Abstract
Background
Fructose-rich diets are linked to the epidemic of metabolic syndrome and co-morbidities including nephropathy. Phytochemicals are increasingly being used for medicinal purposes due to their perceived safety compared to conventional drugs. Although previously shown to beneficially programme metabolism, these phytochemicals might have a negative impact on metabolic health when introduced early in life. We investigated whether neonatal administration of curcumin to rats would impact their response in adolescence to a high-fructose diet. Sprague-Dawley pups (n = 128) were administered either 0.5% dimethyl sulphoxide, curcumin (500 mg kg−1), fructose (20%, w/v) or a combination of curcumin and fructose from post-natal day 6 to 21. Each group was then subdivided into two; one had tap water, while the other had fructose (20%, w/v) to drink for 6 weeks.
Results
There were no differences (p > 0.05) in the fasting blood glucose, triglycerides, cholesterol, plasma insulin and adiponectin concentrations across the groups. The renal corpuscular, glomerular tuft and Bowman capsular areas were similar (p > 0.05) across the treatment groups, in both sexes. Post-weaning fructose alone induced tubular secretions and mesangial proliferation in the kidneys which were prevented by curcumin.
Conclusions
Despite the observed benefits in adolescence, the impact of curcumin on renal health beyond adolescence needs to be explored.
Similar content being viewed by others
Background
The epidemic of childhood obesity (Ludwig 2018) is a public health crisis affecting communities in developed and developing countries (LBD Double Burden of Malnutrition Collaborators 2020). Consequently, there is an increase in the occurrence of metabolic syndrome (MetS) in children and adolescents (Grabia et al. 2021). Childhood MetS has the potential to be projected into adulthood (Magnussen et al. 2018), thereby further increasing the adult prevalence of MetS (Kaur 2014).
Increased consumption of fructose-rich diets and sedentary lifestyles have been identified as major drivers of this global obesity epidemic (Mamikutty et al. 2015; Hannou et al. 2018) that is also linked to interactions between genetic and environmental factors (Kelishadi et al. 2014). Ding et al. (2018) reports that the cardiometabolic risk factors that cluster together in MetS are also independent risk factors for chronic kidney disease (CKD). The consumption of a high-fructose diet has been shown to cause structural and functional changes in the kidneys that increase the risk of developing CKD in both human and animal experimental models (Yu et al. 2018) The assessment of kidney function using surrogate markers such as plasma concentrations of urea and creatinine has limited ability to detect early disease as their concentrations only become elevated when more than 50–60% of renal function is lost (Scarfe et al. 2015). The histopathological analysis of renal tissues reveals early changes that may point to a developing kidney disease (Scarfe et al. 2015). Thus, although invasive, kidney biopsies and histological assessment have long been considered the gold standard for investigating renal function.
Early life stressor events, especially those occurring during gestation and the suckling phases, can cause epigenetic changes that could then result in the permanent alteration of the physiology of the individual, a phenomenon called developmental programming (Collden et al. 2015). Such stressor events include nutritional, hormonal or pharmacological interventions (Patel and Srinivasan 2002). For example, when a high-fructose diet was administered to suckling Sprague-Dawley rats, it produced permanent changes in body mass and induced insulin resistance later in adulthood (Huynh et al. 2008).
The environment and the type of diet introduced early in life can thus either initiate or reverse some epigenetic risk markers of MetS (Park et al. 2017). Previous studies have shown the benefits of interventions with natural products and phytochemicals in the suckling period in programming the metabolic health of adolescent and adult rats (Ibrahim et al., 2017; Lembede et al. 2018; Nyakudya et al. 2018). The use of natural products to improve metabolic health is attracting international interest due to their perceived safety, availability and affordability (Bahmani et al. 2014). It is therefore necessary to identify other compounds that can be used to either program or reprogram against the development of adverse metabolic dysfunction. However, despite the well-reported health benefits of natural phytochemicals, there are also several reports of toxicity from their use (Yang et al. 2018; Guldiken et al. 2018). The kidneys are particularly susceptible to injury due to toxic phytochemicals (Brown 2017).
The biologically active polyphenol, curcumin, is a component of turmeric, isolated from the rhizome of Curcuma longa from the ginger family, Zingiberaceae (Sahebkar 2013). Turmeric has been used for several centuries especially in Asia, as a remedy for common ailments including diarrhoea, cough and jaundice (Sahebkar 2013). Curcumin has been shown to have anti-obesity (Shao et al. 2012), anti-diabetic (Panahi et al. 2017), antioxidant (Ali et al. 2018), anti-inflammatory (Elham et al. 2018) and reno-protective (Huang et al. 2018) properties among others.
Due to the wide array of therapeutic properties reported for curcumin against metabolic conditions, including the renal complications of MetS, and the need to identify and validate natural compounds for use in programming or reprogramming against MetS, we hypothesised that orally administering curcumin to suckling pups will protect them later in adolescence against adverse metabolic programming effects of a high-fructose diet pre- and post-weaning without negatively impacting the kidneys.
Methods
Ethical approval, compliance to ARRIVE guidelines and study location
The protocols and procedures used in the study were approved by the Animal Ethics Screening Committee of the University of the Witwatersrand, Johannesburg (AESC 2016/04/18/B) and complied with international ethical guidelines and standards for the care and use of laboratory animals. The ARRIVE guidelines have been used in writing up this manuscript. The study was conducted in the Central Animal Service (CAS) research facility of the University of the Witwatersrand.
Chemicals and reagents
Dimethyl sulphoxide (DMSO; Sigma-Aldrich, Missouri, USA), used as the vehicle solvent, was made up to a final concentration of 0.5% in distilled water. Curcumin (Sigma-Aldrich, Missouri, USA) 500 mg was dissolved in 10 ml of a 0.5% DMSO solution. However, the curcumin did not dissolve completely and was therefore administered as a suspension. Fructose (Nature’s choice, Randvaal, South Africa) solution was made to 20% w/v using 0.5% DMSO in distilled water in the pre-weaning period and tap water post-weaning. The commercial rat chow was from Epol (Epol®, Centurion, South Africa).
Study animals, housing and general care
Six-day-old male and female Sprague-Dawley pups from fourteen dams that were sourced from the Central Animal Services Unit of the University of the Witwatersrand were used in the study. Each dam had a litter size of between 8 and 12 pups. The suckling pups nursed freely with their dams while housed in perspex cages which were lined with wood shavings and shredded paper for environmental enhancement. The dams had ad libitum access to pelletised rat chow (Epol®, Centurion, South Africa) and plain tap water. The ambient temperature in the room was maintained at 26 ± 2 °C with adequate ventilation and a 12-h light cycle (lights on at 07.00 h).
At post-natal day 21, the rats were weaned from their dams and placed in individual cages. The weaned rats were allowed ad libitum access to normal rat chow and either tap water or fructose solution (20%, w/v) as their drinking fluid. The dams were returned to stock.
Study design
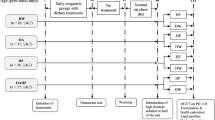
The study was carried out in two interventional phases (see Fig. 1). The date of delivery of the pups was taken as day zero. The pups were allowed to acclimatise to the study environmental conditions till post-natal day (PN) six. The first phase of the study was conducted in the pre-weaning period from PN six to PN 21, and it was aimed at inducing programming with curcumin and fructose. The second phase of the interventions extended from PN 21 and continued for 6 weeks until PN63 (late adolescence). The aim of the second phase of the experiment was to determine whether the initial interventions would have any effect on the response of the rats to a high-fructose diet post-weaning.
The litters (n = 128, males = 65, females = 63) from fourteen Sprague-Dawley dams were used in the study. During the first phase of the study, the pups were allocated in a split-litter pattern to four treatment groups replicated by sex, namely:
-
Group 1 (negative control): administered 10 ml kg−1 of 0.5% DMSO
-
Group 2: administered curcumin, 500 mg kg−1 body mass. Our choice of dosage was based on previous studies that have used a similar dose which had anti-obesity (Ejaz et al. 2009) and anti-diabetic (Miao et al. 2015) effects.
-
Group 3: administered fructose solution (20% w/v)
-
Group 4: administered curcumin 500 mg kg−1 + fructose solution (20%, w/v)
All the treatments were administered once daily at a volume of 10 ml kg−1 daily via oral gavage.
In the second phase of the study, the rats from each of the initial four groups were further subdivided into two subgroups and weaned onto normal rat chow. One of the subgroups had tap water, and the other had fructose solution (20%, w/v/) as their drinking fluids ad libitum. The treatments in phase two were administered for 6 weeks until PN 63. Thus, at post-weaning, there were eight groups of rats which were replicated by sex (Fig. 1).
Body mass measurements
The pups were weighed daily during the first phase of the study to adjust their treatments to ensure constant doses. In the second phase, the rats were weighed twice weekly to monitor their growth and general health.
Terminal procedures
On post-natal day 62, the rats were fasted overnight (12 h) and then weighed. A drop of blood collected via a pin prick to the tail vein was used to determine the fasting blood glucose (FBG) concentration using a calibrated glucometer (Contour Plus™, Bayer Corporation, Mishawaka, USA). The length of the rats, from the nasal opening to the anus (Poudyal et al. 2010), and abdominal circumferences of the rats were measured using a tape measure (Novelli et al. 2007). The body mass index of the rats was computed using the formula:
The rats were then euthanised with sodium pentobarbitone (150 mg kg−1 body mass, Euthapent; Kyron laboratories, South Africa). Blood was drawn via cardiac puncture and transferred into heparinised blood collecting tubes (Becton Dickinson Vacutainer Systems Europe, Meylan Cedex, France). The blood was centrifuged (Hermle Z 230A, B Hermle AG, Germany) at 4000×g at 4 °C for 15 min, and the plasma was collected for biochemical and hormonal assays. The kidneys, pancreas, visceral and epididymal (in male rats) fat pad were removed and weighed. The kidneys were preserved in 10% phosphate-buffered formalin for histology.
Determination of the plasma concentrations of adiponectin, insulin and computation of HOMA-IR
Plasma concentrations of adiponectin and insulin were determined using a rat adiponectin ELISA kit (Elabscience ® Rat ADP/Acrp30 ELISA kit, Houston, TX, USA) and rat insulin ELISA kit [Elabscience ® INS (Insulin) ELISA kit, Houston, TX, USA], respectively. The homeostatic model of insulin resistance was computed using the following formula:
Determination of the plasma concentration of triglycerides and total cholesterol
Plasma concentration of triglycerides (TGs) and total cholesterol was determined using a colorimetric analyser (IDEXX Catalyst Dx™ connected to an IDEXX Vet Lab station, IDEXX Laboratories Inc., Westbrook, Maine, USA).
Histomorphometry of the kidneys
The kidney samples were processed using an automatic tissue processor (Micron STP 120, Thermo Fischer Scientific, USA) and then embedded in paraffin wax. The samples were sectioned onto glass slides at 3 µm thickness using a rotary microtome (Leica Biosystems, USA) and stained with Masson’s trichrome (Bancroft and Gamble 2008) for histopathological assessment. Representative photomicrographs of the MT-stained sections were acquired using an Olympus BH2 RFCA microscope (Olympus corporation, Japan). ImageJ area tool was used to measure the renal corpuscular (RCA) and glomerular tuft (GTA) areas. For each renal corpuscle, the Bowman’s capsule area (BCA) was determined by subtracting the glomerular tuft area from the renal corpuscular area (BCA = RCA minus GTA). From each group, at least 20 renal corpuscles and glomeruli that were clearly visible were acquired at 40× magnification.
Statistical analyses
Data were analysed using the GraphPad Prism version 7.0 statistical software (GraphPad Software Inc., San Diego, CA, USA). Following normality test, data were confirmed to bear Gaussian distribution with symmetry around the mean. Accordingly, data were expressed as mean ± standard error of mean (SEM). One-way analysis of variance (ANOVA) was used to compare the means between all the eight study groups separately in the male and female rats. Significance between groups was identified using the Bonferroni post hoc. A statistical significance was assumed when p < 0.05.
Results
Effect of curcumin and fructose on body mass
The male and female rats gained significant body mass (P < 0.0001), from induction through weaning and termination (Table 1). However, there was no significant difference (p > 0.05) across the treatment groups at the different time points in both sexes.
Effect of curcumin and fructose on markers of adiposity
In both sexes, there were no differences observed (p > 0.05) across the treatment groups in all the measured parameters (Table 2).
Effect of curcumin and fructose on plasma concentration of adiponectin, fasting blood glucose, insulin concentration and homeostatic model of insulin resistance
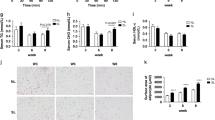
There were no significant differences (p > 0.05) in the plasma concentration of adiponectin across the treatment groups in both sexes (Fig. 2a, b).
Changes in plasma adiponectin concentration at post-natal day 63 of a male and b female rats administered curcumin during suckling. Data expressed as mean ± SEM, n = 7–9 per group. Using ANOVA and Bonferroni post hoc test, there were no differences (p > 0.05) in the plasma adiponectin concentration of the rats across the treatment groups. DMSO + TW = 10 ml kg−1 of a 0.5% dimethyl sulphoxide solution as neonates and plain tap water post-weaning, DMSO + FW = 10 ml kg−1 of a 0.5% dimethyl sulphoxide solution as neonates and fructose (20%, w/v) as drinking fluid post-weaning, CC + TW = curcumin (500 mg kg−1 in 0.5% DMSO) as neonates and plain tap water post-weaning, CC + FW = curcumin (500 mg kg−1 in 0.5% DMSO) as neonates and fructose (20%, w/v) as drinking fluid post-weaning, FW + TW = fructose (20%, w/v) as neonates and plain tap water post-weaning, FW + FW = fructose (20%, w/v) as neonates and fructose (20%, w/v) as drinking fluid post-weaning, CCFW + TW = curcumin (500 mg kg−1) and fructose (20%, w/v) in 0.5% DMSO as neonates and plain tap water post-weaning, CCFW + FW = curcumin (500 mg kg−1) and fructose (20%, w/v) in 0.5% DMSO as neonates and fructose (20%, w/v) post-weaning
In both sexes, fasting blood glucose, plasma insulin and HOMA-IR were similar (p > 0.05) across the treatment groups (Table 3).
Effect of curcumin and fructose on fasting plasma triglycerides and total cholesterol
There was no significant difference (p > 0.05) in the fasting plasma triglycerides and total cholesterol across the treatment groups in both sexes (Table 4).
Effect of curcumin and fructose on the masses of the kidneys and pancreata
The absolute and relative (%BM) masses of the kidneys and pancreas of adolescent male and female Sprague-Dawley rats are presented in Table 5. There were no differences (p > 0.05) observed across the treatment groups in both sexes in the measured viscera.
Effect of curcumin and fructose on kidney histomorphology
Table 6 shows the renal corpuscular area (RCA), glomerular tuft area (GTA) and bowman capsular area (BCA) of male and female rats. Although there were no significant differences in the RCA, GTA and BCA of both male and female rats across the treatment groups, these parameters showed a trend of being lower in the fructose fed rats.
Figure 3a, b shows representative photomicrographs of MT-stained kidney tissues of male and female Sprague-Dawley rats. In both male and female rats, the negative control groups (DMSO + TW) showed normal glomerular, tubular and interstitial architecture. The males that received fructose post-weaning only (DMSO + FW) and as double hit neonatally and post-weaning (FW + FW) showed some mesangial proliferation and mild tubular secretions; the same group of females showed increased tubular secretions. In both male and female rats, neonatal administration of curcumin prevented the post-weaning fructose only induced changes in kidney tissues. Additionally, the kidney samples from male and female rats that were administered curcumin during suckling and no fructose at all showed mild-to-moderate tubular secretions.
Kidney histology (MT staining) of representative adolescent a male and b female rats administered curcumin during suckling. The black arrows point to mild-to-moderate tubular secretions, while the green arrows point to mesangial proliferation. DMSO + TW = 10 ml kg−1 of a 0.5% dimethyl sulphoxide solution as neonates and plain tap water post-weaning, DMSO + FW = 10 ml kg−1 of a 0.5% dimethyl sulphoxide solution as neonates and fructose (20%, w/v) as drinking fluid in the growing period, CC + TW = curcumin (500 mg kg−1 in 0.5% DMSO) as neonates and plain tap water in the growing period, CC + FW = curcumin (500 mg kg−1 in 0.5% DMSO) as neonates and fructose (20%, w/v) as drinking fluid in the growing period, FW + TW = fructose (20%, w/v) as neonates and plain tap water in the growing period, FW + FW = fructose (20%, w/v) as neonates and fructose (20%, w/v) as drinking fluid in the growing period, CCFW + TW = curcumin (500 mg kg−1) and fructose (20%, w/v) in 0.5% DMSO as neonates and plain tap water in the growing period, CCFW + FW = curcumin (500 mg kg−1) and fructose (20%, w/v) in 0.5% DMSO as neonates and fructose (20%, w/v) in the growing period. Scale bar in DMSO + TW = 100 µm applies to all the figures
Discussion
In several studies using adult rodents, a high-fructose diet was shown to induce features of metabolic syndrome. For instance, when fructose (10%, w/v) was fed to adult male Sprague-Dawley rats for 8 weeks, it increased the concentrations of fasting blood glucose, insulin, triglycerides, total and low-density lipoprotein cholesterol (Fadlalla and Khojah 2017). Mamikutty et al. (2014) also found features of obesity such as hypertrophy of adipocytes, elevated levels of serum triglycerides and hyperglycaemia when they administered a 20% fructose w/v solution for 8 weeks to adult male Wistar rats. However, in the current study, both the pre-weaning and post-weaning administration of fructose did not induce overt features of MetS. In this regard, the terminal body masses, BMI, visceral fat (absolute and relative to body mass) and epididymal fat pad mass (in males) were all similar across the treatment groups in both sexes. Additionally, the concentrations of insulin and adiponectin and metabolic substrates (FBG, TG and total cholesterol) were similar across the treatment groups in both male and female rats.
There are possible explanations for the absence of the effects of a high-fructose diet in the neonatal and growing rats. Firstly, it is important to state that the target for this study was the adolescent age group, and thus the use of suckling pups. In children and adolescents, the epidemic of overweight and obesity (Orlando et al. 2018) is strongly linked to increased consumption of high-sugar foods (Magriplis et al. 2021). Suckling pups and growing rats have a higher body surface area-to-volume ratio compared to adult rats which results in a higher metabolic rate and subsequent oxidation of the excess fructose without manifesting its deleterious effects (Tillman et al. 2014). The resistance of young rats to fructose-induced metabolic derangements such as dyslipidaemia and hyperglycaemia is considered a protective mechanism acquired early in life (Ghezzi et al. 2012), which may be due to the efficiency of the GLUT4 transporters which are the insulin-sensitive glucose transporters (Ghezzi et al. 2012). The GLUT5 transporters in the small intestines responsible for shuttling fructose across membranes are few and immature from birth till weaning (Patel et al. 2015; Xu and Ghishan 2018), although they may precociously mature before weaning when fructose is administered early (Castello et al. 1995; Ferraris 2001). Unfortunately, in the present study the GLUT5 transporters were not assayed at weaning to determine their level of expression.
In another study, alteration in neonatal nutrition of suckling rats in the form of high carbohydrate milk formula resulted in differences in body mass only at about post-natal day 100 (Patel and Srinivasan 2010). The age of the rats in the current study at termination was 63 days (adolescent/early adulthood) which might explain the absence of the features of MetS.
Though the quantitative scoring of three areas (RCA, GTA and BCA) of the kidney tissue of the adolescent rats did not show any significant differences across treatment groups in both sexes, the tendency for a reduction in the measured parameters in fructose fed rats needs to be explored in studies extending beyond adolescence. Visual histopathological analysis of the representative slides from the groups showed some interesting findings. We believe that the histopathological changes were only becoming apparent in some of the rats but were masked when the quantitative analysis was done using a larger number of renal corpuscles and glomeruli. Whereas the post-weaning administration of fructose appeared to induce histopathological changes in the kidney tissues of the rats, oral administration of curcumin during suckling showed promise in preventing the development of those changes in both sexes. Curcumin has previously been shown to reverse the vacuolar degeneration of glomerular cells associated with cyclosporin A (Huang et al. 2018) and cadmium tetrachloride-induced nephrotoxicity (Hismiogullari et al. 2015). We thus speculate that the neonatal administration of curcumin to the rats may have epigenetically programmed the rats’ kidneys to resist the adverse effects of a high-fructose diet post-weaning. However, this requires further investigation.
In rats, the kidneys though functionally immature at birth undergo rapid development and attain optimal functioning at or around weaning (Seely 2017). The neonatal kidney is characterised by reduced number and functionality of the tubules and consequently reduced kidney functions such as glomerular filtration rate, tubular secretion and absorption compared to adults (Seely 2017). Our neonatal interventions with curcumin and fructose may have overwhelmed the scanty tubules and affected their proper functioning on a long-term basis and thus resulting in the increased tubular secretions observed in the kidney sections.
The masses of the kidneys and pancreas were also similar across treatment groups in both sexes. Since organ weights are used in toxicological studies as an indication of early deleterious effects of compounds (Sellers et al. 2007; Shafaei et al. 2015), it could be inferred that both curcumin and fructose did not exert toxic effects on the weighed organs.
Conclusions
Oral administration of curcumin and fructose during suckling, followed by a high-fructose diet post-weaning, did not negatively affect the general metabolic health of adolescent male and female rats. However, the administration of curcumin during suckling prevented the fructose-induced histological changes in the kidneys of the rats. The neonatal administration of curcumin alone did not alter BCA, GTA and RCA. Thus, curcumin could be used with caution in neonates to programme against fructose-induced renal injury later in life.
Availability of data and material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MetS:
-
Metabolic syndrome
- CKD:
-
Chronic kidney disease
- PN:
-
Post-natal day
- DMSO:
-
Dimethyl sulphoxide
- CC:
-
Curcumin
- FW:
-
Fructose
- NRC:
-
Normal rat chow
- PTW:
-
Plain tap water
- FBG:
-
Fasting blood glucose
- BMI:
-
Body mass index
- HOMA-IR:
-
Homeostatic Model Assessment of Insulin Resistance
- TGs:
-
Triglycerides
- SEM:
-
Standard error of mean
- ANOVA:
-
Analysis of variance
- RCA:
-
Renal corpuscular area
- GTA:
-
Glomerular tuft area
- BCA:
-
Bowman capsular area
- GLUT4:
-
Glucose transporter 4
- GLUT5:
-
Glucose transporter 5
References
Ali BH, Suhail AS, Yousuf AS, Jamila AK, Shadia AB, Mohammed A et al (2018) Curcumin ameliorates kidney function and oxidative stress in experimental chronic kidney disease. Basic Clin Pharmacol Toxicol 122(1):65–73
Bahmani M, Zargaran A, Rafieian-Kopaei M, Saki K (2014) Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pac J Trop Med 7:S348–S354
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Elsevier Health Sciences
Brown AC (2017) Kidney toxicity related to herbs and dietary supplements: online table of case reports. Part 3 of 5 series. Food Chem Toxicol 107:502–519
Castello A, Guma A, Sevilla L, Furriols M, Testar X, Palacin M et al (1995) Regulation of GLUT5 gene expression in rat intestinal mucosa: regional distribution, circadian rhythm, perinatal development and effect of diabetes. Biochem J 309(1):271–277
Collden G, Balland E, Parkash J, Caron E, Langlet F, Prevot V et al (2015) Neonatal overnutrition causes early alterations in the central response to peripheral ghrelin. Mol Metab 4(1):15–24
Ding C, Yang Z, Wang S, Sun F, Zhan S (2018) The associations of metabolic syndrome with incident hypertension, type 2 diabetes mellitus and chronic kidney disease: a cohort study. Endocrine 60(2):282–291
Ejaz A, Wu D, Kwan P, Meydani M (2009) Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr 139(5):919–925
Elham A, Abbas MA, Thomas J, Amirhossein S (2018) Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: a nature-made jack-of-all-trades? J Cell Physiol 233(2):830–848
Fadlalla E, Khojah E (2017) Effect of aqueous extract of Tamarindus indica L. seeds and crocus sativus on fructose-induced metabolic syndrome in rats. Adv Nutr 8(1):10
Ferraris RP (2001) Dietary and developmental regulation of intestinal sugar transport. Biochem J 360(2):265–276
Ghezzi AC, Cambri LT, Botezelli JD, Ribeiro C, Dalia RA, Rostom-Mello MA (2012) Metabolic syndrome markers in wistar rats of different ages. Diabetol Metab Syndr 4(1):16. https://doi.org/10.1186/758-5996-4-16
Grabia M, Markiewicz-Żukowska R, Socha K (2021) Prevalence of metabolic syndrome in children and adolescents with type 1 diabetes mellitus and possibilities of prevention and treatment: a systematic review. Nutrients 13(6):1782
Guldiken B, Ozkan G, Catalkaya G, Ceylan FD, Yalcinkaya IE, Capanoglu E (2018) Phytochemicals of herbs and spices: health versus toxicological effects. Food Chem Toxicol 119:37–49
Hannou SA, Haslam DE, McKeown NM, Herman MA (2018) Fructose metabolism and metabolic disease. J Clin Invest 128:545–555
Hismiogullari AA, Hismiogullari SE, Karaca O, Sunay FB, Paksoy S, Can M et al (2015) The protective effect of curcumin administration on carbon tetrachloride (CCl4)-induced nephrotoxicity in rats. Pharmacol Rep 67(3):410–416
Huang J, Yao X, Weng G, Qi H, Ye X (2018) Protective effect of curcumin against cyclosporine A-induced rat nephrotoxicity. Mol Med Rep 17(4):6038–6044
Huynh M, Luiken JJ, Coumans W, Bell RC (2008) Dietary fructose during the suckling period increases body weight and fatty acid uptake into skeletal muscle in adult rats. Obesity 16(8):1755–1762
Ibrahim KG, Chivandi E, Mojiminiyi FBO, Erlwanger KH (2017) The response of male and female rats to a high-fructose diet during adolescence following early administration of Hibiscus sabdariffa aqueous calyx extracts. J Dev Orig Health Dis 8(6):628–637
Kaur J (2014) A comprehensive review on metabolic syndrome. Cardiol Res Pract. https://doi.org/10.1155/2014/943162
Kelishadi R, Mansourian M, Heidari-Beni M (2014) Association of fructose consumption and components of metabolic syndrome in human studies: a systematic review and meta-analysis. Nutrition 30(5):503–510
LBD Double Burden of Malnutrition Collaborators (2020) Mapping local patterns of childhood overweight and wasting in low- and middle-income countries between 2000 and 2017. Nat Med 26(5):750–759
Lembede BW, Erlwanger K, Nkomozepi P, Chivandi E (2018) Effect of neonatal orally administered S-allyl cysteine in high-fructose diet fed Wistar rats. J Dev Orig Health Dis 9(2):160–171
Ludwig DS (2018) Epidemic childhood obesity: not yet the end of the beginning. Pediatrics 141:e20174078. https://doi.org/10.1542/peds.2017-4078
Magnussen CG, Fraser BJ, Raitakari OT (2018) Pediatric metabolic syndrome: long-term risks for type 2 diabetes and cardiovascular disease. In: Freemark M (ed) Pediatric obesity contemporary endocrinology. Humana Press, Cham, pp 511–526
Magriplis E, Michas G, Petridi E, Chrousos GP, Roma E, Benetou V et al (2021) Dietary sugar intake and its association with obesity in children and adolescents. Children 8(676):1–14
Mamikutty N, Thent ZC, Sapri SR, Sahruddin NN, Mohd Yusof MR, Haji Suhaimi F (2014) The establishment of metabolic syndrome model by induction of fructose drinking water in male wistar rats. BioMed Res Int. https://doi.org/10.1155/2014/263897
Mamikutty N, Thent ZC, Haji SF (2015) Fructose-drinking water induced nonalcoholic fatty liver disease and ultrastructural alteration of hepatocyte mitochondria in male Wistar rat. BioMed Res Int. https://doi.org/10.1155/2015/895961
Miao M, Guo L, Tian S, Wang T (2015) Effects of curcumine on antioxidation in diabetic rats. Pak J Pharm Sci 28:371–373
Novelli E, Diniz Y, Galhardi C, Ebaid G, Rodrigues H, Mani F et al (2007) Anthropometrical parameters and markers of obesity in rats. Lab Anim 41(1):111–119
Nyakudya T, Mukwevho E, Nkomozepi P, Erlwanger K (2018) Neonatal intake of oleanolic acid attenuates the subsequent development of high fructose diet-induced non-alcoholic fatty liver disease in rats. J Dev Orig Health Dis 9(5):500–510
Orlando A, Cazzaniga E, Giussani M, Palestini P, Genovesi S (2018) Hypertension in children: role of obesity, simple carbohydrates, and uric acid. Front Public Health. https://doi.org/10.3389/fpubh.2018.00129
Panahi Y, Khalili N, Sahebi E, Namazi S, Karimian MS, Majeed M et al (2017) Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: a randomized controlled trial. Inflammopharmacology 25(1):25–31
Park J-H, Kim S-H, Lee MS, Kim M-S (2017) Epigenetic modification by dietary factors: implications in metabolic syndrome. Mol Aspects Med 54:58–70
Patel SM, Srinivasan M (2002) Metabolic programming: causes and consequences. J Biol Chem 227(3):1629–1632
Patel MS, Srinivasan M (2010) Metabolic programming due to alterations in nutrition in the immediate postnatal period. J Nutr 140(3):658–661
Patel C, Douard V, Yu S, Tharabenjasin P, Gao N, Ferraris RP (2015) Fructose-induced increases in expression of intestinal fructolytic and gluconeogenic genes are regulated by GLUT5 and KHK. Am J Physiol-Regul Integr Comp Physiol 309(5):R499–R509
Poudyal H, Campbell F, Brown L (2010) Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate-, high fat-fed rats. J Nutr 140(5):946–953
Roy SM, Fields DA, Mitchell JA, Hawkes CP, Kelly A, Wu GD et al (2019) Body mass index is a better indicator of body composition than weight-for-length at age 1 month. J Pediatrics 204:77–83
Sahebkar A (2013) Why it is necessary to translate curcumin into clinical practice for the prevention and treatment of metabolic syndrome? BioFactors 39(2):197–208
Scarfe L, Rak-Raszewska A, Geraci S, Darssan D, Sharkey J, Huang J et al (2015) Measures of kidney function by minimally invasive techniques correlate with histological glomerular damage in SCID mice with adriamycin-induced nephropathy. Sci Rep 5:13601. https://doi.org/10.1038/srep13601
Seely JC (2017) A brief review of kidney development, maturation, developmental abnormalities, and drug toxicity: juvenile animal relevancy. J Toxicol Pathol 30(2):125–133
Sellers RS, Mortan D, Michael B, Roome N, Johnson JK, Yano BL et al (2007) Society of toxicologic pathology position paper: organ weight recommendations for toxicology studies. Toxicol Pathol 35(5):751–755
Shafaei A, Esmailli K, Farsi E, Aisha AF, Majid AMSA, Ismail Z (2015) Genotoxicity, acute and subchronic toxicity studies of nano liposomes of Orthosiphon stamineus ethanolic extract in Sprague Dawley rats. BMC Complement Altern Med 15(1):360. https://doi.org/10.1186/s12906-015-0885-z
Shao W, Yu Z, Chiang Y, Yang Y, Chai T, Foltz W et al (2012) Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS ONE 7(1):e28784. https://doi.org/10.1371/journal.pone.0028784
Tillman EJ, Morgan DA, Rahmouni K, Swoap SJ (2014) Three months of high-fructose feeding fails to induce excessive weight gain or leptin resistance in mice. PLoS ONE 9(9):e107206. https://doi.org/10.1371/journal.pone.0107206
Xu H, Ghishan FK (2018) Chapter 10: Molecular physiology of gastrointestinal function during development. In: Said HM (ed) Physiology of the gastrointestinal tract, 6th edn. Academic Press, Cambridge, pp 235–269
Yang B, Xie Y, Guo M, Rosner MH, Yang H, Ronco C (2018) Nephrotoxicity and Chinese herbal medicine. Clin J Am Soc Nephrol 13(10):1605–1611
Yu Y-W, Li M-X, Zhang Z-Y, Yu H (2018) The deficiency of CX3CL1/CX3CR1 system ameliorates high fructose diet-induced kidney injury by regulating NF-κB pathways in CX3CR1-knock out mice. Int J Mol Med 41(6):3577–3585
Acknowledgements
The authors acknowledge the assistance of the entire staff of the Central Animal Service Unit, University of the Witwatersrand, Johannesburg, in the general care and welfare of the animals. We also acknowledge the assistance of Dr Umar Mohammed of the Department of Histopathology and Morbid Anatomy of Usmanu Danfodiyo University for his technical assistance in reviewing the histology slides.
Funding
This study received grants from the Faculty of Health Sciences Research Committee of the University of the Witwatersrand, Johannesburg (KGI, Grant Number: 001.251.8521101.5121105.000000. 0000000000.4550), and the National Research Foundation of South Africa (KHE, Grant Number: IRF 2010041900009).
Author information
Authors and Affiliations
Contributions
KGI conceptualised the study and wrote the initial draft of the manuscript. KGI, EC and KHE designed the study, collected and analysed the data. PN performed the histology. All the authors corrected, read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocols and procedures used in the study were approved by the Animal Ethics Screening Committee of the University of the Witwatersrand, Johannesburg, Republic of South Africa (AESC 2016/04/18/B), and complied with international ethical guidelines and standards for the care and use of laboratory animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, K.G., Chivandi, E., Nkomozepi, P. et al. Neonatal orally administered curcumin: impact on the metabolic response and renal histology of Sprague-Dawley rats fed a high-fructose diet until adolescence. Bull Natl Res Cent 46, 153 (2022). https://doi.org/10.1186/s42269-022-00845-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-022-00845-6