Abstract
Background
Anti-glomerular basement membrane (GBM) disease and ANCA-associated vasculitis (AAV) diseases are rare. It is associated with variable renal manifestations and increased mortality, thus requiring early aggressive treatment to minimize adverse outcomes and improve prognosis.
Case presentation
We present the case of a male patient with 1-month onset of asthenia, adynamia, oliguria, and weight loss. Initial laboratory findings were indicative of severe kidney dysfunction. The urinalysis showed active sediment, but the urinary tract ultrasound was unaltered. As these findings were consistent with rapidly progressive glomerulonephritis, he received steroid pulses, and given the severity of the condition, renal replacement therapy was initiated. Other diagnostic tests revealed MPO-ANCA antibody levels of 26 mg/dl, pANCAs 1/320, and anti-GBM of 8 mg/dl. Kidney biopsy evidenced necrotizing glomerulonephritis with extracapillary proliferation in 90% of the glomeruli. The patient received plasma exchange (PE) therapy and intravenous (IV) cyclophosphamide (CYC) cycles; however, he presented with severe alveolar hemorrhage requiring the completion of 21 PE sessions and 3 CYC boluses. Pulmonary symptoms resolved, but the patient persisted dependent on dialysis. During the outpatient follow-up, monthly CYC were prescribed until circulating antibody levels were normal; however, the patient did not recover full kidney function and remained dependent on renal support.
Conclusions
Anti-GBM and AAV diseases are rare; therefore, anti-GBM antibodies should be screened simultaneously in patients with ANCA positive, especially in older patients, due to the early morbidity and mortality typical of anti-GBM disease with comparable disease severity it represents.
Similar content being viewed by others
Background
Anti-glomerular basement membrane (GBM) disease, also known as "Goodpasture syndrome," is an autoimmune vasculitis that affects small vessels through the deposit of IgG antibodies against the glomerular and alveolar basal membranes which are visualized as linear deposits in the GBM. The target of these autoantibodies is the non-collagen-1 domain of type IV collagen on the GBM, which presents changes in the structure of the α-345 hexamer, leading to a conformational change that triggers an autoimmune response (Gulati and McAdoo 2018). ANCA-associated vasculitis (AAVs) is a necrotizing vasculitis with few-to-none immune deposits, also affecting small vessels, and is associated with specific antibodies for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA) (Jennette and Nachman 2017).
Anti-GBM and AAV diseases are different, but both can have kidney and lung manifestations and can be associated with lung–kidney syndrome (Philip et al. 2021). They present clinically as rapidly progressive glomerulonephritis (RPGN), often accompanied by alveolar bleeding (McAdoo et al. 2017; Levy et al. 2001). It constitutes a medical emergency and is associated with increased morbidity and mortality. When patients present both types of antibodies, the condition is called "double positive."
Whereas anti-GBM disease and AAVs are rare, their coexistence is a well-recognized entity that occurs more frequently than expected by chance (McAdoo et al. 2017). The two antibodies are antigenically different, and the phenomenon is not due to cross-reactivity (Short et al. 1995). It has been observed that these "double-positive" patients have a higher probability of presenting glomerulosclerosis and severe interstitial fibrosis with tubular atrophy, uncertain prognosis, and a higher risk of relapse (McAdoo et al. 2017; Zahir et al. 2021).
In current clinical practice, the diagnosis is obtained through serological tests that seek to detect the presence of antibodies and kidney biopsy to identify their specific histopathological changes (McAdoo et al. 2017). The treatment aims to rapidly clear circulating autoantibodies with plasma exchange therapy (PET), prevent further antibody production, and suppress inflammation using immunosuppression and corticosteroids (Prendecki and Pusey 2019).
This report aims to describe the case of a "double-positive" patient presenting with lung–kidney syndrome and to review the literature to describe the disease.
Case presentation
A 69-year-old male patient with a past medical history of smoking of 5.5 pack-years until 29 years ago presented with a 1-month onset of asthenia, adynamic, emesis, oliguria, and weight loss. Serological laboratories at admission revealed severe kidney dysfunction: creatinine 10.39 mg/dL, BUN 91.9 mg/dL, urinalysis showed proteinuria 150 mg/dL, hematuria 450 uL, hematies and leukocyturia, cell blood count with normocytic anemia, moderate hyperkalemia, and blood gases with metabolic acidosis with elevated anion gap but without hyperlactatemia. X-ray and tomography of the chest were normal, and kidney ultrasound showed kidneys of standard shape, size, and echogenicity, without dilatation of collecting systems.
Clinical symptoms, test results, and imaging findings were consistent with a rapidly progressive glomerulonephritis. The initial treatment included 500 mg IV of methylprednisolone pulses for 72 h, previous deworming, and renal replacement therapy.
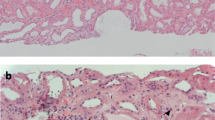
Further laboratory tests were obtained to establish the etiology: ANAs and anti-DNA (-) were negative, normal C3 and C4 complement, and positive ANCAsp of 1/320 were found. The kidney biopsy described a necrotizing glomerulonephritis with cellular and fibrocellular extracapillary proliferation in 90% of the glomeruli, accompanied by lysis of the basement membrane of Bowman's capsule, severe tubulointerstitial nephritis, and minimal changes of chronicity (Figs. 1, 2). The IFI described linear deposits of IgG +++, kappa +++, and lambda +++ in the capillary basement membrane, and the electron microscopy ruled out the presence of immune complexes. The serological studies confirmed the presence of anti-GBM IgG antibodies (8 mg/dl), and ANCAS by ELISA showed an MPO-ANCA of 26.3 units.
We considered that the patient was undergoing anti-glomerular basement membrane disease with the coexistence of vasculitis associated with pANCA and MPO, limited to the kidney. The treatment of choice was PET and cyclophosphamide (Fig. 3).
After 12 sessions of PET, the patient presented with cough and hemoptysis, dyspnea, and required supplemental oxygen; he was monitored in the ICU but did not require ventilatory support. The chest's high-resolution tomography (H-RCT) evidenced diffuse alveolar bleeding and pleural effusion (Fig. 4). The fiberoptic bronchoscopy demonstrated traces of bleeding in all bronchial segments without endobronchial lesions, an extended bronchoalveolar lavage with a hemorrhagic background with the presence of few squamous cells, abundant macrophages (80%) and polymorphonuclear neutrophils (20%), otherwise negative for Neumocistis Jirovecii, BAAR, fungi, or tumors. At this point, the level of anti-GBM IgG antibodies was 2.0 mg/dl. The patient continued receiving treatment with systemic steroids, intravenous cyclophosphamide every 15 days for the first three doses, followed by monthly doses, and prolonged PET until the completion of 21 sessions.
After a prolonged hospital stay and strict therapeutic protocols, the patient presented satisfactory progress, improved breathing mechanics, resolution of hemoptysis and stabilization of hemoglobin levels, and negative ANCAs; thus, anti-GBM antibodies remained positive. The patient was discharged dependent on renal replacement therapy. During the outpatient follow-up, the patient continued stable with no new hospitalizations; however, he continued with dialytic dependence after five months of treatment with IV cyclophosphamide and steroids. It is essential to highlight that at the last serological control, the anti-GBM antibody level remained negative with cyclophosphamide management.
Discussion
The double-positivity phenomenon is a rare condition that has been only described in short series (Philip et al. 2021). Previous reports describe a prevalence of 5–14% in patients with vasculitis, 20–40% in patients with anti-GBM, and 2% in patients with rapidly progressive glomerulonephritis within the Caucasian population, and a prevalence of 9.36% in patients with vasculitis among the Asian population (Zhao et al. 2007). The prevalence in Latin America and the Hispanic population remains unknown.
The association mechanisms between AAV and anti-GBM suggest that AAV may act as a trigger for anti-GBM disease. In a study performed using the serum of 30 patients diagnosed with anti-GBM disease, almost all patients presented with either PR3-ANCA or MPO-ANCA before developing anti-GBM antibodies (Olson et al. 2011). It appears like the initial pathogenic element is the endothelial damage associated with the presence of ANCAs, allowing the exposure of GBM antigens that are not usually exposed, with the consequent formation of autoantibodies against these antigens (Segelmark and Hellmark 2019). The production of these autoantibodies at a low level may persist for years before an acute rise presents and weeks to months before the onset of clinical symptoms (Olson et al. 2011).
A systematic review of the literature documented a mean age of presentation of 57.1 (4–86) years, with 51.8% male patients, a mean duration of symptoms of 15.8 ± 36 weeks, and 66% of patients showing constitutional symptoms such as adynamia, anorexia, weight loss, and fever, like patients with AAV. The clinical presentation is frequently severe as 91.8% of patients developed acute kidney injury, with a mean creatinine level at diagnosis of 9.8 (0.79 -38.4) mg/dl; 64% required dialysis; and 50.7% presented alveolar hemorrhage at the initial presentation (Philip et al. 2021).
One of the most extensive series that was conducted in Northern Europe and published by McAdoo et al. (2017) found that the severity of the disease in patients with anti-GBM disease alone was comparable to "double-positive" patients as the presentation of alveolar hemorrhage. In "double-positive" patients, the frequency of MPO-ANCA antibodies is higher than PR3-ANCA (70% vs. 48% p < 0.01); this has been documented previously in another study where 82% of patients had MPO-ANCA antibodies (Levy et al. 2004). Likewise, in the kidney biopsy, there was a greater tendency to find sclerotic glomerular lesions (median 15% vs. 0% p = 0.188) as well as more significant evidence of interstitial fibrosis and tubular atrophy (median 27% vs. 5% p < 0.001). Patients with MPO-ANCA present with more chronic injury, evidenced by more extensive interstitial fibrosis, tubular atrophy, glomerulosclerosis, and fibrous or fibrocellular crescents (Zonozi et al. 2018). Advanced fibrosis may result from delayed diagnosis and different mechanisms of injury in MPO-ANCA compared to PR3-ANCA patients (Cortazar and Niles 2020). The renal biopsy of our patient showed mainly extracapillary proliferations with cellular and incipient fibrocellular crescents. To find lesions in the same state of development in all or nearly all glomeruli, at least from the histopathological point of view, should raise concern about the mechanism of injury. This means it happened at the same time in an aggressive manner, as described in patients with the presence of anti-GBM antibodies. When the mechanism of injury is through multiple hits, the glomerular crescentic lesions are in different states of evolution. (Cellular, fibrocellular and fibrous crescents are present at the same time.)
The features of kidney injury in "double-positive" patients correspond mainly to those observed in patients with the anti-GBM disease, with active and diffuse crescent-forming glomerulonephritis, almost always associated with linear deposits of IgG (Philip et al. 2021). A study conducted by Zhao et al. found a high percentage of glomeruli with crescents > 85% (p = 0.034) in "double-positive" patients was associated with poor prognosis (Zhao et al. 2007). This might indicate that the proportion of regular crescents and glomeruli in anti-GBM disease are prognostic predictors, as patients with crescents of more than 90% and dialysis dependence at presentation are less likely to recover renal function (Levy et al. 2001). A prognostic classification based on histopathological findings for ANCA-associated glomerulonephritis might be proposed. However, whether these observations apply to "double-positive" patients is uncertain (McAdoo et al. 2017). Given that anti-GBM disease is the dominant phenotype in early disease of double-positive cases, histology could aid in deciding how aggressive the treatment of renal manifestations should be. Nonetheless, aggressive treatment modalities should be implemented, regardless of renal prognosis, in the presence of alveolar hemorrhage.
As mentioned previously, the prognosis of this entity is poor. In another systematic review, the authors found that only 38.7% of patients remain free of dialysis during the first year of follow-up. The relapse rate per year of the "double positives" is higher than patients with isolated anti-GBM but lower than AAV patients, with rates of 28.9%, 0 to 3%, and 34.1 to 45%, respectively. Correspondingly, the 5-year mortality rate is higher among "double positives" than isolated AAVs or anti-GBM disease, with rates of 44.4%, 26%, and 8%, respectively (Philip et al. 2021). The survival rate is overall affected if the presentation of the disease is severe, determined by the requirement of renal replacement therapy and alveolar hemorrhage (McAdoo et al. 2017).
Clinically, the "double-positivity" phenomenon implies that these patients experience the early morbidity and mortality typical of anti-GBM disease with comparable disease severity. At the same time, in the long term, the rate of disease relapses is similar to AVV (McAdoo et al. 2017; Levy et al. 2004; Geetha and Jefferson 2020). An early and accurate diagnosis of "double positivity" is essential to establish a therapeutic strategy. As the initial presentation is typically severe, the patients might receive the same approach as those with isolated anti-GBM (Prendecki and Pusey 2019). In addition to a combination of steroids and cyclophosphamide, removing anti-GBM antibodies by plasma exchange therapy (PET) might improve patient survival (Zhao et al. 2007). The rationale for PET arises from the pathogenic role of antibodies, leading to a rapid decrease in anti-GBM and ANCA titers, with the resolution of alveolar hemorrhage and other features of vasculitis. In most patients undergoing PET and immunosuppression, anti-GBM antibodies drop to undetectable levels in 2 weeks; therefore, the minimum length of PET should be 10 to 20 days. In the context of alveolar hemorrhage and kidney biopsy, plasma should be used as replacement fluid (Padmanabhan et al. 2019). In the present case, despite the initial aggressive immunosuppressive treatment and the use of PET (21 PET sessions and four months of management with cyclophosphamide plus steroid), the patient progressed to end-stage kidney disease; this might be related to the severity of the initial presentation (need for renal replacement therapy, alveolar hemorrhage) and the involvement of 90% of the glomeruli as shown in the histology.
Conservative management should be considered in patients without alveolar hemorrhage who are oliguric and have advanced kidney disease requiring dialysis, especially if they have a very high proportion of crescents (85—100%) on renal biopsy (KDIGO 2021).
Unlike anti-GBM disease alone, "double-positive" patients will have relapse rates comparable to AAV patients; therefore, immunosuppressive management should be administered as maintenance therapy to prevent relapses (KDIGO 2021; McAdoo et al. 2017).
Conclusions
Anti-GBM and AAV diseases are rare; therefore, anti-GBM antibodies should be screened simultaneously in patients with ANCA positive, especially in older patients. These patients present a hybrid phenotype at presentation, experiencing early morbidity and mortality typical of anti-GBM disease with comparable disease severity. At the same time, the long-term course is characterized by relapses common to AAV disease. Predictors of poor outcomes include the severity of renal injury (dialysis at presentation), alveolar hemorrhage, and a high percentage of glomeruli with crescents. All of these must be considered when determining the therapeutic approach since the main objective of the treatment will be to limit the damage to other organs but not to preserve renal function.
Availability of data and materials
All the data and supporting files have been presented within the case report.
Abbreviations
- GBM:
-
Glomerular basement membrane
- AAV:
-
ANCA-associated vasculitis
- ANCA:
-
Anti-neutrophil cytoplasmic antibodies
- MPO:
-
Myeloperoxidase
- PR3:
-
Proteinase 3
- RPGN:
-
Rapidly progressive glomerulonephritis
- PET:
-
Plasma exchange therapy
- H-RCT:
-
High-resolution tomography
References
Cortazar FB, Niles JL (2020) The fate of plasma exchange and glucocorticoid dosing in ANCA-associated vasculitis after PEXIVAS. Am J Kidney Dis 76(4):595–597
Geetha D, Jefferson JA (2020) ANCA-associated vasculitis: core curriculum 2020. Am J Kidney Dis 75(1):124–137
Group KDIGO GDW (2021) KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int 100(4S):S1–S276
Gulati K, McAdoo SP (2018) Anti-glomerular basement membrane disease. Rheumatic Dis Clin N Am 44(4):651–673. https://doi.org/10.1016/j.rdc.2018.06.011
Jennette JC, Nachman PH (2017) ANCA glomerulonephritis and vasculitis. Clin J Am Soc Nephrol 12(10):1680–1691
Levy JB, Turner AN, Rees AJ, Pusey CD (2001) Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med 134(11):1033–1042
Levy JB, Hammad T, Coulthart A, Dougan T, Pusey CD (2004) Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int 66(4):1535–1540
McAdoo SP, Tanna A, Hrušková Z, Holm L, Weiner M, Arulkumaran N et al (2017) Patients double-seropositive for ANCA and anti-GBM antibodies have varied renal survival, frequency of relapse, and outcomes compared to single-seropositive patients. Kidney Int 92(3):693–702
Olson SW, Arbogast CB, Baker TP, Owshalimpur D, Oliver DK, Abbott KC et al (2011) Asymptomatic autoantibodies associate with future anti-glomerular basement membrane disease. J Am Soc Nephrol 22(10):1946–1952
Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E et al (2019) Guidelines on the use of therapeutic apheresis in clinical practice—evidence-based approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J Clin Apher 34(3):171–354
Philip R, Dumont A, Martin Silva N, de Boysson H, Aouba A, Deshayes S (2021) ANCA and anti-glomerular basement membrane double-positive patients: a systematic review of the literature. Autoimmun Rev 20(9):102885
Prendecki M, Pusey C (2019) Plasma exchange in anti-glomerular basement membrane disease. Presse Med 48(11 Pt 2):328–337
Segelmark M, Hellmark T (2019) Anti-glomerular basement membrane disease: an update on subgroups, pathogenesis and therapies. Nephrol Dialysis Transplant 34(11):1826–1832. https://doi.org/10.1093/ndt/gfy327
Short AK, Esnault VL, Lockwood CM (1995) Anti-neutrophil cytoplasm antibodies and anti-glomerular basement membrane antibodies: two coexisting distinct autoreactivities detectable in patients with rapidly progressive glomerulonephritis. Am J Kidney Dis 26(3):439–445
Zahir Z, Wani AS, Prasad N, Jain M (2021) Clinicopathological characteristics and predictors of poor outcome in anti-glomerular basement membrane disease—a fifteen year single center experience. Ren Fail 43(1):79–89
Zhao J, Yang R, Cui Z, Chen M, Zhao MH, Wang HY (2007) Characteristics and outcome of Chinese patients with both antineutrophil cytoplasmic antibody and antiglomerular basement membrane antibodies. Nephron Clin Pract 107(2):c56-62
Zonozi R, Niles JL, Cortazar FB (2018) Renal involvement in antineutrophil cytoplasmic antibody-associated vasculitis. Rheum Dis Clin North Am 44(4):525–543
Acknowledgements
Not applicable.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
DCAR, IRC, and MAHC performed the clinical examination and diagnosis of the patient, as well as manuscript writing. MIM contributed to literature review and manuscript writing. AAFV performed the histological examination of the kidney biopsy and contributed with the revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All required measures were undertaken to preserve the information's confidentiality. All procedures performed in studies involving human participants were in accordance with the ethical standards of Comité de Ética en Investigación- Universidad del Rosario (DVO005-1866-CV1529) at which the studies were conducted and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for publication
Written informed consent was obtained from the patient included in the study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Isaza-Meza, M., Afanador-Rubio, D.C., Huérfano-Castro, M.A. et al. Rapidly progressive glomerulonephritis secondary to anti-GBM disease associated with MPO-ANCA: a case report. Bull Natl Res Cent 47, 42 (2023). https://doi.org/10.1186/s42269-023-01020-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-023-01020-1