cortese M, Riise T, Engeland A, Ascherio A, Bjørnevik K. Urate and the risk of Parkinson’s disease in men and women. Parkinsonism Relat Disord. 2018;52:76–82.
Li H, Zha X, Zhu Y, Liu M, Guo R, Wen Y. An invert U-shaped curve: relationship between fasting plasma glucose and serum uric acid concentration in a large health check-up population in China. Medicine (Baltimore). 2016;95(16):e3456.
Kanda E, Muneyuki T, Kanno Y, Suwa K, Nakajima K. Uric acid level has a U-shaped association with loss of kidney function in healthy people: a prospective cohort study. PLoS ONE. 2015;10(2):e0118031.
Popert AJ, Hewitt JV. Gout and hyperuricaemia in rural and urban populations. Ann Rheum Dis. 1962;21:154–63.
Sturge RA, Scott JT, Kennedy AC, Hart DP, Buchanan WW. Serum uric acid in England and Scotland. Ann Rheum Dis. 1977;36(5):420–7.
Mikkelsen WM, Dodge HJ, Valkenburg H. The distribution of serum uric acid values in a population unselected as to gout or hyperuricemia: Tecumseh, Michigan 1959–1960. Am J Med. 1965;39:242–51.
Hall AP, Barry PE, Dawber TR, McNamara PM. Epidemiology of gout and hyperuricemia. A long-term population study. Am J Med. 1967;42(1):27–37.
Isomäki HA, Takkunen H. Gout and hyperuricemia in a Finnish rural population. Acta Rheumatol Scand. 1969;15(2):112–20.
Beighton P, Solomon L, Soskolne CL, Sweet B. Serum uric acid concentrations in a rural Tswana community in Southern Africa. Ann Rheum Dis. 1973;32(4):346–50.
Beighton P, Solomon L, Soskolne CL, Sweet B, Robin G. Serum uric acid concentrations in an urbanized South African Negro population. Ann Rheum Dis. 1974;33(5):442–5.
Brauer GW, Prior IA. A prospective study of gout in New Zealand Maoris. Ann Rheum Dis. 1978;37(5):466–72.
Kuzuya M, Ando F, Iguchi A, Shimokata H. Effect of aging on serum uric acid levels: longitudinal changes in a large Japanese population group. J Gerontol A Biol Sci Med Sci. 2002;57(10):M660–4.
Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31(8):1582–7.
Thefeld W, Hoffmeister H, Busche EW, Koller PU, Vollmar J. Normal values of serum uric acid levels in relation to age and sex as determined using a new enzymatic uric acid color test. Dtsch Med Wochenschr. 1973;98(8):380–4.
Akizuki S. Serum uric acid levels among thirty-four thousand people in Japan. Ann Rheum Dis. 1982;41(3):272–4.
Neogi T, Jansen TL, Dalbeth N, Fransen J, Schumacher HR, Berendsen D, et al. 2015 Gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2015;74(10):1789–98.
Cinical and Laboratory Standards Institute (CSLI). Defining, establishing, and verifying reference intervals in the clinical laboratory; aproved guideline—third edition. CLSI Document EP28-A3c. 2008.
Das M, Borah NC, Ghose M, Choudhury N. Reference ranges for serum uric acid among healthy Assamese people. Biochem Res Int. 2014;2014:171053.
Sairam S, Domalapalli S, Muthu S, Swaminathan J, Ramesh VA, Sekhar L, et al. Hematological and biochemical parameters in apparently healthy Indian population: defining reference intervals. Indian J Clin Biochem. 2014;29(3):290–7.
Saathoff E, Schneider P, Kleinfeldt V, Geis S, Haule D, Maboko L, et al. Laboratory reference values for healthy adults from southern Tanzania. Trop Med Int Health. 2008;13(5):612–25.
Abebe M, Melku M, Enawgaw B, Birhan W, Deressa T, Terefe B, et al. Reference intervals of routine clinical chemistry parameters among apparently healthy young adults in Amhara National Regional State, Ethiopia. PLoS ONE. 2018;13(8):e0201782.
Eller LA, Eller MA, Ouma B, Kataaha P, Kyabaggu D, Tumusiime R, et al. Reference intervals in healthy adult Ugandan blood donors and their impact on conducting international vaccine trials. PLoS ONE. 2008;3(12):e3919.
Desideri G, Castaldo G, Lombardi A, Mussap M, Testa A, Pontremoli R, et al. Is it time to revise the normal range of serum uric acid levels? Eur Rev Med Pharmacol Sci. 2014;18(9):1295–306.
Association AD. 2. Classification and diagnosis of diabetes. Diabetes Care. 2019;42(Suppl 1):S13–28.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. Circulation. 2018;24:CIR0000000000000625.
Smith KB, Smith MS. Obesity statistics. Prim Care. 2016;43(1):121–35, ix.
Aquino EM, Barreto SM, Bensenor IM, Carvalho MS, Chor D, Duncan BB, et al. Brazilian Longitudinal Study of Adult Health (ELSA-Brasil): objectives and design. Am J Epidemiol. 2012;175(4):315–24.
Schmidt MI, Duncan BB, Mill JG, Lotufo PA, Chor D, Barreto SM, et al. Cohort profile: Longitudinal Study of Adult Health (ELSA-Brasil). Int J Epidemiol. 2015;44(1):68–75.
Bensenor IM, Griep RH, Pinto KA, Faria CP, Felisbino-Mendes M, Caetano EI, et al. Routines of organization of clinical tests and interviews in the ELSA-Brasil investigation center. Rev Saude Publica. 2013;47(Suppl 2):37–47.
Piccinelli M, Tessari E, Bortolomasi M, Piasere O, Semenzin M, Garzotto N, et al. Efficacy of the alcohol use disorders identification test as a screening tool for hazardous alcohol intake and related disorders in primary care: a validity study. BMJ. 1997;314(7078):420–4.
Cinical and Laboratory Standards Institute (CSLI). Defining, establishing, and verifying reference intervals in the clinical laboratory. Aproved guideline—third edition. ed2008. p. CLSI document EP28-A3c.
Seegmiller JE. The acute attack of gouty arthritis. Arthritis Rheum. 1965;8(5):714–25.
Fedeli LG, Vidigal PG, Leite CM, Castilhos CD, Pimentel RA, Maniero VC, et al. Logistics of collection and transportation of biological samples and the organization of the central laboratory in the ELSA-Brasil. Rev Saude Publica. 2013;47(Suppl 2):63–71.
Fossati P, Prencipe L, Berti G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem. 1980;26(2):227–31.
Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67(6):2089–100.
Chou CT, Lai JS. The epidemiology of hyperuricaemia and gout in Taiwan aborigines. Br J Rheumatol. 1998;37(3):258–62.
Li Y, Stamler J, Xiao Z, Folsom A, Tao S, Zhang H. Serum uric acid and its correlates in Chinese adult populations, urban and rural, of Beijing. The PRC-USA Collaborative Study in Cardiovascular and Cardiopulmonary Epidemiology. Int J Epidemiol. 1997;26(2):288–96.
Collaborators GA. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015–35.
Manthey J, Shield KD, Rylett M, Hasan OSM, Probst C, Rehm J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet. 2019;393:2493–502.
Stöckl D, Döring A, Thorand B, Heier M, Belcredi P, Meisinger C. Reproductive factors and serum uric acid levels in females from the general population: the KORA F4 study. PLoS ONE. 2012;7(3):e32668.
Rodrigues SL, Baldo MP, Capingana P, Magalhães P, Dantas EM, Molina MEC, et al. Gender distribution of serum uric acid and cardiovascular risk factors: population based study. Arq Bras Cardiol. 2012;98(1):13–21.
Stamp LK, Wells JE, Pitama S, Faatoese A, Doughty RN, Whalley G, et al. Hyperuricaemia and gout in New Zealand rural and urban Māori and non-Māori communities. Intern Med J. 2013;43(6):678–84.
Kumar B, Lenert P. Gout and African Americans: reducing disparities. Cleve Clin J Med. 2016;83(9):665–74.
Maynard JW, McAdams-DeMarco MA, Law A, Kao L, Gelber AC, Coresh J, et al. Racial differences in gout incidence in a population-based cohort: atherosclerosis risk in communities study. Am J Epidemiol. 2014;179(5):576–83.
Moulin SR, Baldo MP, Souza JB, Luchi WM, Capingana DP, Magalhães P, et al. Distribution of serum uric acid in Black Africans and its association with cardiovascular risk factors. J Clin Hypertens (Greenwich). 2017;19(1):45–50.
Acheson RM. Social class gradients and serum uric acid in males and females. Br Med J. 1969;4(5675):65–7.
Hanna BE, Hamed JM, Touhala LM. Serum uric acid in smokers. Oman Med J. 2008;23(4):269–74.
Kim SK, Choe JY. Association between smoking and serum uric acid in Korean population: data from the seventh Korea national health and nutrition examination survey 2016. Medicine (Baltimore). 2019;98(7):e14507.
Chen JH, Wen CP, Wu SB, Lan JL, Tsai MK, Tai YP, et al. Attenuating the mortality risk of high serum uric acid: the role of physical activity underused. Ann Rheum Dis. 2015;74(11):2034–42.
Kawamoto R, Katoh T, Ninomiya D, Kumagi T, Abe M, Kohara K. Synergistic association of changes in serum uric acid and triglycerides with changes in insulin resistance after walking exercise in community-dwelling older women. Endocr Res. 2016;41(2):116–23.
Fragala MS, Bi C, Chaump M, Kaufman HW, Kroll MH. Associations of aerobic and strength exercise with clinical laboratory test values. PLoS ONE. 2017;12(10):e0180840.
Acknowledgements
Not applicable.
Funding
The baseline ELSA-Brasil study is supported by the Brazilian Ministry of Health (Science and Technology Department), the Brazilian Ministry of Science and Technology, and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (#01 06 0010.00 RS; #01 06 0212.00 BA; #01 06 0300.00 ES; #01 06 0278.00 MG; #01 06 0115.00 SP; #01 06 0071.00 RJ).
Author information
Authors and Affiliations
Contributions
Study conception and design: MD, IMB, PL, RF. Acquisition of data: MD, IMB, ISS. Analysis and interpretation of data: MD, IMB, PL, ISS, RF. Drafting the article: MD, RF. Revising the article: MD, IMB, ISS, PL, RF. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All contributing ELSA-Brasil centres have obtained approval from their respective local ethics committee for including patients’ data in the database and written informed consent was obtained in those centres.
Consent for publication
Not applicable.
Competing interests
MD, IMB, ISS, PL and RF has nothing to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dório, M., Benseñor, I.M., Lotufo, P. et al. Reference range of serum uric acid and prevalence of hyperuricemia: a cross-sectional study from baseline data of ELSA-Brasil cohort. Adv Rheumatol 62, 15 (2022). https://doi.org/10.1186/s42358-022-00246-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-022-00246-3