Abstract
Background
Due to the increasing usage of plastic, microplastics or plastic particles with a length of 1 to 5 mm are omnipresent in the environment, including freshwater, agricultural soil and seas. The majority of plastic trash is made up of microplastic debris, which has lately started to threaten ecosystems. This calls for immediate management and analysis to ensure the traceability of microplastics in the environment.
The main body of the abstract
The polymeric variety, irregular shape and microscopic size of microplastics make it difficult to quantify their presence in the environment. In this review, the analytical techniques for evaluating microplastics are discussed. The techniques included Fourier transform infrared spectroscopy, Raman spectroscopy, laser diffraction particle, thermal analysis, scanning electron microscope, pyrolysis gas chromatography, dynamic light scattering, and atmospheric solid analysis probe (ASAP) paired with quadrupole mass spectrometry (MS).
Short conclusion
The purpose of the present review is to give a summary of the most efficient cutting-edge techniques for more accurate and precise microplastic examination in the environmental samples.
Similar content being viewed by others
Background
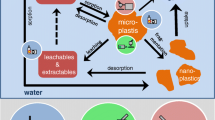
Plastics play a significant function in providing greater convenience in our everyday lives because of qualities like low weight, affordability and durability (Gu et al. 2020). Plastics are non-biodegradable pollutants that are unable to break down naturally, making them an extremely persistent environmental contamination. According to study by Plastics Europe (2019), even though global plastics output has topped 350 million tonnes, scientists warn that if immediate action is not done to curb it, it might reach 500 million tonnes by 2025 (Geyer et al. 2017). Plastic goods disintegrate into microscopic particles known as nanoplastics (length 1 m), microplastics (length 1 m to 5 mm), mesoplastics (length 5 mm to 5 cm), macroplastics (length > 5 to 50 cm) and megaplastics (length > 50 cm) in the environment, including the soil, air, water and other environmental media. Even further, they may be carried by air and water currents. Microplastics may be categorised into two main primary and secondary groups, depending on their source. Plastic pieces that have been dumped into sewage treatment facilities and rivers are considered primary microplastics. Secondary microplastics are produced when massive amounts of plastic trash are fragmented and shrunk by chemical, physical and biological activities (Guo and Wang 2019). The fragments, granules, threads and films are the most typical morphologies of microplastics (Cózar et al. 2014; Huang et al. 2019; Guo et al. 2020).
Significant threats are posed by microplastics to the environment and to living creatures. In the first place, several researches (Wang et al. 2019; Guo et al. 2020; Queiroz et al. 2020; Mu et al. 2022) have shown that microplastics have a long shelf life and are challenging to degrade, which causes significant harm to the ecosystem and animals. It is also possible for microplastics in the environment to undergo a multitude of changes that release biotoxic plastic additives into the atmosphere and lead to secondary pollutants (Liu et al. 2020). Microplastics are extremely likely to carry additional environmental toxins owing to their enormous specific surface area, which might put ecosystems at greater risk (Li et al. 2018; Naqash et al. 2020). One type of substance that may readily enter a person's body through food chains is microplastics (MPs). As a result, it is a significant risk to both people and animals (Gaylarde et al. 2020). They have a wide range of attributes that influence how deadly they are, including their size, composition, and structure. Pirsaheb et al. (2020) found that extremely small, fibre-shaped microplastics are more dangerous in nature. The microplastics formed from these materials, which mostly consist of polyethylene terephthalate (PET), polyethylene (PE) and polypropylene (PP), may, nevertheless, exhibit minimal chemical risk, given that they were designed to provide very minor concerns to human health (Lithner et al. 2011). For instance, metals (such as Cr, Cd, Hg, Sb and Pb) and also flame retardants are typically found in e-waste plastics at per cent (%) concentrations by weight (Li et al. 2019; Turner et al. 2019; Singh et al. 2020).
Microplastics are polymers with a range of chemical compositions, as is widely known. PE, PP, polyvinyl chloride (PVC), polystyrene (PS), polyamide (PA) and PET are some of the microplastics that are regularly found in the environment. The impact of microplastics on plants has been investigated (Zhu et al. 2019). The one that is most usually picked to be assessed as a microplastic is PS. One possible explanation is the fact that the basic PS spheres have small and uniform particle sizes (i.e., 1 m). The consequences of PEs are frequently studied as well because of their widespread usage and persistence as a polymer in the terrestrial environment (de Souza Machado et al. 2018). As a result, from the perspective of ecosystem health, less prevalent plastic products may have an outsized impact and need prioritising for research. For research monitoring microplastics, dependable and comparable standardised sampling and analytical procedures are required (Galgani et al. 2013; Muller et al. 2020). Methods for microplastics analysis are currently being developed, and no uniform strategy has yet been created (Uddin et al. 2020). Determining the types and concentration of microplastic in agriculture is a hot topic at the moment since it is used so often. According to several studies, microplastics have the capability to change the physical characteristics of soil, including adsorption–desorption practises, fertility rate and adverse toxic impacts on terrestrial ecosystems (Brandes et al. 2021; Junhao et al. 2021; Wang et al. 2022).
The significance of each type of data and the information that has to be obtained from samples should be taken into consideration when selecting an appropriate analysis approach among the ones that are accessible. This review summarised the techniques developed and already in use for determining the existence of microplastics in real samples of the environment and speculated on the direction that technology will go in the future. Microplastics frequently consist of combinations of plastic particles with diverse, very complicated compositions. It is possible to figure out the molecular weights, functional groups, structures, and degrees of polymerisation of the polymers present in microplastics, as well as their chemical makeup, by employing a variety of approaches. Microplastic treatment strategies and traceability analysis depend heavily on the chemical composition of microplastics, which must be characterised (Song et al. 2015).
Various physical and chemical characterisation methods of the microplastics shown in Fig. 1 and a variety of techniques are currently and frequently used, including Fourier transform infrared spectroscopy (FTIR) (Song et al. 2015), scanning electron microscopy–energy-dispersive X-ray (SEM–EDX) (Wagner et al. 2017), thermal analysis (Majewsky et al. 2016) and Raman spectroscopy (Araujo et al. 2018). These typical methods as well as a few intriguing technologies have been introduced in this section and are summarised in Table 1.
Methods for identifying and measuring microplastics
Microplastics analysis using microscopy
The morphology of the MPs present in a sample may be identified using visual identification methods such a light microscope or polarising microscopy, according to early investigations on MP counts and physical characterisation (Talvitie et al. 2017). Visual identification approaches often classify MPs into three groups based on their external appearance: fibres, fragments and pellets/microbeads.
Light microscopy
Light microscopy may be used to count the microplastics, which are several hundred micrometres in size. Since microplastics frequently lack shine, their physical response characteristics, such as their unique elasticity or hardness, are employed to identify them. Additionally, fibres, fragments and beads made of microplastics frequently show up in the environment (Abadi et al. 2021). About 70% of the time, microplastic samples are clear (Löder and Gerdts 2015). It is possible to swiftly determine coloured polymers that have had dye added during manufacture using an optical microscope (Dehghani and Moore 2017). It is difficult to define colourless or amorphous plastic particles that are smaller than 100 m. Furthermore, poor sample particle separation may make it difficult to identify microplastics at the microscopic level. Furthermore, it is challenging to distinguish microplastics under a microscope because sediments and biological elements cannot be completely eliminated through chemical degradation. A prior study found that more than 70% of transparent particles and more than 20% of plastic-like particles are misdiagnosed. Since they are similar particles with interfering components, it could be difficult to distinguish between synthetic and natural fibres with a microscope alone. Although cotton fibres are sometimes mistaken for plastic, "destructive tests" have been used to detect the particles. Consequently, a technique has been developed to detect these particles that require bringing heated needles into contact with the particles, which melts the plastic particles (Shim et al. 2017; Hendrickson et al. 2018). The most often used plastic additives are bleaching agents, and they are used in both the textile and plastic synthesis sectors. Due of the fluorescence that bleaching chemicals generally produce, plastics may be identified using a fluorescence microscope. Still, chemicals for paper whitening are also applied. Inaccuracies in detection are still possible since some minerals include chemicals that have the ability to self-fluoresce (Dehghani and Moore 2017).
Polarising microscopy
In toxicity testing, polyethylene (PE) particles have been successfully identified using polarised light microscopy (Mossotti et al. 2021). The transmission of polarised light that may be examined may be affected by the plastic's crystal structure (Abbasi 2021). Depending on the method of manufacturing and the kind of polymer, the degree of crystallinity varies, in the same polymer even. However, tiny microplastic samples are necessary to allow enough polarised light to get through. Samples of opaque microplastic cannot be used with this technique.
Dynamic light scattering
According to Li et al. (2021), nanoplastics are plastic particles having a particle size of less than 1 m. Dynamic light scattering has great promise for the study of nanoplastics. Sorasan et al. (2021) discovered that solar photochemical ageing may transform secondary microplastics into nanoplastics using dynamic light scattering experiments. However, some pollutants in environmental samples might affect the experimental results, much like with laser diffraction particle size analysis.
Laser diffraction particle size analysis
The rapid advancement of material science has resulted in the creation of more sophisticated equipment. Laser diffraction particle size analysis may be used to quickly, precisely and automatically examine the distribution of soil and the sediment particle sizes (Bittelli et al. 2022). The experiment produced exact, finely resolved, and comprehensive results. The approach is essentially non-destructive and allows for the retrieval of crucial samples (Blott et al. 2004). Blott et al. (2004) claim that this technique may be employed to investigate particles that range in size from 0.04 m to 2000 m. However, a number of pollutants in ambient samples might skew the results of the study. Using this technique also aims to isolate and eliminate microplastics from the environment. The size distribution of microplastic particles has not been widely detected using this approach, but as technology advances, it will ultimately play a significant role in this area.
Scanning electron microscopy
Even for exceedingly small particles like nanoplastics, scanning electron microscopy (SEM) may give clear pictures that have been magnified multiple times (Tunali et al. 2020). The texture of the particle surface may easily distinguish organic particles from microplastics, and this can be seen in high-resolution images (Hossain et al. 2019). TEM, or transmission electron microscopy, is another method for locating microplastics. Cross-validating the data from numerous users using a tried-and-true method helps decrease errors even if the visual detection of microplastics by TEM may differ according to the analyser. Energy-dispersive X-ray spectroscopy (EDS) is applied to conduct further research in order to ascertain the elemental makeup of the item. In order to identify microplastics using EDS, antioxidants or additive components such Ca, Na, Al, Mg and Si are frequently collected as markers (Watteau et al. 2018; Wagner et al. 2019). By examining a particle's surface elemental makeup, carbon-rich polymers may be differentiated from inorganic particles (Abadi et al. 2021; Sabri et al. 2021). The quantity of samples that can be processed by SEM and TEM is constrained by the high cost of the equipment as well as the time and labour needed for sample preparation and examination.
Flow cytometry
Adan et al. (2017) assert that the biological and medical fields have made substantial use of flow cytometry. Flow cytometry makes use of laser light that is scattered by particles and recorded in forward or side scattering angles to measure substances range between 0.5 and 40 mm in particle size (Primpke et al. 2018). Kaile et al. (2020) noted that this approach is hardly used for microplastics measurement. Sorasan et al. (2021) employed flow cytometry to track the development of microscopic (1–25 m) microplastics by using Mie's theory to determine the size of microplastic particles from measurements of scattering intensity. The different types of microplastics cannot be distinguished by flow cytometry; it can only detect microplastics with extremely small particle sizes. Furthermore, there is a good chance that certain pollutants in environmental samples will have a negative impact on the accuracy of the outcomes of experiments. In order to identify microplastics with high particle sizes, flow cytometry must be improved. In the future, it is projected that the field of environmental analysis will gain more from the application of flow cytometry.
Spectroscopy-based analyses of microplastics
Spectroscopy can be categorised quantitatively and qualitatively microplastic without damaging the materials. The two most popular spectrum analysis methods are Raman spectroscopy and infrared spectroscopy employing the Fourier transform. Fourier transform infrared spectroscopy (FTIR) may be utilised for identifying microplastics greater than 20 m in size, whereas Raman spectroscopy may be utilised to detect and determine smaller microplastics (Araujo et al. 2018; Schymanski et al. 2018; Prata et al. 2019). FTIR spectroscopy and Raman spectroscopy are widely coupled in order to investigate microplastics (Käppler et al. 2016; Prata et al. 2019; Wright et al. 2019). Today, spectroscopy is frequently used in combination with other methods to count microplastics.
Fourier transform infrared spectroscopy
One can discover more about the specific chemical bonds holding certain particles together by employing Fourier transform infrared spectroscopy (FTIR) spectroscopy. This method makes it simple to identify polymers with a carbon basis. Plastics differ from other organic and inorganic particles in that they have a unique spectrum that results from various bonding configurations in their chemical structure (Avio et al. 2015; Garaba and Dierssen 2018). A trustworthy polymer spectral library may also be utilised to recognise plastics (Jung et al. 2018; Witkowski and Koniorczyk 2018). Using IR spectra to arrange data for every individual plastic decreases the risk of missing plastic particles without a specific colour or texture and prevents the measurement of non-plastic particles. Additionally, FTIR identification indicates the composition of the polymer, which may shed light on the material's source and manner of occurrence (Veerasingam et al. 2020). The conformation of oxygen bonds such as carbonyl groups in the IR spectrum may also be used to measure the degree of oxidation of microplastics (Zhou et al. 2020; Hebner and Maurer-Jones 2020). Micro-FTIR, which possesses both characteristics and permits microscopic viewing of minute plastic-like particles prior to spectroscopic confirmation on a single platform, makes it easier to identify microplastics (Morgado et al. 2021). The FTIR study of microplastics may also employ the transmittance, reflectance and attenuated total reflectance (ATR) modes (Hendrickson et al. 2018). Contrary to the transmission mode, the reflectance and ATR modes do not call for sample preparation procedures for thick and opaque microplastics. Furthermore, unstable spectra can be provided by the ATR mode on erratic microplastic surfaces. The theoretical upper limit on the detection of microplastics is set by the size of the IR beam aperture of the ATR probe. In order to discover microplastics in environmental samples, micro-ATR-FTIR is being employed as a complement to the two tasks of microscopic determination of plastic-like particles and spectroscopy for possible chemical identification (Morgado et al. 2021). However, for microplastics smaller than 50 m, it is sometimes challenging to get a distinct spectrum that can be precisely defined. Since ATR-FTIR measurement is a form of surface contact investigation, the pressure created by the ATR probe could potentially have an impact on delicate microplastics (Silva et al. 2018). Small plastic particles could be hard to find because of adhesion or electrostatic interactions with the probe tip. Each particle must be detected independently in the ATR mode, which also takes a while. Each tiny plastic-like particle must be identified by ATR probes one at a time, and micro-FTIR equipment is pricey. Microplastics occasionally show up as composite materials in environmental samples, are frequently weathered and frequently have complicated chemical compositions. Because of this, obtaining a clean spectrum requires knowledgeable operators for accurate spectrum interpretation. In order to decrease the possibility of false positives while utilising a library, it is also advised to register the variety of surfactants like sodium dodecyl sulphate (SDS) and sodium stearate, which may be mistaken for plastic, together in the FTIR library (Witzig et al. 2020). Because several studies suggest that heavy metals and microbes are adsorbed on the surface of plastics, the sample may need to be cleaned before using FTIR (Mintenig et al. 2017; Jung et al. 2018; Corami et al. 2020). A focal plane array (FPA) detector may be used to obtain FTIR spectra for a range of microplastics in numerous pixel arrays at the same time (Vianell 2020). Mintenig et al. (2017) and Simon et al. (2018) claim that each pixel can offer a unique IR spectra that can be utilised to differentiate between tiny pieces and microplastic fibres. Since there are not enough library data, errors may still happen even when the FPA detector runs for a long time (Primpke et al. 2018). More study is required because PRCs such antioxidants, ink components, plasticizers and label glue may have an effect on the FTIR data. As an illustration, Zhang et al. (2018) created the method known as Fourier transform infrared spectroscopy to increase the effectiveness of identifying microplastics by connecting Fourier transform infrared spectroscopy with microscopy. This innovative method may be used to measure microplastics in environmental samples in addition to measuring microplastics as tiny as 10 m (Li et al. 2020). Additionally, Löder and Gerdts (2015) created a unique technique known as focal plane array detector based micro-Fourier transform infrared imaging employing the focal plane array-based reflectance micro-Fourier transform imaging approach.
Raman spectroscopy
In Raman spectroscopy, each polymer produces a distinct spectrum when exposed to a laser beam since the frequency of the backscattered light changes dependent on the molecular makeup and atom concentration of the target. This spectroscopy may be used to detect plastics since, like FTIR, it offers a polymer composition profile for each sample (Witzig et al. 2020). It may be used to analyse the outcomes of data libraries and algorithms (Anger et al. 2019). Raman spectroscopy and the FTIR approach both include non-destructive chemical analysis and microscopy (Kniggendorf et al. 2019). Raman spectroscopy's smaller laser beam diameter than FTIR's allows it to identify microplastics as tiny as a few micrometres. The benefit of employing non-contact Raman spectroscopy for research is that the microplastic sample is preserved during and after the measurement, allowing for future investigation. However, it can be more challenging to understand the results of each approach in the detection of complex microplastics due to variations in the reactions and spectra of microplastics between Raman spectroscopy and FTIR. This method makes it challenging to determine the target polymer type since additives and colouring agents in microplastics may change the Raman signal (Dowarah and Devipriya 2019). The microplastics' surface curvature may potentially cause accuracy problems. Additionally, it shares FTIR's drawbacks, such as a high cost and the need for expert analysis. Micro-Raman spectroscopy can only distinguish between different materials if a suitable Raman reference spectra for that material are provided in the database and the potential of material misunderstanding has been entered beforehand. Since it could be challenging to detect the desired microplastic, the danger of false positives should be taken into account (Witzig et al. 2020). PE is a chemical that might be mistaken for SDS or sodium stearate. Moreover, by integrating Raman spectroscopy with microscopy, the non-contact, non-destructive analytical technique known as micro-Raman spectroscopy was established. The presence of microplastics in materials may also be quantitatively investigated using micro-Raman spectroscopy. However, because the method can distinguish between particles of various sizes, the maximum number of micro-Raman spectroscopy particles is only 5, 000. As a result, it is quite likely that the measurements of the quantity of microplastics in samples made using micro-Raman spectroscopy (Schymanski et al. 2018; Tsering et al. 2022) will be off. As a result of the field's quick progress, spectroscopy is predicted to eventually take a significant role when analysing and quantifying microplastics chemically at the same time.
Mass spectrometry-based analyses of microplastics
Gas and liquid chromatography combined with mass spectrometry are methods for analysing microplastics by looking at the distinctive products created when microplastics are hydrolysed or pyrolysed (Fabbri et al. 2000; Fischer and Scholz-Böttcher 2017; Wang et al. 2019; Zhou et al. 2020; Li et al. 2021). These mass approaches have a lot of potential for identifying and quantifying microplastics, particularly nanoplastics, due to their high sensitivity.
Inductively coupled plasma mass spectroscopy
Single particle inductively coupled plasma mass spectroscopy (ICP-MS), a novel mass spectrometry technique for measuring microplastics, was developed as a result of advances in isotope tagging technology. This innovative method makes it feasible to quantify the quantity and size of nanoparticles (Laborda et al. 2019). For metal-based nanoparticles in environmental samples, this technique is applied very frequently (Huang et al. 2019; Keller et al. 2018; Cervantes-Avilés et al. 2019).
Considering a comparatively low detection limit of 8.4 105 particles/L, single particle inductively coupled plasma mass spectrometry, for instance, has been used to evaluate the size and number concentration of the model Au-coated microplastics (at submicrometer scale). However, because it relied on an indirect assessment of the Au coating, employing it required lengthy sample preparation (Jiménez-Lamana et al. 2020; Lai et al. 2021). Single-particle inductively coupled plasma mass spectrometry has been demonstrated to be able to evaluate model microplastics particle sizes and number concentrations by monitoring 13C as the process develops (Bolea-Fernandez et al. 2020; Laborda et al. 2021). Liu et al. (2021) utilised single-particle inductively coupled plasma mass spectroscopy in order to quantitatively analyse the particle number concentration (down to 7.1 106 particles/L) with a wide particle size range (800 nm–5 m) and environmentally significant values produced during the photoageing of polystyrene microplastics. It offers enormous promise to investigate the dynamics of microplastics in the ageing process using single-particle inductively coupled plasma mass spectrometry.
Pyrolysis gas chromatography–mass spectrometry
An alternative method for evaluating pyrolysed gases in a range of polymers is pyrolysis–gas chromatography mass spectrometry (Py-GC–MS) (Ceccarini et al. 2018). To evaluate whether a pyrogram acquired from a sample is plastic, it may be compared to the findings of an acknowledged polymer standard. Few plastic (particle) samples are pyrolysed at temperatures above those used in TGA (700 °C), after which they are separated and subjected to GC–MS analysis (Shim et al. 2017). The sizes of these samples range from 0.3 to 7 mg. Styrene butadiene rubber (SBR), polyvinyl acetate (PVA), and poly(acrylonitrile butadiene styrene) are all found in soil and suspended solid particles after thorough investigation. This technique can only recognise microplastics made from derivative products of polystyrene (PS). More investigation is necessary to develop molecular markers for a variety of plastics (Watteau et al. 2018).
Liquid chromatography–tandem mass spectrometry
Liquid chromatography–tandem mass spectrometry (LC–MS/MS) is a dependable method used in medicine and pharmacology and the assessment of environmental acquaintances to minuscule heat-labile, nonvolatile chemicals may be used to recognise few polymers (Gros et al. 2006; Ng et al. 2020). In sewage sludge, for instance, Nylon, PET and polycarbonate-based MPs have been successfully identified and quantitatively assessed using LC–MS/MS (Zhang et al. 2019, 2021; Peng et al. 2020). Since it necessitates depolymerising macromolecules before analysis, this approach is harmful. In contrast to the count, colour, shape and size of NPs and MPs, the analysis method provides information on the mass and number of monomers produced during depolymerisation. Additionally, size fractionation may be employed with the approach to provide details on the size profile distribution.
The majority of polymers lack the distinctive breakdown products required for mass spectrometry measurement even though microplastics commonly contain combinations of several polymer types (Wang et al. 2017; Peng et al. 2020; Li et al. 2021; Zhang et al. 2021). As a result, mass spectrometry cannot yet be used to calculate the overall quantity of microplastics in the environment.
Thermal-based analyses of microplastics
Time and temperature both have an impact on a material's characteristics. Thermal analysis, which looks at the functional relationship of this change, is a crucial technique for material study (Majewsky et al. 2016). Environmental samples are heated before utilising thermal analysis to assess microplastics. The microplastics absorb significant heat as the temperature rises, eventually converting the polymers from a solid state to a liquid or gas form. At that specific temperature, an endothermic peak then manifests (Majewsky et al. 2016). Since various types of polymers have varying thermal stabilities, the composition and type of microplastics and their additives may be investigated using the standard thermograms of polymers (Majewsky et al. 2016).
Differential scanning calorimetry
Analysing materials by examining the thermal characteristics of polymers is made easier using differential scanning calorimetry (DSC) (Tsukame et al. 1997). Currently, polyethylene and other important microplastics are routinely found in the environment, and DSC is widely employed to find them (Castaeda et al. 2014). Due to the fact that DSC only has a few applications, other techniques are usually used with differential scanning calorimetry. It exhibits variations in dissolution, crystallisation, transition temperatures and related enthalpy and entropy to support the physical properties of polymers (Müsellim et al. 2018). Since every plastic item has distinctive characteristics, DSC may be utilised to differentiate between various polymer types (Zainuddin and Syuhada 2020). Because the peaks overlap when DSC identifies microplastics having same melting points (MPs), there are restrictions (Rodriguez Chialanza et al. 2018). It is solely useful for the identification of major microplastics, including PP and PE not all microplastics (Bitter and Lackner 2021). More investigation is necessary to develop molecular markers for a variety of plastics (Watteau et al. 2018).
Thermogravimetric analysis
Thermogravimetric analysis (TGA) is an additional technique for thermal analysis. By evaluating the sample's dependency on time and temperature and measuring the sample's weight loss while it is heated at a certain rate under predetermined ambient and temperature settings, this approach validates the sample's qualitative and quantitative information (Ma et al. 2018). Microplastics in wastewater have been investigated in the past using TGA and DSC. However, only polypropylene (PP) and PE could be distinguished in these tests with confidence. There was no mention of polyamide (PA), polyvinyl chloride (PVC) or polyester (PES). Polyethylene terephthalate (PET) and polyurethane (PU) phase transition signals, for example, are difficult to identify from one another (Majewsky et al. 2016; Sun et al. 2019).
In conclusion, thermal analysis may be added to the analysis process right away without the requirement for time-consuming sample preparation. However, there are also substantial disadvantages to thermal analysis. First off, due to the tendency of polymer branching and other impurities in microplastics to impact the transition temperature of polymers, certain copolymers are difficult to detect by thermal analysis. Furthermore, heat analysis cannot be used to characterise the physical characteristics of microplastics, such as their shape and appearance, because it destroys environmental sample. Therefore, thermal analysis is commonly employed to detect the chemical components of microplastics and to calculate their quantity. This could limit how thermal analysis is applied (Rocha-Santos and Duarte 2015; Majewsky et al. 2016; Shim et al. 2017; Huppertsberg and Knepper 2018; Silva et al. 2018).
Cutting-edge technology
Methods developed via the development of new analytical equipment and the integration of new detection technologies with existing instrumentation should be able to address the present issues in microplastic identification. One issue that has to be overcome in microplastic analysis is the maximum detectable size. The minimal detectable size limit for the current analytical methods is a few micrometres. It is more important than ever to comprehend the presence, destiny, distribution and toxicity of plastics at the nanoscale because smaller plastic particles might cause more dangerous side effects. New methods of identification and protocols for the collection, extraction, purification and concentration of nanoplastics need to be developed as a result.
Atomic force microscopy-infrared and Raman
In conjunction with IR or Raman spectroscopy, atomic force microscopy (AFM) could be a candidate for studying nanoplastics. AFM probes may interact with objects in contact- and non-contact modes, and the technique can provide images with a resolution of just a few nanometres. You may also employ AFM with IR or Raman spectroscopy to display the target material's chemical makeup (Luo et al. 2021a, b). Due to the sample's thermal expansion brought on by IR absorption, the AFM cantilever vibrates. The Fourier transform is used to evaluate the ring-down pattern and quantify the frequency and amplitude of the vibration (Luo et al. 2020). AFM-IR can also record both the target's IR absorption spectra and an image with a spatial resolution of 50–100 nm. IR and AFM spectra of 100 nm PS beads were effectively obtained in earlier research. However, it is challenging and time-consuming to locate a single nanoscale plastic particle target to concentrate on with AFM-IR in an unidentified material. The human search for plastic particles is one of the labour-intensive analytical stages in traditional microplastic analysis methodologies. How rapidly organic and inorganic particles are removed throughout the separation and purification processes determines how long the pretreatment procedure will last. It is possible to miss microscopic, translucent plastic particles, especially during hand identification. Both problems can be solved by automated FTIR/Raman mapping or by monitoring particles with Raman spectroscopy, but both methods need pricey equipment that not many microplastics research laboratories can afford.
Nile red staining of plastics
A simple staining approach may be used to address the issue of minute, transparent particles. Attempts have been made, although the use of Eosin B, Rose Bengal Hostasol Yellow 3G and Oil red EGN is prohibited (Prata et al. 2019). The fluorescent dye 9-diethylamino-5H-benzophenoxazine-5-one is excellent for selectively colouring very hydrophobic microplastics, however. Nile red is an additional choice. It is frequently used to stain physiologically neutral lipids. Only in a hydrophobic environment can Nile red be observed because of its great affinity for neutral lipids and intense fluorescence. Short staining times (10–30 min) and excellent recovery efficiencies (up to 96%) are two benefits of the Nile red staining technique; in addition, a quick bleach wash is performed as needed. This is a helpful first step before conducting a more thorough spectroscopic investigation and is effective for finding hidden microplastics (Erni-Cassola et al. 2017; Simmerman et al. 2020). Following fluorescence microscopy, an FTIR microscope with a fluorescent filter can be used to validate the spectrum identification of the same particle. Combining fluorescence microscopy with FTIR confirmation after Nile red staining might reduce the possibility of missing microplastics in in situ sample identification (Sancataldo et al. 2020). Furthermore, in comparison with only spectroscopy, it takes less time to detect all particles that resemble plastic (Vermeiren et al. 2020). The possibility of co-staining organic chemical molecules is one of the main drawbacks of using Nile red staining on in situ materials. In order to properly prepare the sample for the Nile red stain, extensive cleaning is necessary. Complete organic material removal during the pretreatment stage might be challenging and time-consuming. To get around this, attempts have also been made to remove organic molecules utilising H2O2 or a density separation process utilising, for instance, Fenton reagent or sodium chloride (NaCl) (Al-Azzawi et al. 2020; Li et al. 2020). The efficiency of these techniques, however, is constrained by the large range of densities of plastics.
Near-IR spectra analysis method
NIR spectroscopy has also been considered as a possible evaluation tool for microplastics. The spectrum between 600 and 4000 cm−1 is studied by FTIR, whereas the spectrum between 4000 and 15,000 cm−1 is examined by NIR, according to Zhang et al. (2018). Studies of NIR spectra frequently employ X–H chemical vibration combinations, such as C–H, N–H and O–H. For quantitative analysis, NIR analysis is imprecise and tough to employ. But it can quickly sort and assess a lot of data from plastic sample sets. In order to determine the kind of a sample rather than its number, it is better to use this analytical approach (Paul et al. 2019).
Vis–NIR analysis
A Vis–NIR spectroscopy evaluates how much light is reflected from the surface of the sample in 350–2500 nm range in order to compute the reflectance for each wavelength. Since it takes into account the sample's chemical makeup, this analytical approach may be utilised to quantify microplastics (Corradini et al. 2019). Microplastics like PET, low-density polyethylene (LDPE) and PVC may be recognised using an open Vis–NIR spectral data library for a number of polymers that are often discovered in the environment. However, because it depends on optical detection, biological particles might still be incorrectly categorised as plastic, necessitating human judgement.
Nanothermal analysis
AFM images made with nano-TA probes combine high spatial resolution with local temperature monitoring. Nano-TA is a method for doing this. This makes it possible to understand the thermal behaviour of materials with a spatial resolution of < 100 nm. When a site of interest is chosen during a nano-TA examination, the probe travels to a fixed spot on the sample surface. The probe is extremely sensitive to the microplastic's stiffness (hardness). When the sample surface reaches the glass transition temperature, the tip stops gradually heating up. Since the sample softens during phase inversion, it is largely utilised to calculate the glass transition temperature and research the nanoscale surface characteristics of microplastics. The probe can pierce the sample with this procedure, which is another benefit (Luo et al. 2020). Recent investigations on the features of microplastics altered by the advanced oxidation process using nano-TA and the impacts of TiO2-dyed microplastics' characteristics on ageing (Luo et al. 2020). Understanding the thermal characteristics of distinct locations, such as glass temperature, from the nano-TA research of microplastics facilitates inferring both with the physical and chemical state of each microplastics region. For both amorphous and semi-crystalline polymers such LDPE, PS, polycaprolactone (PCL), PET, polyoxymethylene (POM) and polymethyl methacrylate (PMMA) comparable materials, the phase transition temperature has been investigated using the nano-TA measurement method (Guen et al. 2020).
X-Ray diffraction
XRD patterns were used to examine a range of microplastics. PET has a very poor crystalline quality and large peaks; its highest peak intensity is 2 of 25.7°. PP is a potent crystalline compound with sharp peaks. It has a 31.2° diffraction peak and a 34.3° 2 decrease. Their maximum diffraction peak intensities fall by 21.6°, 24.05° and 27.5° in PE, which is inherently crystalline. These three summits are all very visible and extremely distinct. The emergence of large and wide peaks in PS reveals the material's poor crystalline quality. PS has an intensity peak of 22.6° at a wavelength. The PBT microplastic polymer's pattern is noisy even though it lacks a clear peak and is amorphous in nature (Thakur et al. 2023). According to previous research, the XRD patterns of three different types of microplastics (PE, PVC and PS) show two strong sharp peaks at 2 of 21.1° and 23.4° for PE, no sharp peak for PVC and wide peaks for PS that are defined as having a poor crystalline structure (Ezeonu et al. 2019; Liu et al. 2019; Moura et al. 2023).
Atmospheric solid analysis probe (ASAP)
Although it has been demonstrated that mass spectrometry (MS) is useful for characterising synthetic polymers, it has not yet been extensively investigated for the examination of individual particle microplastics (MPs). Although MPs are currently considered to be widespread contaminants, detailed information on their prevalence is still scarce since MP characterisation processes are difficult to get. This work recommends employing an air solid analysis probe (ASAP) linked to a small quadrupole MS for the chemical analysis of single-particle microplastics in order to completely support complementing staining and image analysis methodologies. Prior to finishing the real polymer characterisation, a two-stage ASAP probe temperature protocol was created with the goal of removing additives and surface impurities. Numerous single-particle MPs, such as polyamides, polyaromatics, polyacrylonitriles, polyolefins, polyacrylates, polycarbonates and (bio)polyesters showed unique mass spectra for the approach. In both full scan and selective ion recording modes, the individual particle size limits of detection for polystyrene MPs were found to be 30 and 5 m, respectively. Also described the results of a multimodal approach to microplastic characterisation. This method uses staining and fluorescence microscopy to initially analyse filtered particles before they are individually probe-picked for ASAP-MS analysis. The procedure provides a comprehensive description of MP contamination, compromising details on particle number, size, shape and chemical composition. The research of MPs in bioplastic water bottles demonstrated clearly the applicability of the well-known multimodal approach (Vitali et al. 2022).
Conclusions
Combinations of different microplastic analytical techniques will be employed to find microplastics in diverse environment matrices. The smaller the microplastics are, the harder they are to locate. Sub-micron analysis is becoming more and more crucial to evaluate the microplastics adverse effects on the environment and human health. It will be essential to create new strategies and enhance current ones as the need for microplastic contamination monitoring grows in order to cut down on the time and effort required for detection. For locating and measuring nanoplastics in environmental samples, it will also be crucial to create trustworthy and useful identification procedures. Future study should concentrate on developing a fully or partially automated analytical strategy that can incorporate image analysis-based approaches to identify the plastic components and detect physical characteristics (such as size and shape) of microplastics. In addition, synthetic cellulose fibres that are sold under the names rayon in the US and viscose in Europe are contaminating the environment in a way that is quite similar to that of microplastics. As a result, this should also be considered while researching microplastics. In order to prevent this error, consideration should also be given to the features that distinguish microplastics from materials that are equivalent to them. In order to identify and eliminate microplastics from environmental samples, research should also continue to introduce and develop innovative dyeing methods, nanotechnology and analytical techniques.
Availability of data and materials
Not applicable.
Abbreviations
- AFM:
-
Atomic force microscopy
- ASAP:
-
Atmospheric solid analysis probe
- ATR:
-
Attenuated total reflectance
- DSC:
-
Differential scanning calorimetry
- EDX:
-
Energy-dispersive X-ray
- FPA:
-
Focal plane array
- FTIR:
-
Fourier transform infrared spectroscopy
- GC:
-
Gas chromatography
- ICP:
-
Inductively coupled plasma
- IR:
-
Infrared
- LC:
-
Liquid chromatography
- MP:
-
Microplastic
- MS:
-
Mass spectrometry
- PA:
-
Polyamide
- PE:
-
Polyethylene
- PET:
-
Polyethylene terephthalate
- PP:
-
Polypropylene
- PVC:
-
Polyvinyl chloride
- SEM:
-
Scanning electron microscope
- TGA:
-
Thermogravimetric analysis
- XRD:
-
X-ray diffraction
References
Abbasi S (2021) Prevalence and physicochemical characteristics of microplastics in the sediment and water of Hashilan Wetland, a national heritage in NW Iran. Environ Technol Innov 23:101782
Adan A, Alizada G, Kiraz Y, Baran Y, Nalbant A (2017) Flow cytometry: basic principles and applications. Crit Rev Biotechnol 37:163–176. https://doi.org/10.3109/07388551.2015.1128876
Al-Azzawi MS, Kefer S, Weißer J, Reichel J, Schwaller C, Glas K, Drewes JE (2020) Validation of sample preparation methods for microplastic analysis in wastewater matrices-reproducibility and standardization. Water 12:2445
Anger PM, Prechtl L, Elsner M, Niessner R, Ivleva NP (2019) Implementation of an open source algorithm for particle recognition and morphological characterisation for microplastic analysis by means of Raman microspectroscopy. Anal Methods 11:3483–3489
Araujo CF, Nolasco MM, Ribeiro AMP, Ribeiro-Claro PJA (2018) Identification of microplastics using Raman spectroscopy: latest developments and future prospects. Water Res 142:426–440. https:// doi. org/ 10. 1016/j. watres. 2018. 05. 060
Avio CG, Gorbi S, Regoli F (2015) Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: first observations in commercial species from Adriatic Sea. Mar Environ Res 111:18–26
Bittelli M, Pellegrini S, Olmi R, Andrenelli MC, Simonetti G, Borrelli E, Morari F (2022) Experimental evidence of laser diffraction accuracy for particle size analysis. Geoderma 409:115627. https://doi.org/10.1016/j.geoderma.2021.115627
Bitter H, Lackner S (2021) Fast and easy quantification of semi-crystalline microplastics in exemplary environmental matrices by differential scanning calorimetry (DSC). Chem Eng J 423:129941
Blott SJ, Croft DJ, Pye K, Saye SE, Wilson HE (2004) Particle size analysis by laser diffraction. Geol Soc, Lond Spec Publ 232:63–73. https://doi.org/10.1144/GSL.SP.2004.232.01.08
Bolea-Fernandez E, Rua-Ibarz A, Velimirovic M, Tirez K, Vanhaecke F (2020) Detection of microplastics using inductively coupled plasma-mass spectrometry (ICP-MS) operated in singleevent mode. J Anal at Spectrom 35:455–460. https://doi.org/10.1039/C9JA00379G
Brandes E, Henseler M, Kreins P (2021) Identifying hot-spots for microplastic contamination in agricultural soils-a spatial modeling approach for Germany. Environ Res Lett 16:104041. https://doi.org/10.1088/1748-9326/ac21e6
Castañeda RA, Avlijas S, Simard MA, Ricciardi A (2014) Microplastic pollution in St. Lawrence river sediments. Can J Fish Aquat Sci 71:1767–1771. https://doi.org/10.1139/cjfas-2014-0281
Ceccarini A, Corti A, Erba F, Modugno F, La Nasa J, Bianchi S, Castelvetro V (2018) The hidden microplastics: new insights and figures from the thorough separation and characterization of microplastics and of their degradation byproducts in coastal sediments. Environ Sci Technol 52:5634–5643
Cervantes-Avilés P, Huang Y, Keller AA (2019) Incidence and persistence of silver nanoparticles throughout the wastewater treatment process. Water Res 156:188–198. https://doi.org/10.1016/j.watres.2019.03.031
Corami F, Rosso B, Bravo B, Gambaro A, Barbante C (2020) A novel method for purification, quantitative analysis and characterization of microplastic fibers using Micro-FTIR. Chemosphere 238:124564
Corradini F, Bartholomeus H, Huerta Lwanga E, Gertsen H, Geissen V (2019) Predicting soil microplastic concentration using vis-NIR spectroscopy. Sci Total Environ 650:922–932
Cózar A, Echevarría F, González-Gordillo JI, Irigoien X, Ubeda B, Hernández-León S, Palma AT, Navarro S, García-de-Lomas J, Ruiz A, Fernández-de-Puelles ML, Duarte CM (2014) Plastic debris in the open ocean. Proc Natl Acad Sci 111:10239–10244. https://doi.org/10.1073/pnas.1314705111
de Souza Machado AA, Lau CW, Till J et al (2018) Impacts of microplastics on the soil biophysical environment. Environ Sci Technol 52:9656–9665
Dehghani S, Moore F (2017) Akhbarizadeh, R. Microplastic pollution in deposited urban dust, Tehran metropolis. Iran Environ Sci Pollut Res Int 24:20360–20371
Dowarah K, Devipriya SP (2019) Microplastic prevalence in the beaches of Puducherry, India and its correlation with fishing and tourism/recreational activities. Mar Pollut Bull 148:123–133
Erni-Cassola G, Gibson MI, Thompson RC, Christie-Oleza JA (2017) Lost, but Found with Nile red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 mµm) in environmental samples. Envrion Sci Technol 51:13641–13648. https://doi.org/10.1021/acs.est.7b04512
Ezeonu S, Ogonnaya E, Nweze C (2019) Electrical and structural properties of polystyrene/graphite composite by direct mixing method. Adv Phys Theor Appl. https://doi.org/10.7176/apta/81-03
Fabbri D, Tartari D, Trombini C (2000) Analysis of poly (vinyl chloride) and other polymers in sediments and suspended matter of a coastal lagoon by pyrolysis-gas chromatography-mass spectrometry. Anal Chim Acta 413:3–11. https://doi.org/10.1016/S0003-2670(00)00766-2
Fischer M, Scholz-Böttcher BM (2017) Simultaneous trace identification and quantification of common types of microplastics in environmental samples by pyrolysis-gas chromatography–mass spectrometry. Environ Sci Technol 51:5052–5060. https://doi.org/10.1021/acs.est.6b06362
Galgani F, Hanke G, Werner S, De Vrees L (2013) Marine litter within the European marine strategy framework directive. ICES J Mar Sci 70:1055–1064
Garaba SP, Dierssen HM (2018) An airborne remote sensing case study of synthetic hydrocarbon detection using short wave infrared absorption features identified from marine-harvested macro- and microplastics. Remote Sens Environ 205:224–235
Gaylarde CC, Neto J, da Fonseca EM (2020) Nanoplastics in aquatic systems - are they more hazardous than microplastics? Environ Pollut 272:115950
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3:e1700782
Gros M, Petrović M, Barceló D (2006) Development of a multi-residue analytical methodology based on liquid chromatography–tandem mass spectrometry (LC–MS/MS) for screening and trace level determination of pharmaceuticals in surface and wastewaters. Talanta 70:678–690
Gu Y, Zhao J, Johnson JA (2020) Polymer networks: from plastics and gels to porous frameworks. Angew Chem Int Ed 59:5022–5049
Guen E, Klapetek P, Puttock R, Hay B, Allard A, Maxwell T, Chapuis PO, Renahy D, Davee G, Valtr M et al (2020) SThM-based local thermomechanical analysis: measurement intercomparison and uncertainty analysis. Int J Therm Sci 156:106502
Guo J, Huang X, Xiang L, Wang Y, Li Y, Li H, Cai Q, Mo C, Wong M (2020) Source, migration and toxicology of microplastics in soil. Environ Int 137:105263. https://doi.org/10.1016/j.envint.2019.105263
Guo X, Wang J (2019) The chemical behaviors of microplastics in marine environment: a review. Mar Pollut Bull 142:1–14. https://doi.org/10.1016/j.marpolbul.2019.03.019
Hebner TS, Maurer-Jones MA (2020) Characterizing microplastic size and morphology of photodegraded polymers placed in simulated moving water conditions. Environ Sci Process Impacts 22:398–407
Hendrickson E, Minor EC, Schreiner K (2018) Microplastic abundance and composition in western lake superior as determined via microscopy, Pyr-GC/MS, and FTIR. Environ Sci Technol 52:1787–1796
Hossain MR, Jiang M, Wei Q, Leff LG (2019) Microplastic surface properties affect bacterial colonization in freshwater. J Basic Microbiol 59:54–61
Huang Y, Zhao Y, Wang J, Zhang M, Jia W, Qin X (2019) LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ Pollut 254:112983. https://doi.org/10.1016/j.envpol.2019.112983
Huppertsberg S, Knepper TP (2018) Instrumental analysis of microplastics- benefits and challenges. Anal Bioanal Chem 410:6343–6352. https://doi.org/10.1007/s00216-018-1210-8
Jiménez-Lamana J, Marigliano L, Allouche J, Grassl B, Szpunar J, Reynaud S (2020) A novel strategy for the detection and quantification of nanoplastics by single particle inductively coupled plasma mass spectrometry (ICP-MS). Anal Chem 92:11664–11672. https://doi.org/10.1021/acs.analchem.0c01536
Jung MR, Horgen FD, Orski SV, Rodriguez CV, Beers KL, Balazs GH, Jones TT, Work TM, Brignac KC, Royer SJ et al (2018) Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar Pollut Bull 127:704–716
Junhao C, Xining Z, Xiaodong G, Li Z, Qi H, Siddique KH (2021) Extraction and identification methods of microplastics and nanoplastics in agricultural soil: a review. J Environ Manage 294:112997. https://doi.org/10.1016/j.jenvman.2021.112997
Kaile N, Lindivat M, Elio J, Thuestad G, Crowley QG, Hoell IA (2020) Preliminary results from detection of microplastics in liquid samples using flow cytometry. Front Mar Sci 7:856–867. https://doi.org/10.3389/fmars.2020.552688
Käppler A, Fischer D, Oberbeckmann S, Schernewski G, Labrenz M, Eichhorn KJ, Voit B (2016) Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal Bioanal Chem 408:8377–8391. https://doi.org/10.1007/s00216-016-9956-3
Keller AA, Huang Y, Nelson J (2018) Detection of nanoparticles in edible plant tissues exposed to nano-copper using single-particle ICP-MS. J Nanopart Res 20:1–13. https://doi.org/10.1007/s11051-018-4192-8
Kniggendorf AK, Wetzel C, Roth B (2019) Microplastics detection in streaming tapwater with raman spectroscopy. Sensors 19:1839
Laborda F, Gimenez-Ingalaturre AC, Bolea E, Castillo JR (2019) Single particle inductively coupled plasma mass spectrometry as screening tool for detection of particles. Spectroc Acta Pt B-Atom Spectr 159:105654. https://doi.org/10.1016/j.sab.2019.105654
Laborda F, Trujillo C, Lobinski R (2021) Analysis of microplastics in consumer products by single particle-inductively coupled plasma mass spectrometry using the carbon-13 isotope. Talanta 221:121486. https://doi.org/10.1016/j.talanta.2020.121486
Lai Y, Dong L, Li Q, Li P, Hao Z, Yu S, Liu J (2021) Counting nanoplastics in environmental waters by single particle inductively coupled plasma mass spectroscopy after cloud-point extraction and in situ labeling of gold nanoparticles. Environ Sci Technol 55:4783–4791. https://doi.org/10.1021/acs.est.0c06839
Lebreton B, Slat F, Ferrar B, Sainte Rose J, Aitken R, Marthouse S, Hajbane S, Cunsolo A, Schwarz A, Levivier K, Noble Debeljak H, Aral R, Schoeneich Argent R, Bramini R, J. (2018) Evidence that the great pacific garbage patch is rapidly accumulating plastic. Sci Rep 8:4666
Li C, Gao Y, He S, Chi H, Li Z, Zhou X, Yan B (2021) Quantification of nanoplastic uptake in cucumber plants by pyrolysis gas chromatography/mass spectrometry. Environ Sci Technol Lett 8:633–638. https://doi.org/10.1021/acs.estlett.1c00369
Li HR, La Guardia MJ, Liu HH, Hale RC, Mainor TM, Harvey E, Sheng GY, Fu JM, Peng PA (2019) Brominated and organophosphate flame retardants along a sediment transect encompassing the Guiyu, China e-waste recycling zone. Sci Total Environ 646:58–67. https://doi.org/10.1016/j.scitotenv.2018.07.276
Li J, Zhang K, Zhang H (2018) Adsorption of antibiotics on microplastics. Environ Pollut 237:460–467. https://doi.org/10.1016/j.envpol.2018.02.050
Li X, Chen L, Ji Y, Li M, Dong B, Qia G, Zhou J, Dai X (2020) Effects of chemical pretreatments on microplastic extraction in sewage sludge and their physicochemical characteristics. Water Res 171:115379
Lithner D, Larsson A, Dave G (2011) Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci Total Environ 409(18):3309–3324. https://doi.org/10.1016/j.scitotenv.2011.04.038
Liu W, Zhao Y, Shi Z, Li Z, Liang X (2020) Ecotoxicoproteomic assessment of microplastics and plastic additives in aquatic organisms: a review. Comp Biochem Physiol Part D Genomics
Liu FF, Liu GZ, Zhu ZL, Wang SC, Zhao FF (2019) Interactions between microplastics and phthalate esters as affected by microplastics characteristics and solution chemistry. Chemosphere 214:688–694. https://doi.org/10.1016/j.chemosphere.2018.09.174
Liu Y, Li R, Yu J, Ni F, Sheng Y, Scircle A, Cizdziel JV, Zhou Y (2021) Separation and identification of microplastics in marine organisms by TGA-FTIR-GC/MS: A case study of mussels from coastal China. Environ Pollut 272:115946
Löder MGJ, Gerdts G (2015) Methodology used for the detection and identification of microplastics-a critical appraisal. In: Bergmann M, Gutow L, Klages M (eds) Marine anthropogenic litter, Springer International Publishing: Cham, 201–227
Luo H, Xiang Y, Li Y, Zhao Y, Pan X (2021a) Photocatalytic aging process of Nano-TiO2 coated polypropylene microplastics: Combining atomic force microscopy and infrared spectroscopy (AFM-IR) for nanoscale chemical characterization. J Hazard Mater 404:124159
Luo H, Xiang Y, Zhao Y, Li Y, Pan X (2020) Nanoscale infrared, thermal and mechanical properties of aged microplastics revealed by an atomic force microscopy coupled with infrared spectroscopy (AFM-IR) technique. Sci Total Environ 744:140944
Luo H, Zeng Y, Zhao Y, Xiang Y, Li Y, Pan X (2021b) Effects of advanced oxidation processes on leachates and properties of microplastics. J Hazard Mater 413:125342
Ma Z, Wang J, Yang Y, Zhang Y, Zhao C, Yu Y, Wang S (2018) Comparison of the thermal degradation behaviors and kinetics of palm oil waste under nitrogen and air atmosphere in TGA-FTIR with a complementary use of model-free and model-fitting approaches. J Anal Appl Pyrolysis 134:12–24
Majewsky M, Bitter H, Eiche E, Horn H (2016) Determination of microplastic polyethylene (PE) and polypropylene (PP) in environmental samples using thermal analysis (TGA-DSC). Sci Total Environ 568:507–511. https://doi.org/10.1016/j.scitotenv.2016.06.017
Mintenig SM, Int-Veen I, Löder MGJ, Primpke S, Gerdts G (2017) Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res 108:365–372
Morgado V, Gomes L, Bettencourt da Silva RJN, Palma C (2021) Validated spreadsheet for the identification of PE, PET, PP and PS microplastics by micro-ATR-FTIR spectra with known uncertainty. Talanta 234:122624
Mossotti R, Dalla Fontana G, Anceschi A, Gasparin E, Battistini T (2021) Preparation and analysis of standards containing microfilaments/microplastic with fibre shape. Chemosphere 270:129410
Moura DS, Pestana CJ, Moffat CF, Hui J, Irvine JT, Lawton LA (2023) Characterisation of microplastics is key for reliable data interpretation. Chemosphere 331:138691. https://doi.org/10.1016/j.chemosphere.2023.138691
Mu X, Qi S, Liu J, Yuan L, Huang Y, Xue J, Qian L, Wang C, Li Y (2022) Toxicity and behavioral response of zebrafish exposed to combined microplastic and bisphenol analogues. Environ Chem Lett 20:41–48. https://doi.org/10.1007/s10311-021-01320-w
Muller YK, Wernicke T, Pittroff M, Witzig CS, Storck FR, Klinger J, Zumbulte N (2020) Microplastic analysis-are we measuring the same? Results on the first global comparative study for microplastic analysis in a water sample. Anal Bioanal Chem 412:555–560
Müsellim E, Tahir MH, Ahmad MS, Ceylan S (2018) Thermokinetic and TG/DSC-FTIR study of pea waste biomass pyrolysis. Appl Therm Eng 137:54–61
Naqash N, Prakash S, Kapoor D, Singh R (2020) Interaction of freshwater microplastics with biota and heavy metals: a review. Environ Chem Lett 18:1813–1824. https://doi.org/10.1007/s10311-020-01044-3
Ng KT, Rapp-Wright H, Egli M, Hartmann A, Steele JC, Sosa-Hernández JE, Barron LP (2020) High-throughput multi-residue quantification of contaminants of emerging concern in wastewaters enabled using direct injection liquid chromatography-tandem mass spectrometry. J Hazard Mater 398:122933
Paul A, Wander L, Becker R, Goedecke C, Braun U (2019) High-throughput NIR spectroscopic (NIRS) detection of microplastics in soil. Environ Sci Pollut Res Int 26:7364–7374
Peng C, Tang X, Gong X, Dai Y, Sun H, Wang L (2020) Development and application of a mass spectrometry method for quantifying nylon microplastics in environment. Anal Chem 92:13930–13935
Pirsaheb M, Hossini H, Makhdoumi P (2020) Review of microplastic occurrence and toxicological effects in marine environment: Experimental evidence of inflammation. Process Saf Environ Protect 142:1–14
Prata JC, da Costa JP, Duarte AC, Rocha-Santos T (2019) Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal Chem 110:150–159
Primpke S, Wirth M, Lorenz C, Gerdts G (2018) Reference database design for the automated analysis of microplastic samples based on Fourier transform infrared (FTIR) spectroscopy. Anal Bioanal Chem 410:5131–5141
Queiroz A, Pedroso GB, Kuriyama SN, Fidalgo-Neto AA (2020) Suband supercritical water for chemical recycling of plastic waste. Curr Opin Green Sustain Chem 25:100364. https://doi.org/10.1016/j.cogsc.2020.100364
Rocha-Santos T, Duarte AC (2015) A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. TrAC Trends Anal Chem 65:47–53. https://doi.org/10.1016/j.trac.2014.10.011
Rodriguez Chialanza M, Sierra I, Perez Parada A, Fornaro L (2018) Identification and quantitation of semi-crystallinemicroplastics using image analysis and differential scanning calorimetry. Environ Sci Pollut Res Int 25:16767–16775
Sabri NH, Muhammad A, Abdul Rahim NH, Roslan A, Abu Talip AR (2021) Feasibility study on co-pyrolyzation of microplastic extraction in conventional sewage sludge for the cementitious application. Mater Today Proc 46:2112–2117
Sancataldo G, Avellone G, Vetri V (2020) Nile Red lifetime reveals microplastic identity. Environ Sci Process Impacts 22:2266–2275
Schymanski D, Goldbeck C, Humpf HU, Fürst P (2018) Analysis of microplastics in water by micro-Raman spectroscopy: release of plastic particles from different packaging into mineral water. Water Res 129:154–162. https://doi.org/10.1016/j.watres.2017.11.011
Shahzad I, Wittchen S, Cepus V (2019) In situ migration analysis and diffusion coefficient determination of bio-based plasticizer from NBR using FTIR-ATR and estimation of migrated plasticizer contents by TGA analysis. Macromol Symp
Shim WJ, Hong SH, Eo SE (2017) Identification methods in microplastic analysis: a review. Anal Methods 9:1384–1391. https://doi.org/10.1039/C6AY02558G
Silva AB, Bastos AS, Justino CIL, da Costa JP, Duarte AC, Rocha-Santos TAP (2018) Microplastics in the environment: challenges in analytical chemistry—a review. Anal Chim Acta 1017:1–19
Simmerman CB, Coleman Wasik JK (2020) The effect of urban point source contamination on microplastic levels in water and organisms in a cold-water stream. Limnol Oceanogr Lett 5:137–146
Simon M, van Alst N, Vollertsen J (2018) Quantification of microplastic mass and removal rates at wastewater treatment plants applying Focal Plane Array (FPA)-based Fourier Transform Infrared (FT-IR) imaging. Water Res 142:1–9
Singh N, Duanb H, Tang Y (2020) Toxicity evaluation of E-waste plastics and potential repercussions for human health. Environ Int 137:105559. https://doi.org/10.1016/j.envint.2020.105559
Song YK, Hong SH, Jang M, Han GM, Shim WJ (2015) Occurrence and distribution of microplastics in the sea surface microlayer in Jinhae Bay, South Korea. Arch Environ Contam Toxicol 69:279–287. https://doi.org/10.1007/s00244-015-0209-9
Sorasan C, Edo C, González-Pleiter M, Fernández-Piñas F, Leganés F, Rodríguez A, Rosal R (2021) Generation of nanoplastics during the photoageing of low-density polyethylene. Environ Pollut 289:117919. https://doi.org/10.1016/j.envpol.2021.117919
Sun J, Dai X, Wang Q, van Loosdrecht MCM, Ni BJ (2019) Microplastics in wastewater treatment plants: detection, occurrence and removal. Water Res 152:21–37
Abadi TR, Abtahi Z, B, Grossart HP, Khodabandeh S, (2021) Microplastic content of Kutum fish, Rutilus frisii kutum in the southern Caspian Sea. Sci Total Environ 752:141542
Talvitie J, Mikola A, Koistinen A, Set¨al¨a O, (2017) Solutions to microplastics pollution – removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res 123:401–407. https://doi.org/10.1016/j.watres.2017.07.005
Thakur B, Singh J, Singh J, Angmo D, Vig AP (2023) Identification and characterization of extracted microplastics from agricultural soil near industrial area: FTIR and X‐ray diffraction method. Environ Qual Manag
Tsering T, Viitala M, Hyvönen M, Reinikainen SP, Mänttäri M (2022) The assessment of particle selection and blank correction to enhance the analysis of microplastics with Raman microspectroscopy. Sci Total Environ 842:156804. https://doi.org/10.1016/j.scitotenv.2022.156804
Tsukame T, Ehara Y, Shimizu Y, Kutsuzawa M, Saitoh H, Shibasaki Y (1997) Characterization of microstructure of polyethylenes by differential scanning calorimetry. Thermochim Acta 299:27–32. https://doi.org/10.1016/S0040-6031(97)00132-9
Tunali M, Uzoefuna EN, Tunali MM, Yenigun O (2020) Effect of microplastics and microplastic-metal combinations on growth and chlorophyll a concentration of Chlorella vulgaris. Sci Total Environ 743:140479
Turner A, Wallerstein C, Arnold R (2019) Identification, origin and characteristics of bio-bead microplastics from beaches in western Europe. Sci Total Environ 664:938–947. https://doi.org/10.1016/j.scitotenv.2019.01.281
Uddin S, Fowler SW, Saeed T (2020) Microplastic particles in the Persian/Arabian Gulf - a review on sampling and identification. Mar Pollut Bull 154:111100
Veerasingam S, Ranjani M, Venkatachalapathy R, Bagaev A, Mukhanov V, Litvinyuk D, Vethamony P (2021) Contributions of Fourier transform infrared spectroscopy in microplastic pollution research: a review. Crit Rev Environ Sci Technol 51(22):2681–2743
Vermeiren P, Munoz C, Ikejima K (2020) Microplastic identification and quantification from organic rich sediments: a validated laboratory protocol. Environ Pollut 262:114298
Vianello AA (2020) Journey into microplastic analysis using FTIR spectroscopy. Ph.D. Thesis, Aalborg University, Aalborg, Denmark
Vitali C, Janssen HG, Ruggeri FS, Nielen MW (2022) Rapid single particle atmospheric solids analysis probe-mass spectrometry for multimodal analysis of microplastics. Anal Chem 95:1395–1401
Wagner J, Wang Z, Ghosal S, Rochman C, Gassel M, Wall S (2017) Novel method for the extraction and identification of microplastics in ocean trawl and fish gut matrices. Anal Methods 9:1479–1490. https://doi.org/10.1039/C6AY02396G
Wagner J, Wang ZM, Ghosal S, Murphy M, Wall S, Cook AM, Robberson W, Allen H (2019) Nondestructive extraction and identification of microplastics from freshwater sport fish stomachs. Environ Sci Technol 53:14496–14506
Wang W, Gao H, Jin S, Li R, Na G (2019) The ecotoxicological effects of microplastics on aquatic food web, from primary producer to human: a review. Ecotox Environ Safe 173:110–117. https://doi.org/10.1016/j.ecoenv.2019.01.113
Wang F, Wang Q, Adams CA, Sun Y, Zhang S (2022) Effects of microplastics on soil properties: current knowledge and future perspectives. J Hazard Mater 424:127531. https://doi.org/10.1016/j.jhazmat.2021.127531
Wang L, Zhang J, Hou S, Sun H (2017) A simple method for quantifying polycarbonate and polyethylene terephthalate microplastics in environmental samples by liquid chromatography–tandem mass spectrometry. Environ Sci Technol Lett 4:530–534. https://doi.org/10.1021/acs.estlett.7b00454
Watteau F, Dignac MF, Bouchard A, Revallier A, Houot S (2018) Microplastic detection in soil amendedwith municipal solid waste composts as revealed by transmission electronic microscopy and Pyrolysis/GC/MS. Front Sustain Food Syst 2:81
Weidner SM, Trimpin S (2010) Mass spectrometry of synthetic polymers. Anal Chem 82:4811–4829. https://doi.org/10.1021/ac101080n
Witkowski H, Koniorczyk M (2018) New sampling method to improve the reliability of FTIR analysis for Self-Compacting Concrete. Constr Build Mater 172:196–203
Witzig CS, Foldi C, Worle K, Habermehl P, Pittroff M, Muller YK, Lauschke T, Fiener P, Dierkes G, Freier KP et al (2020) When good intentions go bad-false positive microplastic detection caused by disposable gloves. Environ Sci Technol 54:12164–12172
Wright SL, Levermore JM, Kelly FJ (2019) Raman spectral imaging for the detection of inhalable microplastics in ambient particulate matter samples. Environ Sci Technol 53:8947–8956. https://doi.org/10.1021/acs.est.8b06663
Zainuddin Z, Syuhada, (2020) Study of analysis method on microplastic identification in bottled drinkingwater. Macromol Symp 391:1900195
Zhang J, Wang L, Kannan K (2021) Quantitative analysis of polyethylene terephthalate and polycarbonate microplastics in sediment collected from South Korea. Jpn USA Chemosp 279:130551
Zhang J, Wang L, Halden RU, Kannan K (2019) Polyethylene terephthalate and polycarbonate microplastics in sewage sludge collected from the United States. Environ Sci Technol Lett 6:650–655
Zhang J, Tian K, Lei C, Min S (2018) Identification and quantification of microplastics in table sea salts using micro-NIR imaging methods. Anal Methods 10:2881–2887
Zhou L, Wang T, Qu G, Jia H, Zhu L (2020) Probing the aging processes and mechanisms of microplastic under simulated multiple actions generated by discharge plasma. J Hazard Mater 398:122956
Zhu F, Zhu C, Wang C et al (2019) Occurrence and ecological impacts of microplastics in soil systems: a review. Bull Environ Contam Toxicol 102:741–749
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval is not required for this study.
Consent for publication
Not required for this study.
Competing interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Randhawa, J.S. Advanced analytical techniques for microplastics in the environment: a review. Bull Natl Res Cent 47, 174 (2023). https://doi.org/10.1186/s42269-023-01148-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-023-01148-0