Abstract
Background
Acute toxicity (LD50) test provides an indication on the range of doses of a substance that could be toxic to the body systems. This study was aimed at determining the LD50 of different extracts of Bombax costatum stem bark and its effects on the histology of liver and kidneys in rats.
Results
Phytochemical screening of n-hexane, ethanol and chloroform extracts of B. costatum stem bark has revealed the presence of alkaloids, flavonoid, saponins, steroid, terpenes, anthraquinones and cardiac glycosides. No mortality was recorded after testing all the different extracts on rats. However, mild body weaknesses and pilo erection were observed in the first hour of extracts’ administration. No statistically significant differences was observed in most of the serum levels of kidney function biomarkers, although a significant decrease (p < 0.05) in bicarbonate and increase (p < 0.05) in urea, respectively, were observed in chloroform extract treated group when compared to control. Further, no marked differences (p > 0.05) were observed in hematological parameters as well as in the liver functions biomarkers in all the 3 extract exposed groups when compared to the control, except significant decrease (p < 0.05) of total bilirubin level in chloroform extract exposed rats. Finally, histological sections of the liver and kidneys showed no aberrations.
Conclusions
Since no mortality and serious clinical manifestation were recorded, it is suggestive that oral acute administration of n-hexane, chloroform and ethanol extracts of B. costatum is greater than 5000 mg/kg. Hence, the plant has high safety margin and can be used within the dose of 5000 mg/kg body weight.
Similar content being viewed by others
Background
In Nigeria and other developing countries around the world, plants are generally consumed as food or for medicinal purposes (Ugwah-Oguejiofor et al. 2019). In most African countries, medicinal plants play a vital role in health care needs of the populace, especially for the treatment of various diseases (Ahmed et al. 2022). The continued use of such plants without proper knowledge of their toxicity and safety is dangerous, hence the necessity to conduct toxicity study on them. The toxicity of a plant is sets of detrimental effects that result from administration of that plant at a single relatively high dosage or small repeated doses over a long period of time on animals or cell lines (Dibong et al. 2015). Pharmacological tests designed to ascertain the degree of such detrimental effects so as to regulate its consumption defined the study of toxicity of that plant (Auti and Kulkarni 2019).
Bombax costatum Pellegr. and Vuillet is one of such plants that are widely consumed in Africa (Lea Blondelle et al. 2022). The Bombax genus is distributed around the tropical region and comprises of about eight (8) different species. Two of the species are found in Africa while the other six species are found in Asia. In Nigeria B. costatum is known by other vernacular names such as “Gurjiya” in Hausa, “Joohi” in Fulfulde (Nazifi et al. 2020) and “Danaa” in Babur (Personal communication). Further, in Nigeria and other African countries, different parts of the plant are used in traditional medicine for the management and treatment of different ailments, to relieve fever, promote lactation, and as stimulant against weakness (Wanbara et al. 2021). The root powder is eaten or applied on the whole body against epileptic seizures (Lea Blondelle et al. 2022). In some countries, the roots and stem bark are used for the treatment of fever, dysentery and dizziness (Lea Blondelle et al. 2022). The bark is used in the management of insanity, treatment of skin diseases, headache, yellow fever and wound healing. The stem bark is used against pain, edema and hernia in Benin (Assogba et al. 2017), while the leaves are taken for the treatment of convulsion (Assogba et al. 2017).
Bombax costatum contains high protein and low content of toxic substances, as the fruit was reported to contain 20–25% protein in Niger, while the seeds contained about 20% oil and high amounts of iron and zinc (Maiga et al. 2005). This implies that the specie could be a good source of iron in case of iron deficiency anemia but there is dearth of information on nutritional content in terms of human nutrition. Hydroalcoholic extract of the plant revealed the presence of secondary metabolites such as; alkaloids, flavonoids, saponins, tannins, anthraquinones, cardiac glycosides, triterpenes and steroids (Nazifi et al. 2020). Although B. costatum is frequently consumed as food or as traditional medicine, there is a dearth of data on the toxicological effect of the plant and thus the need for this study. In this study, single dose oral toxicity of three different extracts of B. costatum, n-hexane (NETR), ethanol (EETR) and chloroform (CETR) were evaluated in rats. Their effects on hematological, liver and kidneys function parameters were ascertained, besides the effects on the histology of vital metabolic organs; liver and kidneys.
Methods
Procurement of plant material and authentication
Bombax costatum Pellegr. and Vuillet stem bark was obtained from Federal Government College, Maiduguri, Borno State with GPS coordinates; Latitude: 11° 49′ 51.9528″ N Longitude: 13° 9′ 3.4812″ E. The plant material was then verified by a botanist at Botany Department, University of Maiduguri, Borno State, Nigeria. The sample was deposited at the herbarium of the Department of Human Anatomy, University of Maiduguri with voucher number UM/HAH/2021/002. The stem bark was then washed with tap water and shade dried at room temperature for 2 weeks.
Preparation of the plant extracts
The dried stem bark of B. costatum was grinded into powder using a mortar and pestle. 600 g of the powder was dissolved in 3 different solvents (200 g in each solvent). The first 200 g in 500 ml of ethanol, the second 200 g in 500 ml n-hexane and third 200 g in 500 ml chloroform for 48 h with occasional shacking as earlier described by (Nazifi et al. 2020). They were then filtered and each of the filtrate was evaporated to dryness at room temperature (23 ± 3 °C). The residues obtained were weighed and kept in a clean, airtight container in the refrigerator. The percentage yield of the extracts were 3.5%, 3.7% and 4.2% for the ethanol, n-hexane and chloroform, respectively.
Experimental animals
Twelve (12) female wistar albino rats weighing between 90 and 140 g were used for the experiment, they were housed at the Animal House Human Physiology Department, University of Maiduguri, Borno State. They were kept in a well aerated cages and given free access to rat chow and water. Simple random sampling method was employed to divide the rats into four groups of three rats each. The study protocol was followed strictly in compliance to the “Guide to the care and use of laboratory animals in research and teaching” as detailed in NIH publications (Council 2011).
Phytochemical screening
Qualitative phytochemical screening was performed on the chloroform, ethanol and n-hexane extracts of B. costatum stem bark, using a standard methods as earlier described (Evans 2009; Chiroma et al. 2022).
Acute toxicity
The up-and-down method as described in the Organization for Economic Co-operation and Development (OECD) guideline 425 was used to determine the oral LD50 of B. costatum stem bark extracts. The limit test method was used because some studies on the plant indicated that the plant is not toxic or has a very low toxicity. A limit dose was set at 5000 mg/kg body weight of the extract (OECD 2008). Twelve (12) female wistar albino rats were randomly grouped into 4 (n = 3) dosed sequentially with 5000 mg/kg body weight of the chloroform, ethanol and n-hexane extracts of B. costatum stem bark, respectively. All the researchers are blinded to the groupings except one, in order to avoid biasness. The rats were fasted for a night before administration of the extract. The rats were then observed periodically for any sign of toxicity and lethargy. The rats were then euthanized after 14 days period following ketamine injection at a dose of 100 mg/kg IP, and blood was collected into plain and EDTA bottles through cardiac puncture. The liver and kidneys were harvested, rinsed in a normal saline (0.9%) and weighed and fixed in 10% formalin for histology. The relative organ weight was also calculated as follows:
Hematological parameters
Hematological parameters were determined with the aid of automated blood analyzer (Landwind, model LW D3600). The machine aspirates and dilutes a blood sample to define variables relating to red blood cells, white blood cells and platelets depending on the diluents used. The machine also computed the hematocrit value in the course of processing the red blood cells based on cumulative pulse height which depends on the red cell volume and number. Hemoglobin was derived from the optical density of about 525 nm after a reaction time of 20–25 s. The modified cyanmethemoglobin method was employed by the machine (Boeringer, 4010, West Germany). The hematological parameters determined were red blood cell count (RBC), white blood cell count (WBC), hemoglobin concentration(Hb), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC).
Biochemical parameters
Biochemical parameters were determined from the rat’s serum. Liver functions biomarkers assessed include, total protein (TP), albumin, alkaline phosphatase (ALP), Aspartate transaminase (AST), Alanine transaminase (ALT), total bilirubin (TB) and conjugated bilirubin (CB), while the kidney functions biomarkers evaluated were sodium, potassium, chloride, bicarbonate, urea and creatinine. All the biomarkers were processed using the available commercial kits according to the manufacturer’s manual.
Histological study
The fixed portions of the liver and kidneys from 10% formalin were processed through routine histological procedure. The tissues were then stained with Hematoxylin and counterstained by Eosin before light microscopic evaluation at a magnification of 200X, by a histopathologist who was blinded to the treatments. Histology was evaluated using the method described by (Drury et al. 1976) with some modifications. Minimum of 3 slides per group were prepared with at least two sections on each and during viewing a minimum of 3 fields were checked on each section.
Statistical analysis
The results were expressed as mean ± standard error of mean (SEM). The data of all the groups were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test using GraphPad Prism version 8.0.2 (ISI, San Diego, CA, USA) software. A p value of < 0.05 was considered as statistically significant.
Results
Phytochemical screening on chloroform, ethanol and n-hexane extracts of Bombax costatum stem bark
Qualitative phytochemical screening for secondary metabolites in chloroform, ethanol and n-hexane extracts of B. costatum stem bark is summarized in Table 1. In all the three extracts, the following phytochemicals were detected; alkaloids, flavonoids, saponins, steroids and terpenes, cardiac glycosides and anthraquinones. Even though tannins were detected in chloroform and ethanol extract of the plant, they are not found in n-hexane extract.
Assessment of signs of toxicity after single dose (5000 mg/kg) administration of chloroform, ethanol or n-hexane extracts of Bombax costatum stem bark to rats
Oral administration of chloroform, ethanol and n-hexane extracts of B. costatum stem bark did not cause mortality in all the group of rats. However, mild presentations of some signs of toxicity such as diarrhea, body weakness, salivation and sleepiness were observed during the 14 days study period as presented in Table 2.
Relative organ weights of rats after single dose (5000 mg/kg) exposure to chloroform, ethanol and n-hexane extracts of Bombax costatum stem bark
Figure 1 shows both liver and kidneys indices of rats after single dose (5000 mg/kg) chloroform, ethanol and n-hexane extracts of B. costatum stem bark. No statistically significant [F (3, 8) = 0.1905, p = 0.900] difference was observed in the relative kidneys weight in all groups of rats exposed to the different extracts of B. costatum when compared to the control group. However, one-way ANOVA showed a statistically significant differences in relative liver weights among the groups of rats [F (3, 8) = 6.492, p = 0.016]. Dunnett’s post hoc revealed a marked increase in relative liver weights in the rats group exposed to n-hexane extract (0.045 ± 0.001, p = 0.018) when compared to the control group of rats (0.032 ± 0.004).
Effects of single dose (5000 mg/kg) administration of chloroform, ethanol and n-hexane extracts of Bombax costatum stem bark on hematological parameters in rats after 14 days
As seen in Table 3, one-way ANOVA showed no statistically significant differences in RBC [F (3, 8) = 2.735, p = 0.1135], Hb [F (3, 8) = 2.686, p = 0.1173], WBC [F (3, 8) = 1.690, p = 0.2457], PCV [F (3, 8) = 2.499, p = 0.1336], MCV [F (3, 8) = 0.5010, p = 0.6920], MCH [F (3, 8) = 0.6115, p = 0.6263], MCHC [F (3, 8) = 0.4357, p = 0.7335] in all the group of rats exposed to single dose of chloroform, ethanol or n-hexane extracts of B. costatum stem bark when compared to the control group of rats.
Effects of single dose (5000 mg/kg) administration of chloroform, ethanol and n-hexane extracts of Bombax costatum stem bark on kidneys function biomarkers in rats after 14 days
The results for kidneys function biomarkers are presented in Fig. 2. One-way ANOVA showed no statistical significant differences in serum levels of sodium [F (3, 8) = 0.2824, p = 0.8368], potassium [F (3, 8) = 3.156, 0.0861], chloride [F (3, 8) = 0.4316, 0.7361] and creatinine [F (3, 8) = 0.09797, p = 0.9589] in the groups of rats that were exposed to single dose of the three extracts tested, when compared to the control group of rats. On the other hand, one-way ANOVA showed a statistical significant differences in serum levels of bicarbonate [F (3, 8) = 11.57, p = 0.0028] and urea [F (3, 8) = 11.68, p = 0.0027] respectively. Dunnett’s post hoc test revealed a significant decrease of bicarbonate levels in chloroform extract treated group of rats (18 ± 0.00, p = 0.0035) when compared to the control (21 ± 0.00) (Fig. 2d). Further, Dunnett’s post hoc also revealed a significant increase in urea levels in chloroform extract treated group of rats (8.2 ± 0.2, p = 0.0019), when compared to the control groups (3.87 ± 0.12) (Fig. 2e).
Effects of single dose (5000 mg/kg) administration of chloroform, ethanol and n-hexane extracts of Bombax costatum stem bark on liver function biomarkers in rats after 14 days
The results obtained for liver function biomarkers are presented in Fig. 3a–g. One-way ANOVA showed no statistically significant differences in the serum levels of albumin [F (3, 8) = 3.519, p = 0.0687], TP [F (3, 8) = 1.144, p = 0.3885], ALP [F (3, 8) = 0.9106, p = 0.4778], AST [F (3, 8) = 1.676, p = 0.2484], ALT [F (3, 8) = 2.261, p = 0.1584] and CB [F (3, 8) = 2.933, p = 0.0994] in rats that were exposed to all 3 different extracts when compared to the control group of rats. However, one-way ANOVA revealed statistically significant difference in serum level of TB [F (3, 8) = 3.596, p = 0.065] among the groups of rats. Dunnett’s post hoc confirmed a significant decrease of TB in chloroform exposed groups of rats (3.0 ± 0.00, p = 0.031) (Fig. 3f) when compared to the control (7.33 ± 0.88).
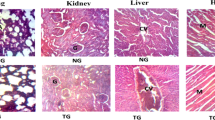
Effects of single dose (5000 mg/kg) administration of chloroform, ethanol and n-hexane extracts of Bombax costatum stem bark on histology of liver and kidneys of rats after 14 days
Histological qualitative analysis of the rat’s liver and kidney tissues were carried-out in order to ascertained any aberration as a result of single dose (5000 mg/kg) exposure to chloroform, ethanol and n-hexane extracts of B. costatum stem bark in rats after 14 days. Figure 4A–D showed photomicrographs of liver tissues from control rats group and those groups that received different extracts of B. costatum. The control rats (Fig. 4A) showed a normal structure of hepatic lobule comprising of radiating plates or cord of hepatocytes (H) forming a network around a central vein (CV). The hepatic cells were polygonal in shape with a conspicuous granular cytoplasm which has a nucleus centrally located with one or two nucleoli besides a number of chromatin materials. The hepatic cords are alternating with thin blood sinusoids and centrifugally extending alone the liver lobules. No noticeable changes were observed in the groups of rats exposed to chloroform, ethanol and n-hexane extracts of B. costatum as they all appeared similar to the control group rats histology (Fig. 4A–D).
Figure 5A–D shows photomicrographs of kidney tissues from control rats group and those groups of rats that received different extracts of B. costatum. The control rats (Fig. 5A) showed the normal histology of the kidneys with the Bowman’s capsule outer layer lined with squamous epithelium and the inner layer formed by podocytes surrounding the glomerulus (G). The Bowman’s space (BS) was wide, clear and not distended. The proximal and distal convoluted tubules are numerous and lined with cuboidal cells with nucleus at the center. There were no visible histological changes seen in the groups of rats that received chloroform, ethanol and n-hexane extracts of B. costatum as they all appeared similar to the control group of rats histology (Fig. 5B–D).
Discussion
In the present study, phytochemical screening of chloroform, ethanol and n-hexane extracts of B. costatum revealed the presence of alkaloids, flavonoids and saponins, steroids and terpenes, cardiac glycosides and anthraquinones in all the three extracts. In addition, chloroform and ethanolic extracts of B. costatum showed the presence of tannins. The present study is in line with preceding study which reported similar observation by noting the presence of flavonoids, alkaloids, tannins, saponins, anthraquinones, cardiac glycosides, steroids and triterpenes in hydromethanolic extracts of stem bark of B. costatum (Lea Blondelle et al. 2022). Various other medicinal plants showed the presence of flavonoids, alkaloids, saponins, cardiac glycosides and steroids. Examples of such include, hydroethanolic leaf extract of Clerodendrum polycephalum, methanolic leaf extract of Colocasia affinis and Eriosema psoraleoides root extract (Chaudhary et al. 2020; Bamikunle et al. 2022). Phytochemicals have both beneficial and harmful effects on the body. Alkaloids are known to have analgesic, anticancer antibiotic, antiparasitic, anesthetic, and spasmolytic properties (Heinrich et al. 2021). Tannins have antibiotic, astringency, hemostatic, antioxidant and pro-oxidant actions (Pizzi 2021). Further, anthraquinones have both pro-oxidant and antioxidant actions. While the antioxidant effect is beneficial to the body systems, however, pro-oxidant effect is harmful. Flavonoids are known for hepatoprotective and anti-inflammatory properties and have a strong antioxidant, anti-proliferative and anti-carcinogenic potentials (Gbenou et al. 2011) and diuretic properties (Vargas et al. 2018). Saponosides have surfactant, antifungal, antibacterial and antiviral properties, they exhibit vein and capillary protective activities and then edematous activity with hormonal activity (Marelli et al. 2016). The medicinal uses and pharmacological potentials of the B. costatum could be as a result of the presence of the phytochemicals mentioned above.
In the present study, the rats exposed to single dose oral administration of the different stem bark extracts of B. costatum at 5000 mg/kg body weight showed mild sign of toxicity with no fatality. Hence, B. costatum is relatively safe up to 5000 mg/kg suggesting that its safety margin is high. Previous studies has also reported that plants extract with LD50 as high as 5000 mg/kg body weight are not harmful (Enenebeaku et al. 2021; Abraham and Ahmad 2021). It is also interesting to note that all the 3 extracts tested does not affect the relative organ weights in kidneys. Further, the chloroform and ethanol extracts of B. costatum do not have significant effects on the relative weights of liver as well. However, n-hexane extract of B. costatum markedly increased the weight of the liver of rats exposed to it. The current study is in agreement with other studies who reported similar findings 14 days after exposure (Porwal et al. 2017). However, Mohammed et al. (2019) reported a statistically significant decrease in relative kidney weight and a statistically significant increase in the relative liver weight of rats only after sub-acute administration of Bombax custatum. These findings may suggest toxicity of to the kidneys and liver after prolong exposure. Several other medicinal plants used in Nigeria were proved to have a high margins of safety in similar studies. Examples of such plants includes; Clerodendrum polycephalum and Guiera senegalensis (Ugwah-Oguejiofor et al. 2019; Ahmed et al. 2022). However, plants like Cuscuta chinensis Lam were shown to have a relatively lower safety margin (Maimaiti et al. 2021).
Blood cells are mainly produced in the bone marrow. Some drugs and chemicals known as hemotoxicants cause reduction in RBCs leading to anemia and some bioactive phytochemicals affect PCV levels (Patrick-Iwuanyanwu et al. 2007). In the present study, exposure of the rats to the different extracts of B. costatum does not affect both qualitative and quantitative hematological parameters. This is suggestive that the extracts have no detrimental effects on bone marrow which is the center for hematopoiesis. The present result revealed presence of saponis in the extracts which are believed to have hemolytic effects (Marrelli et al. 2016). Nevertheless, the normal blood parameters reported might be suggestive of little quantities of saponin in the extracts since quantitative analysis of the phytochemicals was not carried-out in this study.
The kidneys help in the maintenance of homeostasis by reabsorbing vital substances and excreting metabolic wastes (Ahmed et al. 2022). In this study, the different plant extracts do not affect the kidneys function negatively, as no serious derangement was observed in the serum level of sodium, potassium, chloride and creatinine. However, a statistically significant decrease in the serum level of bicarbonate and increase in the serum level of urea were observed in the present study. The increased level of urea is an indicator that the plant extract decreased urea excretion by the kidneys while the decrease in bicarbonate could be an indication that the extract increased bicarbonate excretion by the kidneys as well which may lead to disruption of acid–base balance. These may be a sign of toxicity onset hence, a holistic approach for further studies on the effects of the plant on the physiology of kidneys is recommended. The liver detoxifies harmful substances in the body (Ahmed et al. 2022). The plant does not affect the liver because no statistically significant difference was observed in liver enzymes (Alkaline phosphatase, Aspartate transaminase and Alanine transaminase). An increase in the serum level of the liver function enzymes may be an indication of damaged hepatocytes, thus hepatotoxicity (Mukinda and Eagles 2010). Although there is statistically significant decrease in the serum level of total bilirubin in chloroform extract treated group, lower levels are usually not a concern. Protein synthesis by the liver is not affected in this study, as there were no statistically significant differences in the serum levels of albumin and total protein.
In the present study, the histology of the liver and kidneys did not show any noticeable aberration after exposure to the different extracts tested. The liver shows intact liver parenchyma and hepatocytes with central vein. The kidney shows intact glomeruli, Bowman’s space and juxtaglomerular apparatus. This is suggestive that the extracts is not harmful at the dose administered.
Conclusions
Phytochemical screening of chloroform, ethanol and n-hexane extracts of B. costatum stem bark revealed the presence of flavonoids, alkaloids, tannins, saponins, anthraquinones, cardiac glycosides, steroids and triterpenes. No mortality nor any serious clinical manifestations were seen in the rats after single dose oral administration of the extracts. Hence, the LD50 of n-hexane, chloroform and ethanolic extracts of B. costatum is more than 5000 mg/kg when administered orally. No serious derangements of liver and kidney functions biomarkers were observed, the hematological parameters were also normal besides intact histology of the liver and kidneys. It is therefore safe to say, that the extracts are relatively safe to use.
Availability of data and materials
Data will be given on request through the corresponding author.
Abbreviations
- CONTL:
-
Control
- CETR:
-
Chloroform extract of Bombax costatum
- EETR:
-
Ethanol extract of Bombax costatum
- NETR:
-
N-Hexane extract of Bombax costatum
- Na:
-
Sodium
- K:
-
Potassium
- Cl:
-
Chloride
- HCO3:
-
Bicarbonate
- RBC:
-
Red bood cell count
- WBC:
-
White blood cell count
- Hb:
-
Hemoglobin concentration
- PCV:
-
Packed cell volume
- MCV:
-
Mean corpuscular volume
- MCH:
-
Mean corpuscular hemoglobin
- MCHC:
-
Mean corpuscular hemoglobin concentration
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- CB:
-
Conjugated bilirubin
- TB:
-
Total bilirubin
- TP:
-
Total protein
References
Abraham IG, Ahmad MH (2021) Preliminary sub-acute toxicological assessment of methanol leaves extract of Culcasia angolensis (Araceae) in Wistar rats. Bull Natl Res Centre 45(1):1–10. https://doi.org/10.1186/s42269-021-00686-9
Ahmed AM, Audu HA, Garba SH, Dibal NI, Chiroma SM (2022) Acute toxicity study of Guiera senegalensis J.F. Gmel methanolic leaf extract in Wistar albino rats through oral administration. Comp Clin Pathol. https://doi.org/10.1007/s00580-022-03387-5
Assogba GA, Fandohan AB, Salako VK, Assogbadjo AE (2017) Usages de Bombax costatum(Malvaceae) dans les terroirs riverains de la réserve de biosphère de la Pendjari, République du Bénin. Bois Et Forets Tropiques 333(3):17–33
Auti ST, Kulkarni YA (2019) Acute and 28-day repeated dose oral toxicity study of caraway oil in rats. Drug Metab Pers Therapy. https://doi.org/10.1515/dmpt-2019-0011
Bamikunle MO, ZeziYa’u AUJ (2022) Effect of methanol root extract of Eriosema psoraleoides on biochemical and haematological parameters and cyclooxygenase levels in rats. J Ethnopharmacol 295:115434
Chaudhary A, Sharma S, Mittal A, Gupta S, Dua A (2020) Phytochemical and antioxidant profiling of Ocimum sanctum. J Food Sci Technol 57(10):3852–3863
Chiroma SS, Nazifi AB, Ya’u J, Aliyu M, Bichi LA, Chiroma SM, (2022) Anti-seizure properties of Ipomoea asarifolia (Desr.)(Convolvulaceae) ethanolic leaf extract in laboratory animals. Bull Natl Res Centre 46(1):1–7. https://doi.org/10.1186/s42269-022-00898-7
Dibong SD, Ottou PBM, Vandi D, Ndjib RC, Monkam Tchamaha F, Mpondo ME (2015) Ethnobotanique des plantes medicinales anti hemorroidaires des marches et villages du Centre et du Littoral Cameroun. J Appl Biosc 96:9072–9093
Drury RA, Wallington EA, Cancerson R (1976) Carlton’s histopathologicaltechniques, 4th edn. Oxford University Press, Oxford
Evans WC (2009) Trease and Evans’ Pharmacognosy, 16th edn. Elsevier, London, pp 133–148
Enenebeaku UE, Ukwandu NC, Mgbemena IC, Nwigwe HC, Enenebeaku CK, Duru CE, Ogidi OI (2021) Oral acute toxicity and antimalarial potentials of aqueous and methanolic extracts of roots, leaves and stem of Dictyandra arborescens (Welw.) on Plasmodium berghei infected mice. Bull Natl Res Centre 45(1):1–13. https://doi.org/10.1186/s42269-021-00530-0
Gbenou JD, Ahounou JF, Ladouni P, Agbodjogbe WKDD, Tossou R, Dansou P (2011) Proprietes Anti-Inflammatoires Des Extraits Aqueux De Sterculia setigera Delile Et Du Melange Aframomum melegueta K Schum- Citrus aurantifolia Christm Et Panzer. Int J Biol Chem Sci 5(2):634–641
Heinrich M, Mah J, Amirkia V (2021) Alkaloids used as medicines: structural phytochemistry meets biodiversity—an update and forward look. Molecules 2021:26. https://doi.org/10.3390/molecules26071836
LeaBlondelle KD, Simplice FH, Herve NA, Eglantine KW, Roland RN, Linda DKJ, Balbine KN, Desire GNS, Guillaume CW, Alin C (2022) Antidepressant, anti-amnesic and vasoprotective effect of Bombax costatum Pellegr. & Vuillet aqueous stem bark extract on chronic mild unpredictable stress induced in rat. J Ethnopharmacol 293:115315
Maiga A, Diallo D, Bye R, Paulsen BS (2005) Determination of some toxic and essential metal ions in medicinal and edible plants from Mali. J Agric Food Chem 53:2316–2321
Maimaiti A, Jing-Jing L, Shi L (2021) Investigating the acute and sub-acute toxicity of medicinal Cuscuta chinensis Lam plant. J Ethnopharmacol 273:114005
Marrelli M, Conforti F, Araniti F, Statti GA (2016) Effects of saponins on lipid metabolism: a review of potential health benefits in the treatment of obesity. Molecules 21:1404. https://doi.org/10.3390/molecules21101404
Mohammed N, Anuka JA, Musa AM, Yau J (2019) Acute and sub-chronic toxicity study of methanol stem bark extract of Bombax costatum Pellgr. Et Vuillet (Bombacaceae) in mice. J Herb Drugs 10(30):109–116
Mukinda JT, Eagles FK (2010) Acute and sub-chronic oral toxicity profile of the aqueous extract of Polygala fruticosa in female mice and rats. J Ethnopharmacol 128:236–240
National Research Council (2011) National Institutes of Health Guide for the Care and Use of Laboratory Animals. https://doi.org/10.17226/12910
Nazifi AB, Ahmed A, Hassan FI, Mohammed N, Danbala AA, Odoma S (2020) Comparative anticonvulsant activity of leaf, stem bark and root bark extracts of Bombax costatum Pellegr. and Vuillet in acute models of epilepsy. Trop J Natl Prod Res 4(10):844–849
Organization for Economic Co-operation and Development (OECD) (2008) Acute oral toxicity: up-and-down procedure. OECD Guid Test Chem 425:1–27
Patrick-Iwuanyanwu KC, Wegwu MO, Ayalogu EO (2007) Prevention of CCl4-induced liver damage by ginger, garlic and vitamin E. Pak J Biol Sci 10:617–621
Pizzi A (2021) Tannins medical/pharmacological and related applications: a critical review. Sustain Chem Pharm 22:100481
Porwal M, Khan NA, Maheshwari KK (2017) Evaluation of acute and subacute oral toxicity induced by ethanolic extract of Marsdenia tenacissima leaves in experimental rats. Sci Pharm 85(3):E29
Ugwah-Oguejiofor CJ, Okoli CO, Ugwah MO, Umaru ML, Ogbulie CS, Mshelia HE, Umar M, Njan AA (2019) Acute and sub-acute toxicity of aqueous extract of aerial parts of Caralluma dalzielii N. E. Brown in mice and rats. Heliyon 5:e01179
Vargas F, Romecín P, García-Guillén AI, Wangesteen R, Vargas-Tendero P, Paredes MD, Atucha NM, García-Estañ J (2018) Flavonoids in kidney health and disease. Front Physiol 9:394. https://doi.org/10.3389/fphys.2018.00394
Wanbara N, Taiwe GS, Njapdounke JSK, Sidiki N, Nloga AMN, Ngo Bum E (2021) Anxiolytic and antipyretic properties of the root aqueous extract of Bombax costatum Pellegr. et Vuillet. (Bombacaceae) in mice: implication of behavioural and neurochemical approaches. 02 GSC Biol Pharm Sci 15:140–150
Acknowledgements
Author’s acknowledged the contributions of Pharm. S.S Chiroma of Pharmacist Council of Nigeria, Abuja and Dr. Pharm. A.B. Nazifi of Pharmacology Department, Faculty of Pharmacy Bayero University Kano, for their support and valuable advice during this study.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
AMB, ISM, SHA and SMC conceptualized the work; AMB, AA and BMI carried-out the laboratory animal handling and treatments; SMC, ISM and SHA supervised the work. AMB and SMC performed the data analysis; SMC analyzed the histological slides; AA and BMI carried-out the heamatological analysis, while AMB wrote the draft of the manuscript. SMC, SHA and ISM revised the draft, while all authors approved the final version of the manuscript before submission to journal.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experimental procedures were carried-out strictly in accordance with the “Guide to the care and use of laboratory animals in research and teaching” as detailed in NIH publications (Council 2011).
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bello, A.M., Malgwi, I.S., Adegoke, S.H. et al. Oral acute toxicity study on stem bark extracts of Bombax costatum Pellegr. and Vuillet on wistar albino rats. Bull Natl Res Cent 46, 254 (2022). https://doi.org/10.1186/s42269-022-00944-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-022-00944-4