Abstract
Background
In spite of efforts to control avian influenza (AI) and Newcastle disease (ND) over decades, circulation of the viral causative agents among domestic and feral birds is considered implicated factors for the intermittent outbreaks of AI and ND among domesticated birds as well as commercial poultry flocks in Nigeria. In this study, sera from domestic (Japanese quails) and peri-domestic birds including laughing dove and village weavers were screened for antibodies to low pathogenic AI virus (LPAIV) and ND virus (NDV).
Methods
A competitive ELISA was used to detect anti-AI virus antibodies in the sera of 101 unvaccinated Japanese quails, 30 village weavers and one laughing dove caught for human consumption in Oyo and Osun states, Nigeria. Hemagglutination-inhibiting (HI) antibodies against LPAIV were then detected in the ELISA-positive sera using H3N8, H5N2 and H9N7 subtype-specific antigens. Also, antibodies to NDV were detected and quantified in the sera using HI test.
Results
Seroprevalence of NDV antibodies from tested quail sera was 12.9% (13/101), while AI was 18.8% (19/101) with detection of anti-LPAIV H3N8, H5N2 and H9N7 antibodies. The laughing dove serum was positive for NDV and anti-LPAIV H9N7 antibodies while all sera from village weavers had no detectable LPAIV antibodies, but 26.7% (8/30) were positive for NDV antibodies.
Conclusions
This study provides serologic evidence of infection with LPAIV H3N8, H5N2 and H9N7 as well as Newcastle disease in domestic and peri-domestic birds in southwest Nigeria and highlights the potential role of these birds in the epidemiology of AI and ND.
Similar content being viewed by others
Background
Avian influenza (AI) and Newcastle disease (ND) are viral and often fatal diseases that affect a wide range of avian hosts, irrespective of age. They have been a cause of great set back in poultry productions throughout the world as they cause considerable financial losses with resultant illness in affected flocks, and also for other avian species, mammals and human beings (Capua and Alexander 2006; Alexander 2007; Capua and Munoz 2013). Despite efforts at disease management, circulation of the causative viruses among domestic and feral birds has been documented as a factor responsible for the periodic outbreaks of AI and ND among domesticated birds as well as commercial poultry flocks (Bergervoet et al. 2019; Shi and Gao 2021). Avian influenza A virus has also infected humans, most of whom had direct contact with infected birds or environments contaminated with secretions or excretions from infected birds (WHO 2008; Wang et al. 2009; Li et al. 2014). In addition, there is fear for silent carriers to be the causative agent of antigenic reassortment resulting in new influenza strain (Bergervoet et al. 2019). Similarly, exposures to NDV mostly in laboratory workers and vaccination crews have been shown to cause mainly conjunctivitis (Munir et al. 2012).
Globally, several species of domestic and feral birds are shown to be predisposed to avian influenza viruses (AIVs) infection with migrating and aquatic birds constituting the main reservoir of these viruses (Bergervoet et al. 2019). In particular, the occurrences of these infections have predominantly been reported in poultry in either the highly pathogenic or low pathogenic forms. Seroconversion is usually the primary indication of low pathogenic avian influenza (LPAI) infection in domestic poultry and may be the solitary evidence of infection with some subtypes of LPAI (Clark and Hall 2006). Furthermore, most LPAIVs produce mild to moderate disease in commercial rearing settings, especially when complicated by secondary pathogens, immunosuppression, and stress factors in the environment (Li et al. 2014).
Virtually all domestic and feral bird species are susceptible to infection with Newcastle disease (ND) virus (Alexander and Senne 2008). Wild birds seem to be the reservoir of low virulent strains, whereas poultry are the most likely reservoir of virulent viruses. However, virus exchange between these reservoirs represents a risk of both bird populations (Apopo et al. 2020). However, free-living migratory species, such as waterfowls or white storks, may carry virulent ND virus strains without obvious contact with poultry (Kaleta and Kummerfeld 2012; Yuan et al. 2013).
The clinical signs observed in infected birds vary widely and are dependent on viral factors like pathogenicity (which depends on virulence and tropism of the virus), host factors (age, immune status and species), concurrent infections, route of exposure, duration and magnitude of the infection dose, and external factors such as social and environmental stress (McFerran and McCracken 1998; Capua and Marangon 2006).
Birds other than domestic chickens such as ducks and turkeys have been shown to be possible sources of the spread of AI and ND viruses in Nigeria (Coker et al. 2014; Oluwayelu et al. 2015). More so, different studies have highlighted the importance of routine surveillance in establishing the epidemiological characteristics of these diseases in the country which may help to estimate the local disease burden and possibly inform prevention strategies (Adene et al. 2006; Aiki-Raji et al. 2015; Oluwayelu et al. 2017). However, there is a little report of the detection of AI and ND in quails which is popularly domesticated for meat and egg production as well as laughing dove and village weavers which are peri-domestic birds commonly seen around households and farms in Nigeria. Therefore, this study was designed to evaluate the presence of AIV and NDV antibodies in domesticated Japanese quails and free-living laughing dove and village weavers in Osun and Oyo states, southwest Nigeria.
Methods
Sample collection
Blood samples collected via the jugular vein were randomly obtained from apparently healthy, unvaccinated 132 birds comprising of 101 Japanese quails from ten flocks in Osun state, as well as 30 village weavers from a live-bird market and one laughing dove caught for human consumption in Oyo state, Nigeria. Sera were separated from the collected blood samples and stored at − 20 °C until analyzed. A cross-sectional study was employed and samples were randomly collected from the birds.
Serology
All the sera were screened for the quantitative detection of anti-nucleoprotein antibodies to Avian influenza virus (AIV) using a commercially available competitive enzyme-linked immunosorbent assay (ELISA) kit (BioNote Inc., Korea). The test was carried out according to the manufacturer’s instruction, and results were read at a wavelength of 450 nm using an Optic Ivymen System (Model 2100C) microplate ELISA reader (Biotech SL, Spain). For each sample, the percentage inhibition (PI) was calculated from the obtained optical density values. Samples with PI ≥ 50 were considered positive. These positive samples were then subjected to hemagglutination inhibition (HI) test for subtype-specific AIV antibodies using available reference antigens comprising a panel of LPAI H3N8, H5N2 and H9N7 viruses and 4 hemagglutinating units of each antigen according to standard procedure (OIE 2014). Also, antibodies to NDV were detected and quantified in the 132 sera using the HI test as described by Durojaiye and Adene (1988).
Statistical analysis
The differences in LPAIV and NDV antibodies seroprevalence between domestic and peri-domestic birds were evaluated by Chi-square (X2) test using Graph Pad software (Graph Pad prism version 5, CA, USA). The level of statistical significance was P < 0.05.
Results
The occurrence of NDV antibodies in sera from quails, laughing dove and village weaver birds was 12.9% (13/101), 100% (1/1) and 26.7% (8/30), respectively. NDV antibodies titer range was 16–64, while for LPAI antibodies, 18.8% (19/101), 100% (1/1) and 0% were detected in quails, laughing dove and village weavers, respectively (Table 1).
Of the 19 ELISA-positive samples in quails, five (5) were positive for anti-LPAIV H9N7 antibodies only, 11 for anti-LPAIV H3N8 and H9N7 antibodies, and three (3) for antibodies against all the three LPAIV subtypes, indicating a mixed infection. The laughing dove serum was positive for only anti-LPAIV H9N7 antibodies while all sera from village weavers had no detectable LPAIV antibodies. Compared to the domesticated birds, the peri-domestic birds had significantly lower LPAIV antibody prevalence, with P value of 0.043 and odds ratio (OR) of 7.0 (95% CI 0.89–54.2) (Table 2).
Discussion
Despite efforts for prevention and control of viral transmission between different species of birds, there are still intermittent outbreaks of AI and ND among domesticated birds as well as commercial poultry flocks in Nigeria (Adene et al. 2006; Oluwayelu et al. 2014). This has highlighted the importance of continuous surveillance for these viral infections in different bird species in the country to update the disease situation in order to adopt suitable preventative measures (Coker et al. 2014; Adebiyi and Fagbohun 2017).
In Nigeria where avian influenza disease outbreaks have been reported (Adene et al. 2006) and also, where despite vaccinations against Newcastle disease is routinely carried out, outbreaks have been noted (Aldous and Alexander 2001), the detection of LPAIV (H3N8, H5N2 and H9N7) and NDV antibodies in the sera of apparently healthy domesticated Japanese quails as well as LPAIV H9N7 and NDV in peri-domestic laughing dove and NDV in village weavers is an indication of previous exposure to both viruses and show possible involvement of these domesticated and peri-domestic birds in the epidemiology of LPAI and ND in the study area. Consequently, these birds may play a part in the continued occurrence of these diseases by possibly shedding the viruses into the environment. More so, given that vaccination against AI is currently not permitted in the country, the detection of antibodies to LPAIV H3N8, H5N2 and H9N7 in apparently healthy quails and LPAIV H9N7 in laughing dove in this study indicates that LPAI H3N8, H5N2 and H9N7 circulate in these domesticated and peri-domesticated birds in Osun and Oyo states, southwest Nigeria. This is corroborated by previous studies that detected antibodies against LPAIV H3N8 and H5N2 in turkeys (Oluwayelu et al. 2015) as well as H5N2 in ducks (Coker et al. 2014) in southwest Nigeria and further supported by reports that showed that Japanese quails are susceptible to a wide range of LPAIV subtypes (Makarova et al. 2003).
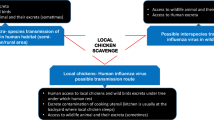
Quails have been suggested to serve as an intermediate host that provides an environment in which influenza viruses can generate variants that can be transmitted to other poultry (Xu et al. 2007), and also buttressed by the fact that they carry sialic acid receptors functional for binding of avian and human influenza viruses (Wan and Perez 2006; Costa et al. 2012).Therefore, the detection of antibodies to H3N8, H5N2 and H9N7 in quails in this study may pose a threat to veterinary and public health because the co-circulation of these avian influenza subtypes in the same susceptible bird population may result in the emergence of novel viruses as a consequence of natural reassortment and raises concerns on its control and public health implications of such co-circulation (Shakal et al. 2014).
In this study, it was observed that the odds of anti-AIV antibodies detection were 7 times higher in domestic than in peri-domestic birds. This may possibly be due to the varied susceptibility to AI virus among domestic and peri-domestic bird species, as well as the propensity of domesticated quails to be exposed to birds from numerous supply sources having a greater tendency of circulating these viruses and could serve as a source of infection (Killian 2009). Consequently, quails have been considered as important carriers for AI and ND viruses (Makarova et al. 2003; Lima et al. 2004).
Peri-domestic birds such as Laughing doves and village weavers are free-living birds seen around poultry houses and also around free-range birds, it is likely that these birds acquired AI and/or ND seropositivity via contact with other infected domestic or wild birds that were shedding the viruses. Also, previous studies have associated free-living birds as possible reservoirs of these viruses for domestic poultry (Meseko et al. 2007; Snoeck et al. 2011; Bergervoet et al. 2019). In addition, the seropositivity may be as a result of intermittent interface of these birds with vaccinated commercial poultry and free-range birds leading to circulation of ND vaccine viruses due to such (Oluwayelu et al. 2014). Moreover, it has been reported that ND transmission is intensified by contact between birds of different species usually practiced by birds being caged together in rural markets (Apopo et al. 2020).
Conclusions
This study provides serologic evidence of infection with LPAIV (H3N8, H5N2 and H9N7) and NDV in Japanese quails and laughing dove as well as NDV in village weavers in southwest Nigeria. The findings highlight the potential role of domestic and peri-domestic birds in the epidemiology of AI and ND, and also stress the need for continuous monitoring of different avian species in order to provide an early warning system for implementation of AI and ND control strategies.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. The raw data are available from the authors upon request.
Abbreviations
- AI:
-
Avian influenza
- AIV:
-
Avian influenza virus
- ND:
-
Newcastle disease
- NDV:
-
Newcastle disease virus
- LPAI:
-
Low pathogenic avian influenza
- LPAIV:
-
Low pathogenic avian influenza virus
- ELISA:
-
Enzyme-linked immunosorbent assay
- HI:
-
Hemagglutination inhibiting
- PI:
-
Percentage inhibition
References
Adebiyi AI, Fagbohun AF (2017) Infectious bronchitis virus in captured free-living, free-range and intensively reared birds in southwest Nigeria. Folia Vet 61(1):23–26
Adene DF, Wakawa AM, Abdu PA, Lombin LH, Kazeem HM, Fatihu MY, Saidu L, Joannis T, Adeyefa CAO, Obi TM (2006) Clinico-pathological and husbandry features associated with the maiden diagnosis of avian influenza in Nigeria. Nig Vet J 27(1):32–38
Aiki-Raji CO, Adebiyi AI, Agbajelola VI, Adetunji SA, Lameed Q, Adesina M, Adekanye G, Omidokun F, Fagbohun O, Oluwayelu DO (2015) Surveillance for low pathogenic avian influenza (LPAI) virus in live bird markets in Oyo and Ogun States, Nigeria. Asian Pac J Trop Dis 5(5):369–373
Aldous EW, Alexander DJ (2001) Detection and differentiation of Newcastle disease virus (avian paramyxovirus type 1). Avian Pathol 30:117–128
Alexander DJ (2007) Summary of avian influenza activity in Europe, Asia, Africa, and Australasia, 2002–2006. Avian Dis 51:161–166
Alexander DJ, Senne DA (2008) Newcastle disease. In: Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DE (eds) Diseases of poultry, 12th edn. Blackwell Publishing, Ames, pp 75–100
Apopo AA, Kariithi HM, Ateya LO, Binepal YS, Sirya JH, Dulu TD, Welch CN, Hernandez SM, Afonso CL (2020) A retrospective study of Newcastle disease in Kenya. Trop Anim Health Prod 52:699–710
Bergervoet SA, Pritz-Verschuren SBE, Gonzales JL, Bossers A, Poen MJ, Dutta J, Khan Z, Kriti D, van Bakel H, Bouwstra R, Fouchier RAM, Beerens N (2019) Circulation of low pathogenic avian influenza (LPAI) viruses in wild birds and poultry in the Netherlands, 2006–2016. Sci Rep 9:13681
Capua I, Alexander DJ (2006) The challenge of avian influenza to the veterinary community. Avian Pathol 35:189–205
Capua I, Marangon S (2006) Control of avian influenza in poultry. Emerg Infect Dis 12(9):1319–1324
Capua I, Munoz O (2013) Emergence of influenza viruses with zoonotic potential: open issues which need to be addressed: a review. Vet Microbiol 165:7–12. https://doi.org/10.1016/j.vetmic.2013.01.044
Clark L, Hall J (2006) Avian influenza in wild birds: status as reservoirs and risks to humans and agriculture. Ornithol Mon 60:3–29
Coker T, Meseko C, Odaibo G, Olaleye D (2014) Circulation of the low pathogenic avian influenza subtype H5N2 virus in ducks at a live bird market in Ibadan. Nigeria Infect J Pov 3:38
Costa T, Chaves AJ, Valle R, Darji A, van Riel D, Kuiken T, Majo N, Ramis A (2012) Distribution patterns of influenza virus receptors and viral attachment patterns in the respiratory and intestinal tracts of seven avian species. Vet Res 43:28
Durojaiye OA, Adene DF (1988) Newcastle disease and egg drop syndrome ’76 in guinea fowls (Numidameleagris galeata Pallas). J Vet Med Ser B 35(2):152–154
Kaleta EF, Kummerfeld N (2012) Isolation of herpes virus and Newcastle disease virus from White Storks (Ciconia ciconia) maintained at four rehabilitation centres in northern Germany during 1983 to 2001 and failure to detect antibodies against avian influenza A viruses of subtypes H5 andH7 in these birds. Avian Pathol 41:383–389
Killian ML (2009) National veterinary services laboratories avian influenza and Newcastle disease diagnostics report. In: Proceedings of the 113th annual meeting of the United States Animal Health Association, pp 590–593
Li Q, Zhou L, Zhou M, Chen Z et al (2014) Epidemiology of human infections with avian influenza A (H7N9) virus in China. N Engl J Med 370:520–532
Lima FS, Santin A, Paulillo A, Junior L (2004) Evaluation of different programs of Newcastle disease vaccination in Japanese quail (Couternix couternix). Int J Poul Scie 3:354–356
Makarova NV, Ozaki H, Kida H, Webster RG, Perez DR (2003) Replication and transmission of influenza viruses in Japanese quail. Virol 310:8–15
McFerran JB, McCracken RM (1998) Newcastle disease. In: Alexander DJ (ed) Newcastle disease. Kluwer Academic, Boston, pp 161–183
Meseko CA, Oladokun AT, Shehu B (2007) An outbreak of highly pathogenic avian influenza (HPAI) in a mixed farm by the introduction of a water fowl. Niger Vet J 28(3):67–69
Munir S, Hussain M, Farooq U, Zabid Ullah Q, Afreen M et al (2012) Quantification of antibodies against poultry haemagglutinating viruses by haemagglutination inhibition test in Lahore. Afr J Microbiol Res 6(21):4614–4619
OIE (2014) Manual of diagnostic tests and vaccines for terrestrial animals. Office International des Epizooties (OIE), Paris, 2014. http://www.oie.int/international-standard-setting/terrestrial-manual/access-online/. Accessed 24 Mar 2021
Oluwayelu DO, Adebiyi AI, Olaniyan IT, Ezewele P, Aina O (2014) Occurrence of Newcastle disease and infectious bursal disease virus antibodies in double-spurred Francolins in Nigeria. J Vet Med. https://doi.org/10.1155/2014/106898
Oluwayelu DO, Aiki-Raji CO, Adigun OT, Olofuntuyi OK, Adebiyi AI (2015) Serological survey for avian influenza in turkeys in three states of southwestern Nigeria. Influenza Res Treat. https://doi.org/10.1155/2015/787890
Oluwayelu DO, Omolanwa A, Adebiyi AI, Aiki-Raji CO (2017) Flock-based surveillance for low pathogenic avian influenza virus in commercial breeders and layers, southwest Nigeria. Afri J Infect Dis 11(1):44–49
Shakal MA, Youssef YI, El Zeedy SA, Ibrahim SM, Al Baroudi BM (2014) Surveillance on avian influenza H5N1 and H9N2 subtypes in Egypt 2012–2013. Poul Fish Wildl Sci 2(1):111. https://doi.org/10.4172/pfw.1000111
Shi W, Gao GF (2021) Emerging H5N8 avian influenza viruses. Science 372(6544):784–786
Snoeck CJ, Adeyanju AT, De Landtsheer S, Ottosson U, Manu S, Hagemeijer W, Mundkur T, Muller CP (2011) Reassortant low-pathogenic avian influenza H5N2 viruses in African wild birds. J Gen Virol 92(5):1172–1183
Wan H, Perez DR (2006) Quail carry sialic acid receptors compatible with binding of avian and human influenza viruses. Virology 346:278–286
Wang M, Fu CX, Zheng BJ (2009) Antibodies against H5 and H9 avian influenza among poultry workers in China. N Engl J Med 360:2583–2584
World Health Organization (2008) Cumulative number of confirmed human cases of avian influenza A/ (H5N1) reported to WHO. http://www.who.int/csr/disease/avian_influenza/country/cases_table_2008_01_03/en/index.html
Xu KM, Li KS, Smith GJ, Li JW, Tai H, Zhang JX, Webster RG, Peiris JS, Chen H, Guan Y (2007) Evolution and molecular epidemiology of H9N2 influenza A viruses from quail in southern China, 2000–2005. J Virol 81:2635–2645
Yuan X, Wang Y, Li J, Yu K, Yang J, Xu H, Zhang Y, Ai H, Wang J (2013) Surveillance and molecular characterization of Newcastle disease virus in seafowl from coastal areas of China in 2011. Virus Genes 46:377–382
Acknowledgements
We appreciate the animal owners for allowing sample collection.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AIA and DOO contributed to the study conception and design. Material preparation, data collection and analysis were performed by AIA, OG and AJ. The first draft of the manuscript was written by AIA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was in line with the National code for Health Research Ethics and approved by the Oyo state Ministry of Health Research Ethics Committee (AD13/479/346). The farm owners and traders voluntarily consented to sample collection.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adebiyi, A.I., Gamra, O., Jubril, A. et al. Serological investigation of low pathogenic avian influenza and Newcastle disease virus antibodies in Japanese quails, 30 village weavers and one laughing dove in two states of Nigeria. Bull Natl Res Cent 46, 25 (2022). https://doi.org/10.1186/s42269-022-00710-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-022-00710-6