Abstract
Background
Left ventricular (LV) apical aneurysm associated with aortic valve stenosis (AS) is very rare. We herein report two cases of this entity.
Case presentation
Case 1 was a 75-year-old woman admitted for surgery for severe AS and enlargement of the ascending aorta. Preoperative routine echocardiography and cardiac computed tomography (CT) revealed an unexpected local aneurysm of the LV apex. The patient underwent aortic valve replacement (AVR), LV aneurysm resection, and ascending aorta replacement. Case 2 was a 71-year-old woman diagnosed with severe AS. Preoperative cardiac CT detected LV apical aneurysm that could not be detected by echocardiography. Cardiac catheterization showed a coronary ventricular fistula. The patient underwent AVR and LV aneurysm resection. Given that neither of the two cases had a history of myocardial infarction or obstructive hypertrophic cardiomyopathy, the cause of the local aneurysm of the LV apex was thought to be relative ischemia at the apex due to myocardial hypertrophy and LV pressure overload due to long-term AS. In addition, a coronary artery fistula was suggested to be involved in the relative ischemia of the apex in Case 2.
Conclusions
Echocardiography alone is not sufficient to exclude apical aneurysm, and cardiac CT may be useful. Regarding the surgical indication, it is necessary to consider further cases in the future.
Similar content being viewed by others
Background
In general, the etiology of LV apical aneurysm is myocardial infarction or cardiomyopathy, such as obstructive hypertrophic cardiomyopathy (HOCM). (Papanastasiou et al. 2021; Maron et al. 2008; Rowin et al. 2017). To our knowledge, there has been only one report of local aneurysms of the LV apex associated with AS (Drews et al. 2014). In this article, we report two cases of AS associated with apical aneurysm.
Case presentation
Case 1
The patient was a 75-year-old woman. She was referred to our hospital with a diagnosis of severe AS after a thorough examination.
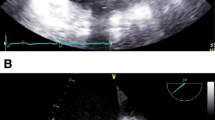
Preoperative echocardiography pointed out the LV apical aneurysm in addition to severe AS (aortic valve area [AVA], 0.54 cm2; mean transvalvular gradient, 56 mmHg; peak velocity, 5.17 m/sec) (Fig. 1). There were no abnormalities in wall motion except for the LV apex aneurysm. The wall thickness of the interventricular septum (IVS) and posterior wall of the LV (LVPW) was 13 mm, indicating afferent hypertrophy of the left ventricle, but there were no obvious findings of HOCM.
Preoperative routine cardiac CT showed a highly calcified aortic valve and a 12-mm apical aneurysm (Fig. 2 A). In addition, the ascending aorta was enlarged to 50 mm. Cardiac catheterization showed no significant stenosis in the coronary arteries.
We performed AVR, apical aneurysm resection, and replacement of the ascending aorta. The wall of the local aneurysm of the LV apex was clearly thinner than the surrounding myocardium and seemed to have been replaced by fibrous tissue. The postoperative course was uneventful. Postoperative echocardiography showed no issues with the performance of the prosthetic valve, and the LV ejection fraction was maintained. Cardiac CT showed no evidence of residual apical aneurysm.
The pathology of the apical aneurysm showed a mixture of normal myocardium and collagen fibers (Fig. 3 A). There was no penetrating fibrosis, which is a finding peculiar to ischemic apex aneurysm, or complex arrangement of myocardium, as seen in obstructive hypertrophic cardiomyopathy.
Case 2
The patient was a 71-year-old woman. She was admitted for surgery for severe AS.
Preoperative echocardiography revealed severe AS (AVA, 0.51 cm2; mean transvalvular gradient, 50 mmHg; peak velocity, 4.89 m/sec) and afferent LV hypertrophy with normal wall motion but no apical aneurysm, as was detected in Case 1, and routine cardiac CT showed a 10-mm aneurysm formation in the apex of the heart (Fig. 2 B) (Fig. 3).
Preoperative cardiac catheterization showed no significant coronary artery stenosis, but a coronary artery fistula from the left anterior descending branch to the ventricle was noted (Fig. 4). We performed AVR and LV aneurysm resection. The intraoperative findings of the apex aneurysm were similar to those in Case 1, with thinning of the aneurysm wall and absence of myocardial tissue on a gross examination. The postoperative course was uneventful.
Azan staining of the pathological tissue showed a mixture of normal myocardium and collagen fibers. As in Case 1, there were no findings suggestive of myocardial infarction or obstructive hypertrophic cardiomyopathy (Fig. 3 B).
Discussion
Myocardial infarction and HOCM are reportedly the most common etiologies of LV apical aneurysm (Papanastasiou et al. 2021; Maron et al. 2008; Rowin et al. 2017; Milton Alcaino et al. 2021). The incidence of ischemic ventricular aneurysms is about 12% to 15%. Most ischemic ventricular aneurysms occur in the anterior wall, which is formed by thinning of the infarcted myocardium and bulging of the myocardial wall due to internal pressure, resulting in a large traffic hole between the LV cavity and the aneurysm (Tokunaga et al. 2021).
An apical aneurysm characteristic of HOCM was found in 2–4.8% of cases reported (Maron et al. 2008; Rowin et al. 2017). In cases of HOCM of the mid-ventricular region, the entire apex is often involved in the aneurysm, rather than the stenosis (Milton Alcaino et al. 2021). Both of our cases had a cystic morphology confined to the apex, which differed from the morphological features of ischemic aneurysm or HOCM. Histopathology also showed no evidence of penetrating fibrosis peculiar to ischemic apex aneurysms, nor was there a complex arrangement of myocardium suggestive of HOCM. There was no history of other inflammatory or traumatic diseases.
To our knowledge, there has been only one report of a local aneurysm of the LV apex associated with AS (Drews et al. 2014). In their study, Drews et al. suggested that myocardial hypertrophy due to AS may have caused relative ischemia in the apex, and prolonged pressure stress on the apex most susceptible to ischemia may have resulted in local aneurysm of the LV apex (Drews et al. 2014). Similarly, in our two cases, LV hypertrophy and long-term pressure load due to severe AS may have caused aneurysm formation in the LV apex, which tended to be in a relative ischemic state (Tokunaga et al. 2021).
In addition, there has been a report that a decrease in the peripheral coronary artery blood flow through a coronary artery fistula may cause a steal phenomenon, resulting in an apex aneurysm (Morishita et al. 2008). Therefore, in Case 2, the ischemic tendency of the anterior descending branch due to coronary ventricular fistula may have been involved in the formation of apical aneurysm.
In recent years, with the widespread use of transcatheter aortic valve implantation (TAVI), cardiac CT, which is useful for diagnosing apical aneurysms, has become more routine. As a result, we believe we can continue to find apical aneurysms that are neither ischemic aneurysms nor HOCM. In Case 1, echocardiography was able to diagnose an apical aneurysm, but in Case 2, it could not be confirmed. The two cases in this study were also eventually diagnosed by cardiac CT. Therefore, echocardiography alone is not sufficient to diagnose an apical aneurysm, so it is possible that such cases have been overlooked in the past.
Existing guidelines do not specifically address the treatment of localized apex aneurysms, such as in our two cases. Therefore, the indications for surgical treatment are controversial. According to the European Society of cardiology guidelines, left ventricular aneurysms may cause arrhythmia and thrombus formation (Steg et al. 2012). Left ventricular aneurysm resection may thus be useful for reducing the risk of arrhythmia and cardiogenic embolism.
In our two cases, we performed apical aneurysm resection in consideration of arrhythmia, cardiogenic embolism, and enlargement of the aneurysm. As there are no established treatment guidelines, the indication for surgery should be determined according to the preoperative reserve capacity in each case, taking into account the morphology of the aneurysm, the presence of arrhythmia, and the possibility of thrombus in the aneurysm. The further accumulation of cases is necessary to determine the indications for surgery in the future.
Conclusions
We experienced two cases of apical aneurysms associated with AS. Because AS causes relative ischemia and prolonged pressure overload in the aortic root due to myocardial hypertrophy, the possibility of apical aneurysm should be considered. Echocardiography alone is not sufficient to exclude apical aneurysm, and cardiac CT may be useful. Regarding the surgical indication, it is necessary to consider further cases in the future.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- LV:
-
Left ventricular
- AS:
-
Aortic valve stenosis
- CT:
-
Computed tomography
- AVR:
-
Aortic valve replacement
- HOCM:
-
Obstructive hypertrophic cardiomyopathy
- AVA:
-
Aortic valve area
- IVS:
-
Interventricular septum
- LVPW:
-
Posterior wall of the left ventricular
- TAVI:
-
Transcatheter aortic valve implantation
References
Drews T, Pasic M, Hetzer R (2014) Left apical aneurysm in a patient with severe aortic valve stenosis. Thorac Cardiovasc Surg Rep 3:9–12
Maron MS, Finley JJ, Bos JM et al (2008) Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation 15:1541–1549
Milton Alcaino I, Juan Aguilar P, Claudia JB (2021) Apical aneurysm in a patient with hypertrophic cardiomyopathy. Rev Med Chil 149(3):472–475
Morishita S, Fukuda N, Fukuda Y et al (2008) Apical aneurysm and abnormal flow signals in the thin apical wall due to coronary artery-left ventricular fistula observed in hypertrophic cardiomyopathy with midventricular obstruction: a case report. J Cardiol Jpn Ed 1:174–177
Noriyuki T, Watanabe T, Kondo K et al (2021) Endoventricular stepwise plication for left ventricular aneurysm. Kyobu Geka 74(2):103–107
Papanastasiou CA, Zegkos T, Karamitsos TD et al (2021) Prognostic role of left ventricular apical aneurysm in hypertrophic cardiomyopathy: a systematic review and meta-analysis. Int J Cardiol 332:127–132
Rowin EJ, Maron BJ, Haas TS et al (2017) Hypertrophic cardiomyopathy with left ventricular apical aneurysm. J Am Coll Cardiol 69:761–763
Steg PG, James SK, Atar D et al (2012) Task force on the management of ST-segment elevation acutemyocardial infarction of the European society of cardiology (ESC) ESC guidelines for themanagement of acute myocardial infarction in patients presenting with STsegment elevation. Eur Heart J 33(20):2569–2619
Acknowledgements
I would like to thank Dr. Nogami for the useful discussions. I am grateful to Dr. Kamohara for carefully proofreading the manuscript.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
The conception or design of the study was provided by EN, and data collection was provided by KS and YT. Data analysis and interpretation were performed by MU, HM, JY, and SK. The paper was written by YK. Important revisions of the paper and approval of the final draft were provided by KK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Research subjects were informed of the purpose and methods of the study, that participation was voluntary and that there would be no disadvantages if they refused, and about the protection of their personal information and withdrawal of consent. Written consent was obtained from the research subjects. Since the case report was anonymized, it was determined that Ethics Committee approval was not required.
Consent for publication
Survey participants were informed in writing about the publication, that participation was voluntary, that they would not be disadvantaged if they refused, and that they would protect their personal information and withdraw their consent, and all agreed in writing.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koga, Y., Kamohara, K., Nogami, E. et al. Two cases of left ventricular apical aneurysm associated with severe aortic stenosis: case report. Bull Natl Res Cent 46, 264 (2022). https://doi.org/10.1186/s42269-022-00954-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-022-00954-2