Abstract
Background
Heart Study has been operating for more than 40 years, and throughout that time it has found a number of risk variables that interact negatively to have an overall negative effect on cardiovascular disease (CVD) with an estimated 17.9 million deaths per year, CVD is the world's leading cause of death.
Main body
In the current study, we present spectrophotometric, chromatographic analysis and bioanalysis methods for qualitative and quantitative evaluation of 15 drugs, including small and large molecules, that the U.S. FDA approved between 2015 and June 2020 to treat CVD’s and in the current review work, they were presented.
Short conclusion
The review's conclusion is that spectroscopic, chromatographic and bioanalysis methods play important role in quality control and standardization of recently approved drugs from 2015 to 2020 for treating CVD’s in its bulk, pharmaceutical dosage form, synthetic mixture or human/rat plasma.
Similar content being viewed by others
Background
Heart Study has been operating for more than 40 years, and throughout that time it has found a number of risk variables that interact negatively to have an overall negative effect on cardiovascular disease (CVD) (Nabel 2003). Experience has revealed that the best method for preventing coronary heart disease is probably a multifactorial one, one that considers all the risk factors (CHD) (Anderson et al. 1991). According to estimates, 17.9 million annual deaths from CVD each year. The collection of heart and blood vessel disorders known as CVDs includes conditions like coronary heart disease, cerebrovascular disease, rheumatic heart disease, and other illnesses. More than four out of every five CVD deaths result from heart attacks and strokes, and one-third of these deaths occur before the age of 70 (WHO report n.d.).
Premature deaths were significantly more common than premature CVD deaths globally (34%) and in Asia (35%) as well as in Europe (22%) and the USA (23%). Ischemic heart disease (IHD) (47%) and stroke (87%) accounted for the majority of CVD deaths (40%). The number of CVD deaths in Asia increased from 5.6 million to 10.8 million during 1990 and 2019, and the proportion of CVD deaths in all deaths increased from 23 to 35%. Furthermore, crude CVD mortality rates increased steadily for both men and women during 1990 and 2019 (Burden and of Disease Collaborative Network. Global Burden of Disease 2019; Zhao 2021).
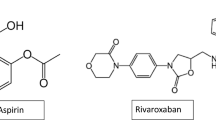
Drug discovery is a multidisciplinary method that is complex and it still presents a wide range of difficulties for the pharmaceutical industry and related sectors (Drews 2000). There were mainly 201 novel molecules authorized for the treatment of CVDs between 1937 and 2013. (Patridge et al. 2016) The US FDA's Centre for Drug Evaluation and Research (CDER) has approved 15 therapeutic medicines for cardiovascular diseases over the past five years, nine of which are small molecules, with the initial three are macromolecules (Table 1 and Fig. 1). The following is a description of the medications approved under this category (Bhutani et al. 2021).
The major goal of developing and validating analytical methods is to demonstrate that they are accurate, specific, precise, and robust for the particular drug (Doltade and Saudagar 2019; Kagawad et al. 2021).
Literature survey reveals that various analytical method have been developed to estimate recently approved drugs from 2015 to 2020 for treating cardiovascular diseases in bulk, tablet dosage form, synthetic mixture and in biological sample. The method consists of UV Spectrophotometric Analysis, Stability indicating RP-HPLC Method, LC/MS/MS, HPTLC, Spectrofluorimetry. Numerous researchers have worked on various spectrophotometric, chromatographic, and bioanalytical analyses, and they have published their findings in a number of journals and scientific databases. A survey of the literature indicated that, as of this writing, no reports on its detailed review about spectrophotometric and chromatographic analysis and bioanalysis of selected recently approved drugs from 2015 to 2020 for treating cardiovascular diseases. Hence, we attempted to complete the current review work since there is a clear need for collective information regarding spectrophotometric, chromatographic, and bioanalytical analysis that will be useful to other researchers and readers. The need to examine and compare the available analytical and bioanalytical tests used to determine these drugs, either alone or in combination, is essential.
Main text
UV Spectroscopy (Verma and Mishra 2018)
Ultraviolet (UV) spectroscopy is an optical spectroscopy technique based on the Beer–Lambert equation, the concentration of the absorbing species in a solution and the path length directly affect the solution's absorbance. It makes use of near-infrared, ultraviolet, and visible light. As a result, it can be used to measure the concentration of the absorber in a solution for a particular path length. Since UV–VIS spectroscopy has been in widespread use for the past 37 years, it has evolved into the most important analytical tool in the modern laboratory. It is important to understand how quickly the absorbance varies with concentration. Other methods could be used in many applications, but none compared to UV–VIS spectroscopy's ease of use, flexibility, precision, speed, and cost-effectiveness. (Table 2).
HPLC methods (LC, RP-HPLC, UPLC) (Saibaba et al. 2016a)
In the pharmaceutical sector, reversed-phase liquid chromatography is the analytical technique that is most frequently utilized liquid chromatographic techniques are used to assess the quality of the drug substance (active pharmaceutical ingredient) and drug product during the drug development process (Table 3).
TLC and HPTLC method (Fenimore and Davis 1981 Feb 1)
Enhanced and improved separation effectiveness and detection limit than thin-layer chromatography (TLC), high-performance thin-layer chromatography (HPTLC) is a sophisticated and automated version of TLC. It is also referred to as flatbed chromatography, planar chromatography, and high-pressure thin-layer chromatography. It is a potent analytical technique that works for both qualitative and quantitative tasks. Depending on the type of solvent solution and adsorbent employed on development plates, separation may be caused through partition, absorption, or both. (Table 4).
LC-MS/MS, LC- MS method (Saibaba et al. 2016b)
Liquid chromatography/Mass Spectrometry (LC/MS) is quickly replacing traditional liquid chromatography as the main method of analysis. It is a powerful analytical technique that combines the liquid chromatography resolving strength and the mass spectrometric detection specificity. Liquid chromatography (LC) is used to separate the components of the sample, and the mass spectrometer is then used to analyze the separated components (MS). The molecular weight, structure, identity, and quantity of particular sample components can be determined using the LC/MS data; charged ions are produced and found by the MS. (Table 5).
Conclusions
Since drug design, bioavailability and safety studies have been greatly influenced by the improvement in quality of life, extremely sensitive and precise analytical techniques are required to meet these objectives. The presented work is focused on the use of various analytical methods such as HPLC (High-Performance Liquid Chromatography), HPTLC (High-Performance Thin-Layer Chromatography), TLC (Thin-Layer Chromatography), UPLC (Ultra Performance Liquid Chromatography), and LC/MS/MS. For the purpose of determining the effectiveness of a medicinal compound in a certain matrix, a critical analytical method should be established for recently approved drugs from 2015 to 2020 for treating cardiovascular disease drug analytes in formulation as well as in API. Various analytical methods detection is appropriate for the examination of recently approved medications from 2015 to 2020 for treating cardiovascular illnesses since it yields precise results at a lower cost than more sophisticated detection methods. This paper provides a summary of the most advanced analytical techniques to estimate the recently approved cardiovascular medications. Analytical chemists would benefit from knowing the essential solvents and their combinations for the tools they have access in the laboratories.
Availability of data and materials
The research work has been carried out by us, and we assure you that it can be provided to you whenever required.
Abbreviations
- HPLC:
-
High-performance liquid chromatography
- HPTLC:
-
High-performance thin-layer chromatography
- UHPLC:
-
Ultra high-performance liquid chromatography
- LC/MS:
-
Liquid chromatography/Mass Spectrometry
References
Agrahari V, Kabra V, Gupta S, Nema RK, Nagar M, Karthikeyan C, Trivedi P (2009) Determination of inherent stability of valsartan by stress degradation and its validation by HPLC. Int J Pharmaceut Clin Res 1(2):77–81
Alamein AA, AM. (2018) Validated eco-friendly chromatographic methods for simultaneous determination of sacubitril and valsartan in spiked human plasma and in pharmaceutical formulation. J Appl Pharmaceut Sci 8(2):011–017
Alexander S, Kumar M (2018) Valsartan. Int J Pharmaceut Clin Res 10(7):186–195
Amara Babu NL, Koganti K, Palakeeti B, Srinivas KS, Rao KP (2021) Development of an efficient stability-indicating LC–MS/MS method for the analysis of selexipag and characterization of its degradation products. Biomed Chromatogr 35(10):e5178
Anderson KM, Odell PM, Wilson PW, Kannel WB (1991) Cardiovascular disease risk profiles. Am Heart J 121(1):293–298
Anjaneyulu N, Kishore RN, Kumar MR, Sneha G (2018) Development and validation of a RP-HPLC method for the simultaneous estimation of valsartan and Sacubitril in rat plasma. Global J Pharmacy Pharmaceut Sci 6(5):105–110
Banda SD (2022) Analytical method development and validation of edoxaban in bulk and pharmaceutical dosage form by rp-hplc
Banu T, Kumar HT, Rao VK, Rao SY (2021) Application of simultaneous equation method for estimation of sacubitril and valsartan in combined dosage form. Asian J Res Chem 14(2):111–114
Bhadru B, Rao VV, Vidhyadhara S (2019) Development and validation of bioanalytical method for the quantitative estimation of selexipag in biological matrices using LC-MS/MS. J Pharm Sci Res 11(7):2722–2727
Bhatia SM, Kokil SU (2009) Determination and validation of valsartan and its degradation products by isocratic HPLC. J Chem Metrol 2009:1–12
Bhutani P, Joshi G, Raja N, Bachhav N, Rajanna PK, Bhutani H, Paul AT, Kumar R (2021) US FDA approved drugs from 2015–June 2020: a perspective. J Med Chem 64(5):2339–2381
Cardiovascular disease. WHO report. Available at: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1
Chinthala K, Kancherla P, Kumar P (2017) 2017, Bioanalytical method development and validation for quantitative estimation of valsartan by LC-MS/MS in human plasma. Asian J Chem 29(7):1482–1486
Chunduri RHB, Dannana GS (2016) Development and Validation of a Reliable and Rapid LC-MS/MS method for simultaneous quantification of Sacubitril and Valsartan in Rat Plasma and its application to a Pharmacokinetic study. Biomed Chromatogr 9:1467–1475
Damle MC, Bagwe RA (2015) Development and validation of stability-indicating hptlc method for ivabradine hcl. Pharma Sci Monitor. 6(1):141–152
Dandamudi S, Rangapuram V (2021) Synchronized analysis of bempedoic acid and ezetimibe in pure binary mixture and their combined tablets by a new stability indicating RP-UPLC method. Int J Health Sci III:7278–7290
Dhiware TK, Patil PA, Mahesh GS (2019) Quantitative estimation of edoxaban by zero and first order area under curve spectrophotometric method in bulk and in-house tablets. World J Pharmaceut Res 8(10):1016–1025
Dhiware TK, Patil PA, Salaraya MG (2019) Development and validation of HPTLC method for determination of edoxaban in bulk and tablet. Asian J Pharmaceut Anal 9(3):161–166
Doltade M, Saudagar R (2019) The analytical method development and validation: a review. J Drug Deliv Therapeut 9(3):563–570
Drews J (2000) Drug discovery: a historical perspective. Science 287(5460):1960–1964
EL-Gizawy SM, Abdelmageeb OH, Omar MA, Deryea SM, Abdel-Megieb AM, (2012) Development and validation of hplc method for simultaneous determination of amlodipine, valsartan, hydrochlorthiazide in dosage form and in spiked human plasma. Am J Anal Chem 2012(3):422–430
El-Masry AA, El-Wasseef DR, Eid M, Shehata IA, Zeid AM (2021) Optimization and validation of a facile RP-HPLC method for determination of betrixaban and lercanidipine in pharmaceutical and biological matrices. J Chromatogr Sci 59(8):785–794
El-Masry AA, El-Wasseef DR, Eid M, Shehata IA, Zeid AM (2022) Development of three ecological spectroscopic methods for analysis of betrixaban either alone or in mixture with lercanidipine: greenness assessment. R Soc Open Sci 9(2):211457
Fenimore DC, Davis CM (1981) High performance thin-layer chromatography. Anal Chem 53(2):252–266
Ghayas S, Muhammad HS, Siddiqui F, Yousuf RI, Masood MA, Fakhsheena A, Bushra R, Bashir L, Naz S, Muhammad IN (2017) Chromatographic method development and validation fort the determination of valsartan in biological fluid. Pak J Pharm Sci
Global Burden of Disease Collaborative Network (2019) Global Burden of Disease Study 2019 (GBD). Institute for Health Metrics and Evaluation (IHME), Results. Seattle, WA, https://ghdx.healthdata.org/gbd-2019 Accessed 2022
Gorumutchu GP, Ratnakaram VN (2018) Oxidative coupling: a tranquil approach for determination of selexipag by visible spectrophotometry. Orient J Chem. 34(6):3112
Gouveia F, Bicker J, Santos J, Rocha M, Alves G, Falcão A, Fortuna A (2020) Development, validation and application of a new HPLC-DAD method for simultaneous quantification of apixaban, dabigatran, edoxaban and rivaroxaban in human plasma. J Pharm Biomed Anal 20(181):113109
Guvvala V, Subramanian VC, Anireddy JS (2019) A study on structural characterization of degradation products of cangrelor using LC/QTOF/MS/MS and NMR. J Pharm Biomed Anal 5(170):327–334
Jasemizad T, Padhye LP (2019) Simultaneous analysis of betrixaban and hexazinone using liquid chromatography/tandem mass spectrometry in aqueous solutions. Methodsx 1(6):1863–1870
Kagawad P, Gharge S, Jivaje K, Hiremath SI, Suryawanshi SS (2021) Quality control and standardization of Quercetin in herbal medicines by spectroscopic and chromatographic techniques. Fut J Pharmaceut Sci 7(1):1–2
Kajal K, Archana T (2022) Simultaneous estimation of sacubitril and valsartan combination of drug in tablet dosage form using hydrotropy by UV spectrophotometry. 7(1), 1–23
Kalyankar GG, Vansiya PH, Bodiwala KB, Lodha SR, Prajapati PB, Ranch KM (2018) Development and validation of spectrophotometric method for the estimation of edoxaban tosylate monohydrate in its synthetic mixture. Am J PharmTech Res. 8(2):296–306
Karla VR, Raghasudha M, Chitta R (2022) Simultaneous determination of bempedoic acid and ezetimibe in rat plasma using HPLC–PDA and its applications to a pharmacokinetic study. Chem Afr 5(4):917–927
Khalid AMA, Mohammed WIN, Ahmed EI-Olemy, Ramzy S (2018) Application of TLC densitometric method for simultaneous determination of sacubitril and valsartan in their newly approved pharmaceutical formulation. Eurasian J Anal Chem, 2017, 13(6
Krishnaiah CH, Reddy AR, Kumar R, Mukkanti K (2010) Stability-indicating UPLC method for determination of Valsartan and their degradation products in active pharmaceutical ingredient and pharmaceutical dosage forms. J Pharm Biomed Anal 53:483–489
Kumar TH, Banu T, Ravindar B, Rasheed SH, Gajji N (2021) Quantification of sacubitril and valsartan in tablet formulation by RP-HPLC method. Int J 1:10–16
Madhuri PL, Kumar TH, Rao YS, Rao K (2019) UV spectrophotometric method for estimation of sacubitril in synthetic mixture. Asian J Res Chem 12(1):7–10
Maheshwari S, Khandhar AP, Jain A (2010) Quantitative determination and validation of ivabradine hcl by stability indicating RP-HPLC method and spectrophotometric method in solid dosage form. Eurasian J Anal Chem 5(1):53–62
Maheshwari K, Rani SS (2022) Validated method for the simultaneous estimation of bempedoic acid and ezetimibein bulk and tablet formulation by RP-HPLC method. World J Pharmaceut Sci 33–41
Mishra K, Prasanna KA, Behera SR (2020) Simultaneous estimation of sacubitril and valsartan in bulk and pharmaceutical dosage form by using rp-hplc. Res J Pharmacy Life Sci 1(2):25–32
Mostafa N, Fayez Y, Farid JF (2016) Validated stability indicating chromatographic methods for determination of Ivabradine hydrochloride in the presence of its acidic degradation product. Int J Res Rev Pharmacy
Motisariya MH, Patel KG, Shah PA (2013) Validated stability-indicating high-performance thin layer chromatographic method for determination of Ivabradine hydrochloride in bulk and marketed formulation: an application to kinetic study. Bull Fac Pharmacy Cairo Univ 51(2):233–241
Moussa BA, Hashem HM, Mahrouse MA, Mahmoud ST (2018) A validated RP-HPLC method for the determination of rosuvastatin in presence of sacubitril/valsartan in rat plasma: application to in vivo evaluation of OATP-mediated drug interaction potential between rosuvastatin and sacubitril/valsartan. Microchem J 1(143):31–38
Moussa BA, Hashem H, Mahrouse MA, Mahmoud ST (2018) Experimental design approach in HPLC method development: application for the simultaneous determination of sacubitril and valsartan in presence of their impurities and investigation of degradation kinetics. Chromatographia 81(1):139–156
Murugan S, Vetrichelvan T (2019) Absorbance ratio and first order derivative spectroscopic methods for simultaneous determination of sacubitril and valsartan in bulk and tablet dosage form. Res J Pharmacy Technol 12(11):5251–5254
Naazneen S, Sridevi A (2017) Development of assay method and forced degradation study of valsartan and sacubitril by RP-HPLC in tablet formulation. Int J App Pharm 9(1):9–15
Nabel EG (2003) Cardiovascular disease. N Engl J Med 349(1):60–72
Nowakowska J, Pikul P, Marszałł M, Ciura K (2017) Application and validation of simple isocratic HPLC-UV-DAD method with dual wavelength detection for Ivabradine determination and its application in the study of stress degradation. J Chem
Parambi DGT, Mathew M, Ganesan V (2011) A validated stability indicating HPLC method for the determination of valsartan in tablet dosage form. J Appl Pharmaceut Sci 1(4)
Patil PA, Raj HA, Sonara GB (2016) Q-absorbance ratio spectrophotometric method for simultaneous determination of atenolol and ivabradine hydrochloride in synthetic mixture. Pharmaceut Biol Eval 3(2):224–230
Patra S, Panda S (2014) Rapid and selective UV spectrophotometric and RP-HPLC methods for dissolution studies of ivabradine controlled-release formulations. Pharmatutor 2(8):201–213
Patridge E, Gareiss P, Kinch MS, Hoyer D (2016) An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discovery Today 21(2):204–207
Pérez M, Ramírez G, Pérez M, Restrepo P (2017) Validation of an analytical method for the determination of valsartan in human plasma by HPLC/UV with addition standard using losartan as an internal standard. Colomb Med 38(1):13–20
Phalguna Y, Jahan N, Indraja N, Kumar SG (2018) Analytical method development and validation for the estimation of sacubitril and valsartan in combined pharmaceutical dosage forms by RP-HPLC. Asian J Res Pharmaceut Sci 8(1):09–16
Prajapati P, Bhayani D, Mehta P (2020) Development and validation of a stability indicating UHPLC method for Sacubitril/Valsartan complex in the presence of impurities and degradation product. J Appl Pharm Sci 10(02):097–107
Prathyusha SM, Deepti CA, Naik RR (2020) Development and validated of spectrophotometric methods for the determination of Selexipag (An anti-hypertensive agent). Res J Pharmacy Technol 13(3):1346–1350
Ragab MA, Galal SM, Korany MA, Ahmed AR (2017) First derivative emission spectrofluorimetric method for the determination of LCZ696, a newly approved FDA supramolecular complex of valsartan and sacubitril in tablets. Luminescence 32(8):1417–1425
Rao KS, Jena N, Rao MEB (2010) Development and validation of a specific stability indicating high performance liquid chromatographic method for valsartan. J Young Pharm 2(2):183–189
Rao GS, Rao SV, Vardhan SVM, Ramchandran D (2013) Development and validation of new UV spectrophotometric assay method for Valsartan in pure and in formulation. J Chem Pharm Res 5(7):229–232
Rao KP, Koganti K, Palakeeti B, Srinivas KS (2021) Related substances method development and validation of an LCMS/MS method for quantification of selexipag and its related impurities in rat plasma and its application to pharmacokinetic studies. SN Appl Sci 3(3):1–2
Rashid MA, Muneer S, Mendhi J, Sabuj MZ, Alhamhoom Y, Xiao Y, Wang T, Izake EL, Islam N (2021) Inhaled Edoxaban dry powder inhaler formulations: Development, characterization and their effects on the coagulopathy associated with COVID-19 infection. Int J Pharm 25(608):121122
Rashid MA, Muneer S, Alhamhoom Y, Islam N (2022) Rapid assay for the therapeutic drug monitoring of edoxaban. Biomolecules 12(4):590
Ratnakaram VN (2018) Diazo coupling for the determination of selexipag by visible spectrophotometry. Int J Green Pharmacy (IJGP). 12(04).
Ravisankar P, Srikanth D, Reddy CV, Rao PR, Babu PS (2018) Development and validation of UV spectrophotometric method for the determination of Edoxaban Tosylate Monohydrate in pharmaceutical dosage form. Indian J Res Pharmacy Biotechnol 6(2):73–78
Reddy PS, Jagarlapudi VS, Sekharan CB (2016) Determination of edoxaban in bulk and in tablet dosage form by stability indicating high-performance liquid chromatography. Pharmaceut Sci 22(1):35
Saibaba SV, Kumar MS, Pandiyan PS (2016a) Mini review on lc/ms technique. World J Pharmacy Pharmaceut Sci 5(4):2381–2395
Saibaba SV, Kumar MS, Pandiyan PS (2016b) mini review on lc/ms technique. World J Pharmacy Pharmaceut Sci 5(4):2381–2395
Sankar PR, Eswarudu MM, Krishna PS, Srikanth D, Babu PS, Rohith N (2021) Novel validated RP-HPLC method for determination of edoxaban tosylate monohydrate in bulk and its pharmaceutical dosage form. J Pharm Sci Res 13(5):232–237
Satheshkumar S, Muruganantham V (2021) A rabit method for quantification of selexipag in human plasma using high performance liquid chromatography with electron spray ionzationtendem mass spectrometry. Ann Roman Soc Cell Biol 15:5689–5707
Smerikarova M, Bozhanov S, Maslarska V, Tournev I (2021) Determination of tafamidis plasma concentrations in amyloidosis patients with Glu89Gln mutation by HPLC-UV detection. J Chromatogr Sci
Tammam MH, Talib NFA (2019) Development and validation of a bioanalytical HPLC method for quantification of valsartan in human plasma and its application in P‟ Cokinetic studies. Anal Chem Lett 672–681
Tarkase KN, Tajane SR, Jadhav MB (2010) Development and validation of UV spectrophotometric method for estimation of valsartan in bulk and tablet dosage form. J Pharm Res 5(4):2344–2346
Tarkase KN, Tajane SR, Jadhav MB (2020) Development and validation of UV spectrophotometric method for estimation of valsartan in bulk and tablet dosage form. J Pharm Res 5(4):2344–2346
Thete PG, Saudagar RB (2018) Quantitative determination and validation of novel derivative spectrophotometric method for estimation of ivabradine hydrochloride in bulk and marketed formulation. Asian J Pharmacy Pharmacol 4(5):697–701
Thete PG, Saudagar RB (2019) Analytical method development and validation for the determination of ivabradine hcl by RP-HPLC in bulk and pharmaceutical dosage form. Asian J Pharmacy Technol 9(2):89–92
Todkar P, Dichwalkar S, Hamrapurkar P (2020) Stability indicating HPLC method for determination of Riociguat in bulk and pharmaceutical dosage form. ISCB Int
Tohidi M, Ramezani M, Mehramizi A (2019) Application of continuous wavelet transform coupled with zero-crossing technique for the simultaneous spectrophotometric determination of sacubitril and valsartan in tablet dosage form. J Chem Health Risks 9(4):331–344
Trefi S, Bitar Y, Gilard V (2019) Separation and quantification of sacubitril-valsartan combination in tablets by a new ion-pair hplc. Res J Pharm Technol 12(3):1017–1022
Vaka S, Parthiban P (2017) New method development and validation for the simultaneous estimation of sacubitril and valsartan in a bulk and pharmaceutical dosage forms. Int J Res 4(1):17–24
Vejendla A, Talari S, Ramu G, Rajani C (2021) Characterization of novel stress degradation products of Bempedoic acid and Ezetimibe using UPLC–MS/MS: development and validation of stability-indicating UPLC method. Fut J Pharmaceut Sci 7(1):1–3
Verma G, Mishra M (2018) Development and optimization of UV-Vis spectroscopy-a review. World J Pharmaceut Res 7(11):1170–1180
Yarra US, Gummadi S (2021) Stability indicating RP-UPLC method for simultaneous quantification of bempedoic acid and ezetimibe in bulk and pharmaceutical formulations. Fut J Pharmaceut Sci 7(1):1–9
Younis SE, El-Nahass SA, Elkhatib MA, Soliman SA, Youssef RM (2020) Gradient HPLC-DAD method for quantification of novel oral anticoagulant “Edoxaban” in plasma: Its selective determination in presence of sixteen co-administered drugs. J Chromatogr B 1(1160):122386
Youssef RM, El-Nahass SA, Soliman SA, Younis SE (2021) Development of hybrid spectrofluorimetric method for simultaneous determination of Valsartan and Sacubitril in LCZ696 tablets. Spectrochim Acta Part A Mol Biomol Spectrosc 15(256):119748
Zarghi A, Shafaati A, Foroutan SM, Movahed H (2008) Rapid quantification of valsartan in human plasma by liquid chromatography using a monolithic column and a fluorescence detection: application for pharmacokinetic studies. Sci Pharm 76(3):439–450
Zhao D (2021) Epidemiological features of cardiovascular disease in Asia. JACC Asia. 1(1):1–3
Zhou L, Zou L, Sun L, Zhang H, Hui W, Zou Q (2018) A liquid chromatographic method for separation of sacubitril–valsartan and their stereoisomeric impurities. Anal Methods 10(9):1046–1053
Acknowledgements
The authors are very thankful to Principal Dr. S. S. Jalalpure and Vice Principal Dr. M. B. Patil for their support and guidance. Authors are also thankful to the department of pharmaceutical Chemistry.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
We have assured that “all authors have read and approved the manuscript.” All the authors have equal contribution and participation in this research work. SG has reviewed all manuscripts on “Spectrophotometric, chromatographic and bioanalysis of selected recently approved drugs from 2015–2020 to treat cardiovascular diseases: an analytical review” he had completed his work under the supervision of SH. SH also helped him in their review work and guides to resolve the complications.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
No competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gharge, S., Koli, R., Gudasi, S. et al. Spectrophotometric, chromatographic and bioanalysis of selected recently approved drugs from 2015–2020 to treat cardiovascular diseases: an analytical review. Bull Natl Res Cent 47, 24 (2023). https://doi.org/10.1186/s42269-023-01000-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-023-01000-5