Abstract
Background
The awareness in the consumption of plant-based food has gained attention in the recent years. Phytochemicals are thought to play a critical role in health promotion and in the prevention and management of chronic diseases. These compounds have reported to produce little or no side effects and are thus significantly used in treating various ailments. d-Pinitol is the chief active compound found in soy and soy products. Several studies have shown the health benefits of d-pinitol such as antioxidant, anti-diabetic, anti-inflammatory and anticancer properties. In this review, an attempt has been made to review the effects of d-pinitol against diabetes mellitus in pre-clinical and clinical studies.
Methodology
Journal articles were sourced and filtered with relevant keywords on “d-pinitol and diabetes mellitus”. Scientific databases, including PubMed, NCBI, Google Scholar, Science Direct, SciFinder and Web of Science, were accessed to identify the most relevant articles on the effect of d-pinitol in diabetes mellitus. The study duration was from September 2021 to February 2022.
Results
This comprehensive review demonstrates the application of d-pinitol against diabetes mellitus. Most of the animal and clinical studies included in this review reported that d-pinitol treatment effectively regulated hyperglycemia and prevented insulin resistance.
Conclusions
d-Pinitol could serve as an effective anti-hyperglycemic agent for the treatment of diabetes mellitus. Further research to study its safety and mechanism of action is recommended in order to employ this compound for clinical trials.
Similar content being viewed by others
Background
Diabetes mellitus is one of the most common non-communicable diseases. It is group of metabolic disorders in which hyperglycemia lasts over a longer period leading to several other complications (Toi et al. 2020). It is characterized by supraphysiological glucose due to the deficiency in insulin secretion or insulin receptor or post-receptor events that results in the disturbances in the biochemical pathways of carbohydrates, fats and protein (Ruiz 2012). Moreover, the impaired metabolism leads to the progression and aggravation of oxidative stress through different mechanisms like glucose autoxidation, protein glycation, etc. (Dos Santos et al. 2019). The total number of people with diabetes is predicted to increase from 171 million in 2000 to 366 million in 2030 (Tao et al. 2015). Studies direct that the incidence of diabetes will continue to rise in the future due to growing prevalence of obesity, accelerating aging populations, environmental factors and sedentary lifestyles (Piché et al. 2020). Chronic blood sugar level and insulin resistance may in turn lead to severe impairment and dysfunction of heart, kidney, liver, eye, nerves, etc., which predominantly lead to mortality (Kautzky-Willer et al. 2016). At present, there is no known cure approaches for diabetes mellitus except in some very specific cases. Several efforts are taken to identify the novel treatment for this condition with minimal side effects and risk factors.
Epidemiological evidence indicates that the regular intake of fruits and vegetables containing polyphenols is beneficial in both preventing and reversing the detrimental effects of several chronic conditions including diabetes mellitus (Gandhi et al. 2020). These phytochemicals and their derivatives are considered to be safer, less harmful than synthetic drugs and non-toxic. Many studies have reported that consumption of soy and soy products offers reasonable protection against different types of ailments in humans (Jayachandran and Xu 2019; Ganesan and Xu 2017). Soy diet contains various biologically active components and has received much attention for their robust beneficial effects in the prevention of diabetes, cardiovascular disorders, obesity, cancer and osteoporosis (Chatterjee et al. 2018).
d-Pinitol (D-PIN) is derived from soybean and the methyl ether of D-chiro-inositol found as large quantities in soy foods (Phillips et al. 1982). D-PIN was isolated from the seed coat, cotyledon and embryo axis of twenty-two different soybean seeds (Poongothai and Sripathi 2013). Several studies have reported the beneficial effects of D-PIN including its antioxidant (Koh et al. 2018), anti-inflammatory (López-Domènech et al. 2018), hepatoprotective (Lee et al. 2019), cardioprotective (Hu et al. 2021) and neuroprotective activities (Alonso-Castro et al. 2019). The objective of the present review is to summarize the beneficial effects of d-pinitol against diabetes mellitus in pre-clinical and clinical studies.
Main text
Chemistry
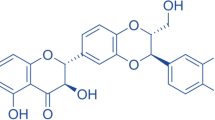
The chemical name of D-PIN is 3-Omethyl-d-chiro-inositol (Fig. 1) (Bhat et al. 2009). It has a molecular formula of C7H14O6, molecular weight of 194.18 g/mol. The melting point of D-PIN is about 187 °C, and boiling point is 317 ± 42.0 °C at 760 mmHg (Anderson 1972). D-PIN has a higher solubility in water, and it is slightly soluble in ethanol (Srivastava et al. 2020).
Bioavailability and metabolism
The pharmacokinetic and bioavailability study (Qiu et al. 2021) results of D-PIN showed that the mean half-life time (t1/2z) was 49.6 ± 23.4 h during intravenous administration of D-PIN and it was 26.9 ± 15.4 h in intragastric administration, suggesting that the metabolism of d-pinitol was a slow elimination phase. This could be due to the enterohepatic circulation process and/or higher plasma protein binding because of the intragastric administration. The apparent volume of distribution (Vz) for D-PIN was 325.2 ± 107.8 L/kg and 1557.50 ± 1329.05 L/kg for intravenous and intragastric administration, respectively. The octanol/water partition coefficient (LogP) of d-pinitol was − 1.89 obtained by ACD/Percepta, indicating that it was a highly polar and hydrophilic compound. Higher values of Vz and small LogP value specified that D-PIN may have higher affinity to plasma protein. The bioavailability of D-PIN was found to be approximately 18.3%.
Effects of d-pinitol against Diabetes mellitus
Pre-clinical studies
Pancreatic protective effect of D-PIN on streptozotocin (STZ)-induced diabetes mellitus
This study was undertaken to investigate the anti-diabetic efficacy of D-PIN in STZ-induced diabetes in male Wistar rats (Sivakumar and Subramanian 2009a). Diabetes was induced in rats by administering single intraperitoneal injection of STZ (50 mg/kg body weight). D-PIN (50 mg/kg body weight) was given to rats orally for 30 days once a day to see the anti-diabetic property. Gliclazide was used as the reference drug to compare the effect of D-PIN. In STZ-induced diabetic rats, FBG (mM) was increased (15.99 ± 0.20), insulin (μU/ml) levels were decreased (5.11 ± 0.28), hemoglobin (g/dL) was decreased (6.77 ± 0.20) and HbA1C (%) was increased (13.73 ± 0.38) when compared to the control rats. D-PIN administered group significantly (P < 0.05) decreased FBG (6.98 ± 0.13), increased insulin levels (12.34 ± 0.74), increased hemoglobin levels (11.47 ± 0.48) and decreased HbA1C (8.55 ± 0.46). The levels of plasma and pancreatic LPO (nM/ml) (10.98 ± 0.66, 81.17 ± 3.43); hydroperoxides (10–5 mM/dL) (16.32 ± 0.63, 29.94 ± 1.45) were increased; antioxidants SOD (1.43 ± 0.46), CAT (6.24 ± 0.39), GPx (3.06 ± 0.16), GST (1.37 ± 0.14), vitamin C (0.60 ± 0.03), vitamin E (0.80 ± 0.04), ceruloplasmin (7.92 ± 0.31) and GSH (16.15 ± 1.21) were decreased in the diabetic induced rats. The levels of these parameters were significantly (P < 0.05) modified in D-PIN supplemented rats. Similarly, the levels of serum AST, ALT and ALP were markedly increased in STZ group when compared to the control animals that was effectively (P < 0.05) restored to near normal levels in D-PIN provided animals. Histological study of pancreas showed intense degranulation of beta cells and vacuolation of pancreatic islets in the STZ-induced diabetic rats. D-PIN-treated rats showed reduced degranulation and vacuolation. The results of this study demonstrated that the antioxidant and anti-peroxidative effect of D-PIN was crucial in protecting the pancreas against diabetes induced oxidative stress.
Effects of D-PIN on insulin resistance through modulating PI3kt/Akt signaling pathway
The study was carried out in Sprague–Dawley rats (Gao et al. 2015). STZ solution (30 mg/kg body weight) was used to induce diabetes mellitus in rats. D-PIN was administered orally at a dose of 30 and 60 mg/kg body weight to determine its effects. Body weight was considerably (P < 0.05) decreased in diabetic rats (380.75 ± 30.73 g) compared to the control rats (496.50 ± 27.18 g). D-PIN-treated rats showed a significant (P < 0.05) improvement in the body weight (424.20 ± 17.87). Fasting blood glucose levels in the diabetic group was markedly (P < 0.05) higher (21.1 ± 4.31 mmol/L) compared to the normal control group (4.8 ± 118 mmol/L). There was a significant decreased in the fasting blood glucose values in the D-PIN supplemented group (16.6 ± 2.56 mmol/L). Insulin sensitivity was measured using fasting serum insulin levels (FINS), HOMA-β and insulin sensitivity index (ISI). FINS was markedly increased in the STZ-induced group (20.07 ± 3.17) and HOMA-β was relatively decreased in the STZ-induced group (20.77 ± 5.07) compared to the control group 13.11 ± 1.55 and 260.88 ± 150.43. In the D-PIN-treated group, significant decrease in FINS was observed (14.08 ± 2.14) without any significant change in HOMA-β (24.54 ± 9.77). ISI was significantly (P < 0.05) increased in diabetic group (− 6.13 ± 0.15) which was significantly (P < 0.05) reversed to near normal in D-PIN-treated group (− 5.40 ± 0.17). Further, mRNA expression of proteins involved in the PI3k/Akt signaling pathway was studied. RT-PCR results showed increased gene expression of glycogen synthase, PI3k and Akt with decreased expression of p110 and p85 in the D-PIN-treated groups compared to the diabetes induced group. Immunohistochemical analysis showed increased pAkt stained regions in the D-PIN-treated group suggesting the activation of PI3k/Akt signaling pathway by D-PIN. Overall results reported that anti-hyperglycemic action of D-PIN could be due to its potential to activate the PI3k/Akt signaling pathway that inhibited insulin resistance in experimental rats.
Effect of D-PIN on STZ-induced renal alterations
Sivakumar et al. (2010a; b) reported the renal protective function D-PIN on STZ-induced renal alterations in Wistar rats. STZ was administered as a single dose (50 mg/kg body weight) intraperitoneally to cause renal damage. D-PIN was orally supplemented for 30 days at a dose of 50 mg/kg body weight. The levels of serum urea (45.18 ± 1.34 mg/dL), uric acid (7.06 ± 0.28 mg/dL) and creatinine (1.23 ± 0.61 mg/dL) along with advanced glycation end products (AGE) were markedly (P < 0.05) increased in STZ-induced animals when compared to the control animals (urea 22.77 ± 0.78; creatinine 0.41 ± 0.31; uric acid 2.62 ± 0.19 mg/dL). The levels of renal MDA, hydroperoxides and protein carbonyls increased and antioxidants SOD (9.44 ± 0.73), CAT (20.93 ± 1.03), GPx (4.04 ± 0.21), GST (2.44 ± 0.17), GSH (20.75 ± 0.88), vitamin C (0.49 ± 0.33) and vitamin E (0.44 ± 0.21) decreased in the STZ challenged rats revealing increased renal oxidative stress. D-PIN-treated rats effectively (P < 0.05) decreased lipid peroxidation and improved cellular antioxidants SOD (14.34 ± 0.43), CAT (34.49 ± 1.10), GPx (6.93 ± 0.09), GST (6.10 ± 0.13), GSH (30.83 ± 0.69), vitamin C (0.95 ± 0.36), vitamin E (0.88 ± 0.37) compared to the STZ rats. Renal histopathological analysis showed enlargement of lining cells of renal tubules, fatty infiltration, capillary tufts and large area of hemorrhage and lymphocyte infiltration in STZ-induced animals. TEM studies revealed larger and dilated glomeruli, partial thickening of Bowman’s capsule, swollen mitochondria, broadened podocyte and vacuolization in D-PIN-untreated animals. D-PIN supplemented group showed significant improvement in the renal cellular architecture with reduced abnormalities. The findings of this study revealed that D-PIN remarkably decreased STZ-mediated hyperglycemia-induced renal oxidative stress by its promising antioxidant function.
D-PIN increases insulin secretion in obese mice
Silva Júnior et al. (2020) studied the role of D-PIN on hepatic lipid metabolism in obese albino mice. Monosodium glutamate (MSG) was used (4 g/kg body weight) for five days to induce obesity in mice. D-PIN treatment (50 mg/kg body weight) was followed for 30 days by oral gavage route. In MSG-induced animals, obesity was evident from increased Lee index (21%) with increased adiposity (P < 0.0001). Weight of retroperitoneal and perigonadal white fats (83%) and interscapular brown fat pad (79%) was significantly (P < 0.0001) higher in MSG-induced mice when compared to the control mice. However, no significant changes in the above parameters were observed in the D-PIN group compared to the MSG group. MSG-induced obese animals showed increased plasma and hepatic levels of total cholesterol, TG compared to the control animals. D-PIN treatment did not affect the circulating lipids in obese mice but significantly (P < 0.05) improved TG content in liver. RT-PCR analysis of hepatic expressions of lipogenic genes showed increased (169%) expressions of sterol regulatory element binding protein (SREBP)-1-C, acetyl CoA carboxylase ((ACC) 146%), fatty acid synthase (FS) (156%), uncoupling protein (UCP)-2 (71%), subunit alpha of AMP-activated protein kinase (AMPKα) (38%) in obese group. These changes were significantly reduced in D-PIN supplemented group SREBP-1 (52%), ACC (41%), FS (43%), UCP-2 (31%), AMPKα (36%). Morphological analysis of pancreatic islet cells in obese mice showed hypertrophic islets with higher islet area and enhanced percentage of medium and large islets compared to control mice. D-PIN treatment increased the percentage of large islets but did not affect pancreatic weight. Results of this study revealed that D-PIN treatment for 30 days in obese mice improved ectopic hepatic fat deposition by improving liver transcription factors and enzymes involved in de novo lipogenesis and by reducing the expression of AMPKα that might have protected against the TG deposition in the liver. Thus, D-PIN increased glucose-induced insulin secretion, which might have in turn enhanced the liver lipogenesis and TG deposition in mice.
Effect of D-PIN on STZ-induced cardiopathy
The modulatory effect of D-PIN on STZ-induced diabetic cardiopathy was reported in SAMP8 and SAMR1 mice (Li et al. 2021). STZ was given intraperitoneally daily for five consecutive days at a dose of 50 mg/kg body weight to induce diabetes mellitus in mice. D-PIN (150 mg /kg body weight) was provided for 10 weeks by oral gavage route. Significant (P < 0.01) decrease in body weight (27%) and increase in fasting blood glucose (28.4 ± 0.02 mmol/L) was observed in diabetic group compared to the normal control group. These changes were markedly (P < 0.01) reverted in D-PIN supplemented groups with body weight increased up to 76% and fasting blood glucose levels decreased (10.3 ± 0.01 mmol/L) when compared to the diabetic group. Cardiac fibrosis was assessed by Masson’s staining that showed increase in cardiac fibroblast with increased collagen synthesis and cardiac hypertrophy in STZ-induced diabetic animals. Reduced abnormalities were seen in D-PIN-treated animals when compared to the diabetic animals. In diabetic induced mice, mRNA expression of cardiac α-myosin heavy chain (MHC), β-MHC, brain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP) was found to be increased; cytoplasmic and nuclear levels of Nrf2 decreased; cardiac MDA levels increased with decrease in antioxidants SOD, GPx and GSH; protein expression of Bcl-2 decreased with increased caspase-3 and caspase 9 expressions. Further, cardiac protein expression of PI3k, phosphor Akt and phosphor-mTOR decreased in STZ-induced group when compared to the control group. All these changes were significantly (P < 0.01) reverted in D-PIN-treated group. Thus, the observations of this study showed that D-PIN significantly improved glycemic control and cellular antioxidants, thereby improving cardiac function by reducing myocardial apoptosis and fibrosis through the PI3k/Akt/mTOR signaling pathway.
Effect of D-PIN on STZ-induced diabetic hyperlipidemia
The protective efficacy of D-PIN against STZ-induced diabetic hyperlipidemia was studied in albino Wistar rats (Geethan and Prince 2008). Single dose of intraperitoneal injection of STZ (40 mg/kg body weight) was given to induce hyperglycemia in experimental rats. D-PIN (100 mg/kg body weight) was administered orally for 30 days to study its ameliorating effect. In STZ-induced animals, body weight was decreased, blood glucose levels were significantly increased, serum lipids such as total cholesterol (225.3 ± 20.5 mg/dL), triglycerides (TG) (225.3 ± 20.5 mg/dL), free fatty acids (FFA) (155.4 ± 12.2 mg/dL) and phospholipids (PL) (192 ± 15.2 mg/dL) were significantly increased compared to the control animals. D-PIN-treated animals showed significant (P < 0.05) decrease in the levels of serum total cholesterol (99.2 ± 8.6 mg/dL), TG (96.3 ± 8.2 mg/dL), FFA (87 ± 6.2 mg/dL) and PL (132.1 ± 8 mg/dL). Similarly, the levels of liver, kidney, heart and brain total cholesterol, TG, FFA and PL were markedly increased in the diabetic hyperlipidemia-induced animals compared to the control animals. D-PIN supplementation effectively (P < 0.05) altered these levels to near normal in the treated group. The levels of serum lipoproteins LDL, VLDL and HDL were significantly decreased in the D-PIN-administered group when compared to the STZ-induced untreated diabetic group. The hyperlipidemia reported in STZ-induced rats could be due to excess fat mobilization from adipose tissue because of underutilization of glucose. D-PIN treatment remarkably facilitated the glucose utilization suppressing the mobilization of fat and prevented hyperlipidemia in the diabetic rats.
Effect of D-PIN on STZ-induced liver damage
The liver protective nature of D-PIN in STZ-induced diabetes in male Wistar rats was reported by Sivakumar et al. (2010a; b). STZ was given at the dose of 50 mg/kg body weight. D-PIN was given orally (50 mg/kg body weight) for 30 days. The levels of liver lipid peroxides (TBARS and hydroperoxides), plasma pro-inflammatory cytokines TNF-α, IL-1β and IL-6 along with nitric oxide and NF-kB p65 unit were markedly (P < 0.05) increased; liver antioxidants SOD (4.42 ± 0.26), CAT (42.90 ± 1.21), GPx (4.58 ± 0.26), GST (4.07 ± 0.97), reduced glutathione GSH (24.56 ± 0.96), vitamin C (0.54 ± 0.03), vitamin E (0.95 ± 0.15) were decreased in STZ-induced diabetic animals when compared to the control animals. D-PIN treatment effectively (P < 0.05) reverted all these changes that was evident by increase in the antioxidant activities/levels of SOD (7.37 ± 0.21), CAT (68.83 ± 1.53), GPx (7.59 ± 0.28), GST (6.48 ± 0.17), vitamin C (1.35 ± 0.05), vitamin E (1.56 ± 0.04), GSH (30.75 ± 0.27). Liver histopathological analysis revealed fibrosis in portal area, microvesicular vacuolization, granular degeneration and distortion in the arrangements of cells around the central vein capillaries leading to loss of concentric arrangement of hepatocytes in STZ-induced rats. Transmission electron microscopy (TEM) of liver showed pyknotic nuclei, increased droplet accumulation, reduced mitochondria and glycogen content in STZ-administered group. D-PIN-treated animals showed marked reduction and improvement in all the abnormalities seen in STZ group in histological and TEM studies. The anti-hyperglycemic and antioxidant function of D-PIN was very important for its hepatoprotective action in diabetic rats.
D-PIN stimulates translocation of GLUT 4 in skeletal muscle in mice
GLUT 4 is an important glucose transporter in the skeletal muscle, heart muscle and adipocytes that is regulated by Insulin. In this study, the efficacy of D-PIN along with myo-inositol to stimulate the translocation of GLUT 4 was reported in C57 BL/mice (Dang et al. 2010). D-PIN (1 g/kg body weight) was supplemented orally to mice 30 min before a post-oral injection of glucose (2 g/kg body weight). Oral glucose tolerance test was conducted in the control and experimental group. The results showed significantly (P < 0.05) increased plasma glucose level after 15 min of glucose ingestion that reached a maximum after 30 min in the diabetic mice. In the D-PIN-treated mice, plasma glucose level was significantly (P < 0.05) decreased after 30 and 60 min; the maximum level reached 15 min after glucose ingestion and significantly returned to normal level sooner than the induced group. The values of HOMA-IR were also significantly (P < 0.05) increased in the diabetes-induced group compared to the control group and D-PIN treatment efficiently (P < 0.05) reduced the HOMA-IR. However, no significant changes were observed in the levels of plasma cholesterol in the treated and untreated groups. Western blot analysis showed increased protein expression of IRβ in the plasma membrane fraction and increased protein expression of GLUT 4 in the tissue lysate. This suggests that D-PIN has potentially stimulated the translocation of GLUT 4 in the treated group compared to the untreated diabetic group. The probable mechanism by which D-PIN activated the translocation of GLUT 4 could be by activating phosphoinositide 3 kinase and/or 5′ AMP-activated protein kinase.
D-PIN modulates hepatic enzymes in STZ-induced diabetes
This study by Sivakumar and Subramaniam (2009b) explored the anti-diabetic activity of D-PIN in rats by modulation of key carbohydrate metabolizing enzymes in the liver. Diabetes mellitus was induced in rats by giving a single intraperitoneal injection of STZ (50 mg/kg body weight). D-PIN was given at different doses (25, 50, 75 and 100 mg/kg body weight) orally (50 mg/kg body weight) for 15, 21, 30 and 45 days. Body weight in diabetic rats was significantly (P < 0.05) decreased compared to the control rats. D-PIN treatment efficiently (P < 0.05) increased body weight in the treated rats. Results of the glucose tolerance test (GTT) showed a sudden increase of blood glucose after glucose load in the diabetic group at 60 min, and this stayed high over the next 60 min, whereas in the D-PIN-treated group the blood glucose was significantly (P < 0.05) decreased at 60 min and 120 min compared to the diabetic group. The fasting blood glucose values in the diabetic group were significantly increased to 298.43 ± 1.45 mg/dL compared to the control group 87.38 ± 0.61 mg/dL. D-PIN-treated group showed significantly decreased fasting blood glucose values (98.65 ± 14.3 mg/dL) compared to the STZ-induced diabetic group. Similarly, the plasma insulin concentration was significantly decreased in the STZ-induced animals (6.42 ± 0.22) compared to the normal animals (14.84 ± 0.15). This was markedly (P < 0.05) improved in D-PIN-supplemented animals (10.58 ± 0.17) compared to the diabetic animals. The HbA1C levels are also raised in the diabetic group to 12.83% that was brought to near normal levels (7.29%) in the D-PIN-treated group. The activities of carbohydrate metabolizing enzymes such as hexokinase (129.62 ± 1.55), pyruvate kinase (102.22 ± 1.17), glucose 6 phosphate dehydrogenase were significantly decreased and lactate dehydrogenase (450.49 ± 2.97), fructose 1,6 bisphosphatase (754.95 ± 3.59), glucose 6 phosphatase (1964 ± 4.82) were significantly increased in the diabetic rats. The activities of these enzymes in the D-PIN such as hexokinase (227.7 ± 0.64), pyruvate kinase (168.7 ± 1.37), lactate dehydrogenase (290.46 ± 2.23), glucose 6 phosphatase (1141.49 ± 3.31), fructose 1,6 bisphosphatase (536 ± 7.91) and glucose 6 phosphate dehydrogenase (492.82 ± 2.72) were significantly brought back to near normal. Moreover, the glycogen content and glycogen synthase activity were significantly decreased in STZ-induced animals compared to the control animals; this was markedly (P < 0.05) restored to near normal in D-PIN-supplemented animals. The results of this study concluded that the glucose lowering ability of D-PIN could be due to its capability to modulate the activities of hepatic carbohydrate metabolizing enzymes and to protect the hepatic architecture.
Reno protective effect in experimental diabetes
Male Wistar rats were used in this study (Sousa et al. 2020). STZ was given at the dose of 65 mg/kg body weight intraperitoneally to induce diabetes. Diabetes was confirmed in the experimental animals using blood glucose values greater than or equal to 200 mg/dL. D-PIN (20 mg/kg body weight) was administered by oral gavage route for eight weeks after 2 months of induction of diabetes. Blood glucose values increased significantly (P < 0.05) in the diabetic group to 306.8 ± 42.2 mg/dL compared to the control group. D-PIN treatment effectively (P < 0.05) lowered the blood glucose values to 234.4 ± 23.5 mg/dL in the treated group. Plasma creatinine levels in the diabetic group increased to 0.88 ± 0.05 mg/dL that was significantly (P < 0.05) brought down to 0.67 ± 0.44 in the treated group. Urinary albumin excretion was significantly increased up to 28% in the STZ-induced diabetic animals that were restored to near normal in the D-PIN-treated animals. Urinary albumin/creatinine ratio and total renal blood flow were significantly (P < 0.05) increased in the diabetic group (4.6 ± 0.9 mg/mg; − 3.4 ± 0.6 mg/g/min) that were remarkably reduced in the D-PIN group to 2.1 ± 0.3 mg/mg and − 2.9 ± 0.7 mg/g/min). Glomerular volume in STZ group was significantly (P < 0.05) (55.2%) increased in D-PIN-treated group compared to the diabetic induced group. PAS staining showed thickening of Bowman’s capsule, hyalinosis of vascular pole, dilated Bowman’s space and hypercellularity in diabetes induced animals. All these abnormalities were effectivity reduced in D-PIN-treated animals. Western blot analysis showed decreased Nephrin protein expression (30%) in STZ animals that was significantly increased in D-PIN-treated animals, increased phosphoThr202/Tyr 204 protein expression (97%) and TGF-β expression (87%) in STZ-induced animals that were significantly decreased in D-PIN-treated animals. Anti-oxidant, anti-proliferative, anti-inflammatory activity of D-PIN was an important reason for its renoprotective and anti-diabetic action.
Clinical studies
Effect of D-PIN against type 2 diabetes mellitus in patients
The effect of D-PIN on glycemic control, insulin resistance and adipocytokine levels in patients with type 2 diabetes mellitus was assessed (Kim et al. 2012). A total of 66 patients (aged 20 to 75 years) diagnosed with type 2 diabetes mellitus and who had been taking oral hypoglycemic drugs in the previous three months were involved in the study. Thirty-three patients were randomly marked and put in the D-PIN (400 mg thrice a day) treatment group, and other thirty-three patients were designated in the placebo group. The levels of plasma FBG, post-prandial blood glucose and mean HbA1C levels were lowered in the D-PIN-treated group compared to the placebo group. But only FBG levels were significantly (P < 0.05) decreased when compared to the placebo group. The index of insulin resistance was measured in the form of HOMA-IR. No significant (P < 0.05) changes were observed in the levels of C-peptide and insulin between placebo and D-PIN supplemented groups. There was no significant (P < 0.05) difference in the levels of adiponectin, FFA and CRP between the D-PIN-treated groups and placebo group. However, leptin levels were significantly (P < 0.05) lowered in the D-PIN supplemented patients compared to the placebo group. Multiple regression analysis revealed that HbA1C level was an important predictor, that showed significant (P < 0.01; r = 0.65) decline in HbA1C levels. The results of the study depicted that D-PIN regulated insulin levels to improve glycemic control and insulin sensitivity in patients with Diabetes mellitus, particularly in in patients with insulin resistance.
Anti-hyperglycemic role of D-PIN on patients with hyperglycemia
Effect of D-PIN isolated from soybean was tested for its anti-hyperglycemic activity in patients with type 2 diabetes mellitus (Kang et al. 2006). This study was conducted on Korean patients with type 2 diabetes mellitus. After overnight fast, patients were given cooked white rice (64.2 gm) containing 50 g of available carbohydrate. D-PIN isolated from soybean (1.2 gm) was supplemented as pre-treatment at 0.60, 120 and 180 min prior to rice ingestion. After the intake of rice, the capillary blood glucose levels increased to 119.3 ± 6.7, 1225 ± 8.0, 108.7 ± 8.0 and 22.5 ± 9.5 mg/dl at 60, 90, 120 and 240 min. D-PIN supplementation decreased blood glucose levels to 116 ± 6.5, 120.14 ± 7.9, 96.4 ± 6.4 and 18.2 ± 10.4 mg/dL at 60, 90, 120 and 240 min. The levels of post-prandial glucose were also significantly (P < 0.05) decreased in patients supplemented with D-PIN. However, the plasma insulin levels were not significantly affected in the D-PIN group (2570 ± 418) compared to the untreated group (2835 ± 608). The results of the study clearly showed that the anti-hyperglycemic effect of D-PIN could be due to its ability to interact with the insulin signaling pathways.
Effect of D-PIN isolated from soybeans on glycemic control in patients
The effect of D-PIN on glycemic control and cardiovascular risk factors was studied in patients with type 2 diabetes mellitus (Kim et al. 2005). Thirty subjects were involved in this study, and D-PIN (600 mg) was given orally daily for 12 weeks. Results showed a significant (P < 0.05) decrease in the concentration of plasma glucose (8.79 ± 0.30 mmol/L), insulin (16.4 ± 1.4 μU/mL), fructosamine (351.5 ± 13.7 μmol/L), HbA1C (8.79%), HOMA-IR (6.44 ± 0.59), LDL cholesterol (3.10 ± 0.23 mmol/L), LDL/HDL cholesterol ratio (2.71 ± 0.32), triglycerides (5.23 ± 0.24 mmol/L), TG/Cholesterol ratio (1.54 ± 0.14) and a significant (P < 0.05) increase in HDL cholesterol (1.21 ± 0.07 mmol/L) in the D-PIN-treated patients. However, there were not significant changes observed in the body weight, waist circumference, body fat content, ALT, AST, BUN and creatinine levels in the D-PIN-treated patients. Therefore, this study exemplified that D-PIN could be an important agent for treating type 2 diabetes mellitus and for curbing the diabetes related cardiovascular complications associated with it.
Effects of D-PIN on patients with glucose metabolism and adipocytokines
This study evaluated the impact of D-PIN on type 2 diabetic patients with poor control with anti-diabetic agents. Twenty-two patients with uncontrolled type 2 diabetes mellitus were selected for this study (Kim et al. 2007). The criteria for diabetes were fasting blood glucose above or equal to 7.8 mmol/L or HbA1C with above or equal to 7.5%. D-PIN was supplemented orally for 12 weeks at 20 mg/kg/day. Before the treatment of D-PIN the fasting blood glucose values were found to be 11.1 ± 2.1 mmol/L, post-prandial glucose 16.5 ± 4.7, HbA1C 9.8%, whereas after D-PIN treatment the values were significantly (P < 0.05) improved with fasting blood glucose 9.4 ± 2.8 mmol/L, post-prandial glucose 12.9 ± 4.1 mmol/L and HbA1C 8.3%. However, no significant changes were found in other parameters like C peptide, total cholesterol, triglycerides, HDL cholesterol, LDL cholesterol, ALT, AST, 24-h urinary protein and 24-h urinary albumin. Similarly, there were no significant changes in the levels of adiponectin, leptin, free fatty acids and CRP in the PIN-treated and untreated groups. The results of the study showed that D-PIN treatment effectively altered glucose metabolism but did not improve lipid parameters and adipocytokine levels in patients with uncontrolled diabetes mellitus.
Effects of D-PIN treatment on Insulin action in subjects with uncontrolled diabetes
A study was conducted in patients to assess the glucose lowering effect of D-PIN in obese subjects (Davis et al. 2000). Twenty-two obese patients with a mean age of 51 ± 12 years were subjected to the study. Abnormal glucose tolerance and BMI > 30 kg/m3 were used as criteria for confirming obesity and diabetes mellitus. D-PIN was given orally to patients for 28 days at a dose of 20 mg/kg/day. The results were summarized as pre- and post-treatment outcomes after D-PIN supplementation. In the pretreated groups, fasting blood glucose was 7.6 ± 0.7 mmol/L, fasting insulin 102 ± 14 pmol/L, HbA1C 6.8%, plasma free fatty acids 0.49 ± 0.04 μmol/mL, total cholesterol 5.28 ± 0.26 mmol/L, LDL 3.26 ± 0.28 mmol/L, HDL 0.85 mmol/L and total cholesterol 2.59 ± 0.26 mmol/L. After D-PIN treatment, there were no significant changes in the fasting blood glucose 7.2 ± 0.7 mmol/L. fasting insulin 101 ± 2.3 pmol/L, HbA1C 6.8%, free fatty acids 0.49 ± 0.04 μmol/mL, total cholesterol 5.09 ± 0.26 mmol/L, LDL 3.23 ± 0.23 mmol/L, HDL 0.84 ± 0.06, total triglycerides 2.26 ± 0.24 mmol/L. Thus, the results of this study showed that D-PIN treatment for 4 weeks did not appreciably change the baseline glucose production, insulin-mediated glucose disposal and major lipid fractions in plasma.
Toxicity studies on D-PIN
Toxicity studies on D-PIN is extremely limited in animal model and human studies did not report any significant toxicity of this compound. A study from Chauhan et al. (2011) analyzed the toxicity of D-PIN in Swiss albino mice. Acute toxicity studies in mice revealed that D-PIN does not induce any mortality and a dose of up to 2000 mg/kg body weight was tolerable in animals. Clinical studies have also shown that D-PIN was well tolerated in humans up to 1200 mg/day (Kim et al. 2012).
Conclusions
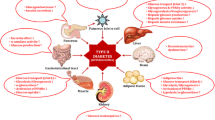
In this review, we have summarized the different mechanisms involved in the protective effect of D-PIN in diabetes mellitus through various animal and human studies (Tables 1, 2) (Fig. 2). All pre-clinical studies included in this review reported an appreciable control of hyperglycemia, glycemic regulation and insulin resistance in the D-PIN-treated animals compared to the diabetic animals. Most of the clinical studies also reported the glucose lowering effect of D-PIN. Moreover, a dosage of up to 150 mg/kg body weight of D-PIN was well tolerated in animal models and 1200 mg/day in case of clinical studies. Studies did not report any substantial toxic effects of D-PIN. The common mechanism attributed to the anti-diabetic effect of D-PIN by most of the studies was its potential antioxidant and anti-peroxidative effect that helped in preventing the diabetes associated complications. Therefore, we suggest that more clinical studies to be undertaken to identify the exact mechanism how D-PIN is able to illicit its anti-hyperglycemic effect and long-term studies are recommended in human to assess the toxicity of D-PIN.
Availability of data and materials
Not applicable.
Abbreviations
- D-PIN:
-
D-Pinitol
- STZ:
-
Streptozotocin
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- GPx:
-
Glutathione peroxidase
- GST:
-
Glutathione-S-transferase
- TG:
-
Triglycerides
- FFA:
-
Free fatty acids
- LDL:
-
Low-density lipoprotein
- HDL:
-
High-density lipoprotein
- VLDL:
-
Very low-density lipoprotein
- PL:
-
Phospholipid
- GSH:
-
Reduced glutathione
- TEM:
-
Transmission electron microscope
- GLUT:
-
Glucose transporter
- GTT:
-
Glucose tolerance test
- FBG:
-
Fasting blood glucose
- CRP:
-
C-Reactive protein
- HbA1c:
-
Glycosylated hemoglobin
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- BUN:
-
Blood urea nitrogen
- LPO:
-
Lipid peroxidation
- MDA:
-
Malondialdehyde
- FINS:
-
Fasting serum insulin levels
- ISI:
-
Insulin sensitivity index
- FS:
-
Fatty acid synthase
- UCP:
-
Uncoupling protein
- BNP:
-
Brain natriuretic peptide
- ANP:
-
Atrial natriuretic peptide
- HOMA-IR:
-
Homeostasis model assessment-estimated insulin resistance
References
Alonso-Castro AJ, Alba-Betancourt C, Rocha-González E et al (2019) Neuropharmacological effects of d-pinitol and its possible mechanisms of action. J Food Biochem 43(12):e13070
Anderson I (1972) The cyclitols. In: Pigman W, Horton D (eds) The carbohydrates, chemistry and biochemistry, vol 1A, 2nd edn. Academic Press, Inc., New York and London
Bhat KA, Shah BA, Gupta KK et al (2009) Semi-synthetic analogs of pinitol as potential inhibitors of TNF-alpha cytokine expression in human neutrophils. Bioorg Med Chem Lett 19:1939–1943
Chatterjee C, Gleddie S, Xiao CW (2018) Soybean bioactive peptides and their functional properties. Nutrients 10(9):1211
Chauhan PS, Gupta KK, Bani S (2011) The immunosuppressive effects of Agyrolobium roseum and pinitol in experimental animals. Int Immunopharmacol 11(2):286–291
Dang NT, Mukai R, Yoshida K et al (2010) d-Pinitol and myo-inositol stimulate translocation of glucose transporter 4 in skeletal muscle of C57BL/6 mice. Biosci Biotechnol Biochem 74(5):1062–1067
Davis A, Christiansen M, Horowitz JF et al (2000) Effect of pinitol treatment on insulin action in subjects with insulin resistance. Diabetes Care 23(7):1000–1005
Dos Santos JM, Tewari S, Mendes RH (2019) The role of oxidative stress in the development of diabetes mellitus and its complications. J Diabetes Res 5:4189813
Gandhi GR, Vasconcelos ABS, Wu DT et al (2020) Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: a systematic review of in vitro and in vivo studies. Nutrients 12(10):2907
Ganesan K, Xu B (2017) A critical review on polyphenols and health benefits of black soybeans. Nutrients 9(5):455
Gao Y, Zhang M, Wu T et al (2015) Effects of d-pinitol on insulin resistance through the PI3K/Akt signaling pathway in type 2 diabetes mellitus rats. J Agric Food Chem 63(26):6019–6026
Geethan PK, Prince PS (2008) Antihyperlipidemic effect of d-pinitol on streptozotocin-induced diabetic Wistar rats. J Biochem Mol Toxicol 22(4):220–224
Hu X, Zhu Y, Xiaoyan LV et al (2021) Elucidation of the mechanism of action of pinitol against pressure overload-induced cardiac hypertrophy and fibrosis in an animal model of aortic stenosis. Biosci Biotechnol Biochem 85(3):643–655
Jayachandran M, Xu B (2019) An insight into the health benefits of fermented soy products. Food Chem 15(271):362–371
Kang MJ, Kim JI, Yoon SY et al (2006) Pinitol from soybeans reduces postprandial blood glucose in patients with type 2 diabetes mellitus. J Med Food 9(2):182–186
Kautzky-Willer A, Harreiter J, Pacini G (2016) Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev 37(3):278–316
Kim JI, Kim JC, Kang MJ et al (2005) Effects of pinitol isolated from soybeans on glycaemic control and cardiovascular risk factors in Korean patients with type II diabetes mellitus: a randomized controlled study. Eur J Clin Nutr 59(3):456–458
Kim MJ, Yoo KH, Kim JH et al (2007) Effect of pinitol on glucose metabolism and adipocytokines in uncontrolled type 2 diabetes. Diabetes Res Clin Pract 77(Suppl 1):S247–S251
Kim HJ, Park KS, Lee SK et al (2012) Effects of pinitol on glycemic control, insulin resistance and adipocytokine levels in patients with type 2 diabetes mellitus. Ann Nutr Metab 60(1):1–5
Koh ES, Kim S, Kim M et al (2018) d-Pinitol alleviates cyclosporine A-induced renal tubulointerstitial fibrosis via activating Sirt1 and Nrf2 antioxidant pathways. Int J Mol Med 41(4):1826–1834
Lee E, Lim Y, Kwon SW et al (2019) Pinitol consumption improves liver health status by reducing oxidative stress and fatty acid accumulation in subjects with non-alcoholic fatty liver disease: a randomized, double-blind, placebo-controlled trial. J Nutr Biochem 68:33–41
Li XL, Xu M, Yu F et al (2021) Effects of d-pinitol on myocardial apoptosis and fibrosis in streptozocin-induced aging-accelerated mice. J Food Biochem 45(4):e13669
López-Domènech S, Bañuls C, de Marañón AM et al (2018) Pinitol alleviates systemic inflammatory cytokines in human obesity by a mechanism involving unfolded protein response and sirtuin 1. Clin Nutr 37(6 Pt A):2036–2044
Phillips DV, Dougherty DE, Smith AE (1982) Cyclitols in soybean. J Agric Food Chem 30(3):456–458
Piché ME, Tchernof A, Després JP (2020) Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res 126(11):1477–1500
Poongothai G, Sripathi SK (2013) A review on insulinomimetic pinitol from plants. Int J Pharm Bio Sci 4:992–1009
Qiu J, Yan X, Liao Y et al (2021) An UPLC-MS/MS method for quantification of d-pinitol in rat plasma and its application to a pharmacokinetic and bioavailability study. J Chromatogr B Analyt Technol Biomed Life Sci 15(1163):122498
Ruiz J (2012) Diabète [Diabetes mellitus]. Rev Med Suisse 8(324):88–90
Silvajúnior JAD, Silva ACVFD, Figueiredo LS et al (2020) d-Pinitol increases insulin secretion and regulates hepatic lipid metabolism in Msg-obese mice. An Acad Bras Cienc 92(4):e20201382
Sivakumar S, Subramanian SP (2009a) d-Pinitol attenuates the impaired activities of hepatic key enzymes in carbohydrate metabolism of streptozotocin-induced diabetic rats. Gen Physiol Biophys 28(3):233–241
Sivakumar S, Subramanian SP (2009b) Pancreatic tissue protective nature of d-pinitol studied in streptozotocin-mediated oxidative stress in experimental diabetic rats. Eur J Pharmacol 622(1–3):65–70
Sivakumar S, Palsamy P, Subramanian SP (2010a) Attenuation of oxidative stress and alteration of hepatic tissue ultrastructure by d-pinitol in streptozotocin-induced diabetic rats. Free Radic Res 44(6):668–678
Sivakumar S, Palsamy P, Subramanian SP (2010b) Impact of d-pinitol on the attenuation of proinflammatory cytokines, hyperglycemia-mediated oxidative stress and protection of kidney tissue ultrastructure in streptozotocin-induced diabetic rats. Chem Biol Interact 188(1):237–245
Sousa LGF, Cortez LUAS, Evangelista JSAM et al (2020) Renal protective effect of pinitol in experimental diabetes. Eur J Pharmacol 5(880):173130
Srivastava K, Tiwari M, Dubey A et al (2020) d-Pinitol—a natural phytomolecule and its pharmacological effect. Int J Pharm Life Sci 11(5):6609–6623
Tao Z, Shi A, Zhao J (2015) Epidemiological perspectives of diabetes. Cell Biochem Biophys 73(1):181–185
Toi PL, Anothaisintawee T, Chaikledkaew U et al (2020) Preventive role of diet interventions and dietary factors in type 2 diabetes mellitus: an umbrella review. Nutrients 12(9):2722
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript. AP planned and wrote the manuscript, VMK helped in preparing the tables, and NC helped in preparing the figures.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Authors declare that there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pandi, A., Kalappan, V.M. & Chandrashekar, N. Effects of d-pinitol on diabetes mellitus: an updated review. Bull Natl Res Cent 46, 130 (2022). https://doi.org/10.1186/s42269-022-00820-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-022-00820-1