Abstract
Background
Water pollution due to the disposal of heavy metals in water bodies is one of the most global concerns that adversely affect the ecosystem and human health because of their non-biodegradability and potential of accumulation. Copper is one of the most present metals in the environment released mainly from disposing of agricultural fertilizers and pesticides, mining operations, chemical, pharmaceutical, and paper manufacturing industries into stream bodies. The elevated exposure to Cu(II) causes severe toxicity, diabetes, anemia, kidney disorders, liver damage, and death.
Main body
Several researchers developed detection methods and techniques for Cu(II) detection in the different water samples and sources to ensure that Cu(II) concentration falls within the acceptable limit range, including atomic and molecular spectrophotometry, mass spectroscopy, sensors, voltammetry, and chromatography. This review spotlights recent Cu(II) detection trends in water samples, highlighting their detection limits, advantages, and disadvantages.
Conclusion
Based on the water sample, detection limit, method cost, an appropriate analysis can be used. Recent trends of Cu(II) detection in water samples include atomic and molecular spectrophotometry, mass spectroscopy, sensors, voltammetry, and chromatography. The principle, definitions, experimental work, advantages, and disadvantages of each method are discussed and highlighted.
Similar content being viewed by others
Background
Water scarcity is one of the global issues that highly affects the living quality and risks many lives. The dramatic growth in industrialization and population drives the continuous need for water for consumption and generates wastewater streams contaminated with various pollutants. Wastewater streams are considered the most predominant sources that contaminate drinking water, groundwater, and seawater with heavy metals. Although some metals in trace limits are beneficial for many biological processes, their presence at a higher level can cause adverse consequences on the whole ecosystem. For example, different types of fish accumulate heavy metals in their livers and muscle tissues (Kinuthia et al. 2020; Sobhanardakani et al. 2011). The impacts of heavy metals will not only be on marine creatures; however, human health might also be affected significantly if these contaminants entered the food or the biological chain at an elevated level (Pesavento et al. 2019; Wołowiec et al. 2019).
Copper (II) is one of the most common substances in the environment among heavy metals. Cu(II) is an essential micronutrient for several animals and plants and can be naturally accumulated in the food chain (Briffa et al. 2020). The primary sources of Cu(II) in the environment are agricultural fertilizers and pesticides, mining operations, chemical, pharmaceutical, and paper manufacturing industries (Wołowiec et al. 2019). Besides, people with in-house-copper plumbing systems are exposed to elevated Cu(II) levels in the drinking water than others due to pipe corrosion (Government of Western Australia 2016; Pesavento et al. 2019). It is worth mentioning that the accepted level of Cu(II)in drinking water is1.3 mg L−1 (US-EPA 2009; WHO 2004).
With the continuous world's Cu(II) production, Cu(II) quantities in the environment, especially soluble Cu(II) compounds, which are the greatest threat to people's health, have increased, leading to adverse environmental effects. Long-term Cu(II) exposure can cause severe toxicity, diabetes, anemia, kidney disorders, liver damage, and death (da Costa Lopes and Bogel‐Łukasik 2015; Government of Western Australia 2016; Malik et al. 2019; Taylor et al. 2020). These adverse consequences arise the need for detection methods or technologies of Cu(II) in different water samples and resources, determining if the Cu(II) limit is acceptable or not, mitigating any risk for human lives.
Main text
Many methods have been developed to detect copper (II) Cu(II) in various water samples, including spectrophotometric methods (Awual and Hasan 2015; Fu and Yuan 2007; Satheesh and Rao 2016), voltammetric methods (Liu 2017), sensors (Li et al. 2019), selective membranes.(Zamani et al. 2007), and others (Seher et al. 2020; Wang et al. 2014a, 2019; Yetimoğlu et al. 2010). However, no previously published article comprehensively reviewed recent trends of Cu(II) detection in water samples, including advances in atomic and molecular spectrophotometry, mass spectroscopy, sensors, voltammetry, and chromatography.
Therefore, this review aims to comprehensively discuss these technologies for different water samples and evaluate their benefits and drawbacks. Figure 1 illustrates a hierarchic graph of these methods.
Atomic spectrometric methods
Atomic spectrometric methods (ASM) use spectral dispersion equipment to isolate the determinable element signals (Broekaert 2006). ASM can be classified according to the sort of spectral dispersion, either absorption or emission. In atomic absorption, the ground-state atoms of metals absorb light at particular wavelengths to allow electronic transitions in their electron orbitals. On the other hand, in atomic emission, thermal energy creates excited-state atoms that emit light to return to the ground state. Atomic emission has the advantage of the possibility of measuring several different atomic species simultaneously (Bachmann and Miller 2020). ASM can also be classified according to the element signals and their detection, either photo-spectroscopy or mass-spectroscopy.In mass-spectroscopy, the atom is ionized with an electric charge to gain electrons. Then analytes are identified and quantified using the m/z generated from a sample (Thomas 2019).
Atomic absorption spectroscopy
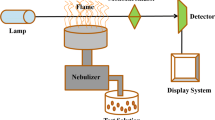
Atomic absorption spectrometry (AAS) has been widely used for analyzing heavy metals. ASS includes several techniques in which free atoms in a gaseous state absorb specific optical radiation, determining the atomic composition. AAS is classified according to the atomization methods into Graphite Furnace Atomic Absorption Spectrometry (GFAAS), Flame Atomic Absorption Spectrometry (FAAS), Cold Vapor Atomic Absorption Spectrometry (CVAAS), and Hydride Atomic Absorption Spectrometry (HAAS) (Baysal et al. 2013; Michalke and Nischwitz 2013; Zhang et al. 2020). The concentration levels of detected elements present in the sample can be evaluated based on the absorption intensity(Baysal et al. 2013; Zhang et al. 2020). AAS can detect over 70 elements in samples with different physical states, such as solid or solution phases. AAS, FAAS, and GFAAS are the applicable standard techniques for detecting Cu(II) in different liquid samples. A block diagram of AAS is illustrated in Fig. 2.
Graphite furnace atomic absorption spectrometry (GFAAS)
GFAAS, also known as Electro-Thermal Atomic Absorption Spectrometry (ETAAS), is one of the atomic spectrometric methods in which a small quantity of the sample is placed in a graphite tube that is heated to dissolve, decompose, volatilize, ash, and atomize the sample, respectively(Holcombe and Borges 2006). The detection is conducted by passing light from a line source, which is a characteristic of the element, through the graphite tube. The absorbance resulting from the existence of the free analyte atoms in the gas state is measured.
GFAAS provides reliable and accurate results, but the measurement takes a relatively long time, and the number of detectable elements is less compared to other AAS methods (Malik et al. 2019; Zhang et al. 2020). GFAAS has been used for heavy metals detection, including Cu(II) in water samples. Huang and Shih (1993) detected Cu(II) in seawater samples using GFAAS. The method provides high precision and accuracy for Cu(II) detection, where the detection limit of Cu(II) was between 0.3–0.4 µg L−1 with the injection of a 20 out of µl of seawater, which was further reduced to 0.07 µg L−1 when multiple injections were utilized (injection of five 20 µl of seawater).
Flame atomic absorption spectrometry (FAAS)
FAAS is a popular method that is widely used for the analysis of trace metals. The sample is aspirated through a tube to the nebulizer, broken into a fine aerosol. Then, the aerosol is carried to the flam by a carrier gas and broken up into its separate atoms. The atomization of the analyte in solution is performed using a mixture of oxidant gas and fuel gas, in which the fuel gas is also considered a carrier gas(Švehla et al. 2019). Nitrous oxide-acetylene or air-acetylene are the two types of combustion flame gases used depending on the thermal stability of the analyte, where acetylene-nitrous oxide (dinitrogen oxide) flame provides a temperature of 3000 °C, while for air-acetylene flame provides a temperature of 2300 °C(Baysal et al. 2013).
FAAS is extensively utilized for the analysis of high concentrations of trace metals in various samples. The detection limits of the FAAS technique are usually within the ppm range, and the analysis of the present elements in the sample takes few seconds (10 to 15 s) (Baysal et al. 2013). Many studies and reviews have been conducted for the detection of Cu(II) utilizing FAAS. Khayatian et al. (2018) conducted an experimental work using FAAS for the detection of Cu(ll) and Pb(ll) in wastewater samples from refineries. Outcomes of this study showed detection limits of 4 ppb and 11 ppb for Cu(ll) and Pb(ll), respectively. Researches have been conducted to boost the FAAS detection limit. Pourabdollah and Mokhtari (2014) enhanced the detection limit of Cu(II) in samples of the river and sewage water to ng mL−1by solid-phase extraction. Bagherian et al. (2019) used a liquid–liquid microextraction technique to enhance the detection limit of Cu(II) to 0.60 μg L−1 for 10 ml of water solution sample. Cloud point extraction allowed the determination of Cu(II) with a detection limit of 1.5 µg L−1 (Lemos et al. 2007) and 0.04 µg L−1 (Goudarzi 2007). Dispersive Liquid–Liquid Micro-Extraction-Slotted Quartz Tube (DLLME-SQT) could detect Cu(II) in wastewater, tap, and seawater samples with 0.67 µg L−1 (Özzeybek et al. 2017). Spiking the samples can help in a broad range of concentrations between 21 and 1400 µg L−1 at 324.754 nm, 249.215 nm, and 224.426 nm (Lima et al. 2010). Other related studies were covered in other literature (Citak and Tuzen 2013; Fathi et al. 2010; Ouyang et al. 2010; Saber Tehrani et al. 2005). Table 1 summarizes using of AAS to detect Cu(II) in various water samples.
Atomic emission spectroscopy
In contrast to AAS, thermal energy is used to create excited-state atoms in Atomic Emission Spectroscopy AES. The excited atoms emit light to relax to the ground state. AES has the advantage of measuring several different atomic species simultaneously (Bachmann and Miller 2020). AES methods include a flame photometer that uses a cooking gas flame to measure sodium, potassium, lithium, X-ray spectroscopy, and Inductively Coupled Plasma (ICP) that uses plasma to achieve a high temperature between 6000 and 10,000 K can analyze over 70 elements.
Although X-ray spectroscopy has been reported to detect Cu(II), it requires preconcentration before the analysis through complicated treatment methods, including precipitation by Ammonium-Pyrrolidine-Dithiocarbamate (APDC) (Tomic et al. 1985) and chelating solid-phase extraction disk (Hou et al. 2003), as well as it is not a standard technique for determination of Cu(II). Hence, X-ray spectroscopy will be skipped in this review, in addition to the flame photometer that is not used for detecting Cu(II). On the other hand, most heavy and earth alkaline elements are determined using ICP characterized by high temperature, electron density, excitation, and ionization capability (Deng et al. 2017). ICP can be classified according to the detection method into Optical Emission Spectrometry (ICP-OES) and Mass Spectrometry (ICP-MS). The difference between ICP-OES and ICP-MS is that, while the detection in ICP-OES depends on the emitted photons, ICP-MS depends on the mass to charge ratio m/z (Poirier et al. 2016). It is noteworthy that a combination of OES and MS has been investigated by many researchers (Deng et al. 2017; Garbarino et al. 1989; Sawatari et al. 1995). This combination allows analysts to benefit from exceptional, independent features of each detector, which will result in more accurate analysis and a broader linear range of calibration curves, as reported by Deng et al. (2017).
Although X-ray spectroscopy.
Inductively coupled plasma-optical emission spectrometry (ICP-OES)
The principle of elemental analysis using ICP-OES depends on electromagnetic radiation emissions at wavelength characteristics of a particular element and the intensity, which indicates the element concentration. These electromagnetic radiation emissions result from atomic relaxation to the ground state of their thermal excitation by the plasma. Inert argon gas entered into ICP-OES is subjected to a high voltage spark due to Radio Frequency (RF), which creates a magnetic field at 27 or 40 MHz, resulting in ionization and plasma formation in the plasma torch (Jung et al. 2019; Rutzke 2018). Plasma torch composites of concentric quartz or ceramic tubes in which stable and sustainable plasma can reach a high temperature between 6000 and 10,000 K are produced. The ICP-OES can be configurated as either radially or axially viewed. Axial view provides better detection limits, whereas radial view results in a higher upper linear range. The samples are usually introduced to the instrument as an aerosol after converting from liquid through a nebulization process (Liu et al. 2021). The detector measures the emitted line intensity. The Components layout of radial and axial ICP-OES is illustrated in Fig. 3.
Components layout of a radial ICP-OES and b axial ICP-OES instrument (Rutzke 2018)
Many research works exist on ICP-OES. Donati et al. reviewed the advances and applications of ICP-OES (Donati et al. 2017). Other researchers conducted experimental work to detect various metals, including Cu(II), in water samples (Cui et al. 2011; Ranjbar et al. 2012). Giersz et al. (2017) could detect volatile and non-volatile elements using photochemical vapor generation pneumatic nebulization in the programmable temperature spray chamber (PCVG–PN–PTSC). Dimpe et al. (2014) suggested optimizing the operation conditions in ICP-OES to enhance the detection limit of Cu(II) in water samples. Suggestions include selecting a wavelength of 324.754 nm, RF power to be1400 W, and plasma argon flow rate to be 0.95 L min−1. Ranjbar et al. (2012) proposed using Ionic liquid-based dispersive liquid–liquid microextraction combined with ICP-OES to determine trace quantities of metal, including Cu, Co, Ni, Mg, and Zn in environmental water samples, including wastewater, drinking water, and fish farming pond water. The proposed optimum operating conditions are RF power of 1.65 kW, nebulizer pressure of 140 kPa, and a Cu spectral line of 323.754 nm.
Inductively coupled plasma-mass spectrometry (ICP-MS)
ICP-MS has similar components and operational mechanisms described in ICP-OES, such as the sample introduction and plasma generation. However, In ICP-MS, after the samples' aerosol is transferred into the high-temperature plasma that generates ions, the generated ions are extracted through the interface region into electrostatics ions (ion optics), as shown in Fig. 4. The ion optics is responsible for focusing the ion beam into the quadrupole analyzer, where the separations of ions take place upon their mass to charge ratio, which is subsequently measured at the detector (Chen et al. 2020). ICP-MS can detect a wide range of heavy metals from different water sources and their isotopes. Several types of ICP-MS are used for detection, such as Multi-Collector Inductively Coupled Plasma Spectrometry (MC-ICP-MS), which have a multidetector and can collect and analyze isotopes precisely. Another type is High Resolution Inductively Coupled Plasma Mass Spectrometry (HR-ICP-MS). This type has an electric and magnetic sector for ion separation and focusing (Einschlag and Carlos 2013).
Schematic diagram of ICP-MS instrument (Gilstrap Jr 2009)
Many researchers suggest preconcentration of the heavy metal in samples before their analysis by ICP-MS. For example, Xing et al. (2019) indicated that 50-time pre-concentrates of test samples using 250 mg of NOBIAS chelate resins before ICP-MS analysis accurately detect the heavy metals including Cu (II), Cd (II), Mn (II), and Zn (II). O'Sullivan et al. (2013) also suggests preconcentration of the heavy metal, including Co, Cu, Ni, and Zn, using solid-phase extraction by injecting the sample through a column of chelating sorbent. An optimized single-particle ICP-MS (spICP-MS) technique was utilized by Venkatesan et al. (2018) to analyze Pb, Fe, Sn, Cu, Ag in tap water samples. spICP-MS is a time-resolved analysis where the particles are detected as pulses above the elemental background signal. The instrument revealed that Cu(II) was detected in only 25 water samples at a concentration range of 15–136 ng L−1. Table 2 summarizes ICP-OES and ICP-MS techniques.
Molecular spectrophotometric methods
Molecular spectrophotometric methods measure the absorption of ultraviolet (UV), visible, and near-infrared (NIR) electromagnetic waves by a molecule to identify and quantify the substance of interest. These molecules, known as a chromophore (or chromogen), absorb light at a specific wavelength producing peak absorbance, in which its area is proportional to the quantity of analyte (Bachmann and Miller 2020). Detection of Cu(II) using molecular spectrophotometric methods depends on the type of reagent that leads to chromophore formation.
Spectrophotometric techniques have been used to detect Cu(II) in wastewater (Awual and Hasan 2015; Fu and Yuan 2007; Satheesh and Rao 2016), where different types of reagents or ligands have been studied to estimate Cu(II) concentrations in water using a chromogenic reagent. For example (Alharthi and Al-Saidi 2020) synthesized 4-amino-3-mercapto-6-[2-(2-thienyl) vinyl]-1,2,4-triazin-5(4H)-one (AMT) as chromogenic reagent. The experiment was first done by dissolving a specific amount of CuNO3·3H2O in deionized water to prepare a stock solution of C Cu(II) (50 µg mL−1). Then HACH LANGE (Model DR-6000) spectrophotometer was used to record electronic spectra and absorbance values of the ligand and its Cu complex, while Perkin Elmer CHNS/O analyzer model 2400 Series II was used to specify the elemental composition of the legend and its complex with Cu. To characterize the complexity of Cu (II) and AMT different techniques have been used in this study, such as Fourier Transform Infrared (FTIR), scanning electron microscope (SEM), Energy Dispersive X-Ray Analysis (EDX), Thermogravimetric analysis (TGA), and erythrocyte sedimentation rate (ESR). The rapid color production (a brown product) caused by AMT's reaction with Cu(II) in less than 10 s at a wide range of pH values was concluded to be a sensitive chemosensor for Cu(II) monitoring in water samples a detection limit of 0.011 µg mL−1. As a result, the suggested spectrophotometric approach is suitable for many laboratories' rapid and routine analyses. Another chromogenic reagent, azo-Schiff base 1-((4-(1-(2-hydroxyphenylimino)ethyl)-phenyl) diazenyl) naphthalene-2- ol (HPEDN), was studied by Raafid et al. (2020) in which it was complexed with Cu(II) and displayed a brown color with maximum absorption at λmax of 500 nm. In the concentration range of 1.7 to 5.4 g mL−1. The obtained results were compared to those resulting from the flame atomic absorption spectrometry process, revealing that both methods produced similar results.
A third chromophoric ligand for Cu(II) ions is chloro (phenyl) glyoxime, which was investigated by Turkoglu and Soylak (2005) to determine trace quantities of Cu(II) using Hitachi Model 150–20 UV–VIS double beam spectrophotometer. The procedure followed in this study was started by applying 1000 µl of 0.05% chloro (phenyl) glyoxime solution to ten micrograms of Cu(II) in a 25-mL calibrated flask. Borate buffer was used to keep the pH of the solution 4.0. The solution was then combined and diluted using distilled water. A spectrophotometer was used to calculate the absorbance of this solution after 10 min against a reagent blank at 290.5 nm. This procedure showed a high molar absorptivity value, low detection limit (10 µg L−1), easily applied to detect Cu(II) in actual natural water samples. In addition, a study by Sharma et al. (2010) showed that a maximum absorbance could be achieved through using a novel UV spectrophotometric method (Shimadzu UV–visible 160 A spectrophotometer) based on complex formation of Cu(II) with reagent cefixime immediately in 1,4-dioxan-distilled water at ambient temperature. Less number of reagents utilized in this study without acid, buffer solution, and heating of reaction mixture besides too rapid analysis showed that the proposed method could analyze Cu (II) in natural water samples with 3.19 × 10–2 µg mL−1as detection limit. For high precision and accuracy, another reagent that can react with Cu(II) under the same spectrophotometric technique is 1-(2-pyridylazo)-2-naphthal in a highly acidic solution at pH 2.40 to 2.55 to produce pink chelate with maximum absorption at 550 nm and a detection limit of 0.1–2.5 μg mL−1. Cu(II) can be detected using this method in industrial wastewater (Sarker 2010).
To avoid using hazardous solvents for Cu(II) detection in water samples, which may harm the environment. A study by Hashem et al. (2011) used noncancerous (green chemistry) and non-expensive reagents such as 5-(4-nitrophenylazo) salicylic acid (NPAS) and 2,2’-Dipyridyl(Dp) as two ligands that made a high stable ternary complex of Cu(II) at λmax 525 by Job's method and achieved 0.63–5.04 mg L−1 of Cu(II) determination.
Furthermore, reagent polyethyleneimine (PEI) has several advantages over other reagents, including fast detection, high sensitivity, good selectivity, a broad linear response spectrum, and low cost. The Cu(II) ion was found to have a higher selectivity by PEI than other ions investigated (Wen et al. 2017). They used polyethyleneimine to produce a dark blue cuprammonium complex characterized by two absorption peaks at 275 and 630 nm with a detection limit for Cu(II)equal to 566 nM. On the other hand, using Cu(II) piperazine showed simple, accurate, and effective Cu(II) detection in water and industrial wastewater (Kavitha et al. 2013).
The spectrophotometric determination of the Cu(II) content has also been determined by using the sodium salt of 4-phenylsemicarbazone1,2-naphthoquinone-4-sulfon as a legend, which can be easily synthesized by using a water–ethanol solution for its recrystallization (Zagurskaya-Sharaevskaya and Povar 2015). It was observed that the detection limit of the Cu(II) ions in tap water by spectrophotometric be 0.012 mg L−1, and the concentration was about 0.0400 mg L−1 same as what was given by the voltammetric method. It was recommended to examine the used legend in this study in vivo applications because no effect was observed on biological systems. Table 3 summarizes different types of reagents to detect the Cu(II) using spectrometric methods and their detection limits.
Sensors
Using sensors for analysis has recently attracted attention due to their low cost, high efficiency, and great sensitivity. Advances of current sensor technologies are discussed in this section, including electrochemical, colorimetric, and optical fiber chemical sensors.
Electrochemical sensors
Electrochemical sensors are a great addition to the trace detection of heavy metals because of their robustness, selectivity, fast analysis, and user-friendly devices. The principle of these sensors is to use electrodes through electrochemical technologies, such as anodic stripping voltammetry, for heavy metals detection. The main advantages of electrochemical sensors include their inexpensive, sensitivity, and ease of operation. However, designing electrodes using new and economical materials is essential for further improvement in the precision of the analysis and the results obtained (Pujol et al. 2014).
Romero-Cano et al. (2019) discussed the electrochemical detection of Cu(II) in water samples. Carbon paste electrodes formulated from bio-template (grapefruit peels) were used and functionalized with the carboxyl group. The Cu(II) detection involves three steps, as shown in Fig. 5. The first stage includes the adsorption of Cu(II) on the oxygenated groups of the used substance. Oxygenated groups provide protons to Cu(II), while water molecules recompense the other Cu(II) coordination. In the second step, a potential of − 0.4 V (vs. Ag/AgCl) is applied to the medium, reducing the Cu(II) on the electrode surface. This step is followed by observing an oxidation signal (about − 0.2 V) due to Cu re-dissolution on the electrode surface, indicating Cu existence in the solution.
Mechanism of Cu(II) detection (Romero-Cano et al. 2019)
Timoshenko et al. (2020) developed a selective sensor for electrochemical detection of Cu(II) ions in the nanomolar concentration in biological systems. In this technique, electrodes with a 10 μm diameter were functionalized with modified superoxide dismutase (E2Zn2SOD), where E represents the free active site of Cu Cu(II). The reaction between Cu(II) and E2Zn2SOD has high specificity, guarantees high accuracy, selectivity, and sensitivity.
Many other studies discussed various electrodes, such as modifying bulk electrodes with graphitic carbon particles, nanocomposite of Cetrimonium bromide and ZnSe QD, and carbon electrode with immobilized silver hexacyanoferrate nanoparticles. These investigations revealed the positive impacts of sensors characterized by high sensitivity and lower detection limits (Fattahi et al. 2014). It is noteworthy that the combination of electrochemical biosensors could enhance sensor efficiency, selectivity, sensitivity, and detection limit, such as using gold nanomaterial for detecting Cu(II) in wastewater (Ejeian et al. 2018; Gumpu et al. 2015).
Colorimetric sensor
Recently, colorimetric sensors with gold and silver nanoparticles have played an essential role in several areas such as chemical, biological and environmental fields because they are simple, sensitive, and highly biocompatible. Ma et al. (2011)discussed the colorimetric determination of Cu(II) during the generation and synthesis of silver and dopamine NPs. Dopamine is described as having the multifunctional ability, such as its ability to reduce metal ions to metal NPs (e.g., Ag+). Dopamine can decrease the silver ions to monodispersed AgNPs and operate the AgNPs generated. The oxygen and nitrogen in the dopamine can coordinate with the Cu(II) ion, and thus the AgNPs functionalized with dopamine can coordinate with the Cu(II) ion. An aggregation of AgNPs and a color-changing response might occur due to the binding reaction of dopamine and Cu(II). Therefore, in one step, production, modification of AgNPs, and the determination of Cu(II) ion can be achieved. Figure 6 displays the primary mechanism of colorimetric detection of Cu(II) ions with a nanosilver/dopamine sensor. When silver nitrate is added to the dopamine solution, the solution color turns yellow, indicating monodispersed AgNPs. Changing the pH using NaOH facilitates the adjustment of the reaction rate.
Mechanism of colorimetric detection of Cu(II) ion (Ma et al. 2011)
Chen et al. (2015) reported the main steps for the Cu(II) AuNPs-based colorimetric detection process, including the synthesis of AuNPs, the modification of AuNPs, and then the detection of Cu(II). However, this process is complicated, and there is a need for better and more effective sensors. Wang et al. (2014b) discussed heavy metal detection in aqueous solutions using the colorimetric sensor. Rhodamine was used as the base colorimetric matter, doped with CuCl2, CrCl2, NiCl2, and the change in the final solution's color indicates a change in the solution properties such as pH, electrical conductivity, and optical properties.
Optical fiber chemical sensor
Optical transduction, which includes various Surface Plasmon Resonance (SPR) or localized SPR (LSPR) based sensing devices, has recently been proposed for heavy metals detection. SPR has many advantages, such as its high sensitivity, selectivity, cost efficiency, and fast rate. Moreover, it is an effectual marker-free technique used to determine the interface between an immobilized receptor and a substrate. It has also been used for many decades for detecting different types of metals, including Cu(II) (Pesavento et al. 2019). The detection technique is based on the diversity of the receptor layer's refractive index at the metal surface, wherein the surface plasmon resonance is getting agitated once the metal ion comes together with the receptor. Pesavento et al. (2019) developed an SPR sensor for Cu(II) detection in drinking water. The sensor was based on the D-shaped plastic optical fiber (POF) using D, L-penicillamine as the receptor. POF with a core of poly-methylmethacrylate and a fluorinated polymer layer was modified to recognize the optical sensor platform. The mechanism of the sensor can be obtained in three steps, as shown in Fig. 7.
Mechanism of Optical Fiber Chemical sensor (Pesavento et al. 2019)
The first step includes producing a D-shaped region on the plastic optical fiber by eliminating cladding and part of the core through a basic polishing procedure based on two polishing papers. In the second step Microposit, S1813 photoresist is turned on the exposed plastic optical fiber core using a rotating coater device to determine a layer between the metal and the plastic optical fiber. This layer can enhance the performance as it has a higher refractive index than that of the plastic optical fiber. According to Cennamo et al. (2011), the spin coating method permitted the accumulation of a consistent photoresist layer around 1.5-µm thickness. A thin gold film was spluttered in the third and last step. The procedure of spluttering was replicated three times thru operating an electric current of 60 mA, at a pressure of 0.05 mbar, for 35 s, therefore, obtaining a 60 nm thick layer (20 manometers of gold for every single step).
Abdullah et al. (2017) studied the use of optical biosensors. He mentioned that the main principle of biosensors is the use of biological substances such as enzymes, proteins, and receptors to detect various elements, including heavy metals (e.g., Cu(II)). It is used to measure the biological response, thus generating an electrical signal equivalent to the concentration of the measured substance. In recent years, a cell-free optical biosensor was developed by Gupta et al. (2019) to detect a wide range of copper and mercury present in wastewater by using genetically modified plasmids. In the same study, luciferase and emerald-green fluorescent protein genes were used firefly separately as a detector, which resulted in an identical detection limit value of mercury equal to 1 ppb in Escherichia coli and the cell-free system. Table 4 summarizes the detection limits and pH medium for each type of sensor in water samples.
Voltammetric method
Electrochemical practices are getting more attention in today's world as an optimal method for Cu(II) detection in water samples. These techniques have many advantages such as low-cost equipment, easy procedure to follow, appropriateness for real-time detection, and transportability methods compared to others (Barshan-Tashnizi et al. 2013). The following sections explain two selected techniques in the voltammetry category.
Differential pulse anodic stripping voltammetry technique
Electrochemical examination based on anodic stripping voltammetry (ASV) is an excellent method for detecting heavy metals such as Cu(II) in water samples. The advantage of this method is that it can monitor minimal concentrations of metals by the electrochemical amplitude on the electrode surface in a short time using relatively cheap equipment (Javanbakht et al. 2009; Rehacek et al. 2008). There are limited studies on detecting Cu(II) using ASV, but one good study was by Zhuang et al. (2011), who used a gold microelectrode to detect Cu (II) in water samples using the differential pulse ASV technique. The water samples are first acidified by 0.01 M HNO3 as a backing electrolyte for Cu(II) detection purposes in the experimental work. After that, all samples are collected in acid-cleaned High-Density Polyethylene (HDPE) taster bottles at a temperature around -4 °C. Then, a gold microelectrode is used as a working electrode with a diameter of 25 μm and is motivated in H2SO4 by cyclic sweeping voltammetry in range from (− 0.15 to 1.5 V) at a specific scan rate. Anodic stripping resolution contains two main stages. Cu (II) is reduced to Cu(II) ion on the microelectrode surface at − 0.4 V and required time in the first stage. Moreover, the next stage is to oxidize the Cu(II) ion during sweeping analysis from (0–0.6) V. In order to quantify Cu(II), the current of the stripping peak should be measured in this method. Differential pulse (ASV) is accomplished for Cu(II) determination. The optimum conditions for differential pulse anodic stripping voltammetry method are − 0.4 V for accumulation potential, 210 s accumulation time, 0.05 V amplitude, 0.1 pulse width, and 10 s for pulse time (Zhuang et al. 2011). Results of the proposed method can be reported by comparing the anodic peak of the voltammetric curve of the gold microelectrode in electrode without Cu(II) (dashed line) with the linear sweeping voltammetry curve in HNO3 (0.01 M) solution having 1um of Cu(II) at fifty mV/s scan rate (solid line). Fast chemical redox of Cu(II) is a logical reason behind the difference between the two mentioned curves, as shown in Fig. 8. So, it can be concluded that gold microelectrode is an excellent choice to discover Cu(II) sensitively.
Anodic peak of the voltammetric curve of the gold microelectrode in electrode without Cu(II) (dashed line) and the linear sweeping voltammetry curve in HNO_3 (0.01 M) solution having 1 μm of Cu(II) at fifty mV/s scan rate (solid line) (Zhuang et al. 2011)
Fast Fourier transformation continuous stripping cycle voltammetry
In this technique, Cu(II) ion is determined using the Fast Fourier transformation continuous stripping cycle voltammetry method by implementing a carbon paste electrode. A supported liquid membrane has been implemented in order to recover Cu(II) from the feed sample (first chamber) with 2-Amino-N-(2-Pyridyl Methyl)-Benzamide as an organic ligand (Fig. 9). Because of the pH difference between the two existing chambers, Cu(II) is transferred into the receiving chamber, in which Cu(II) ions can be detected using the Fast Fourier Transformation CCV with carbon paste electrode within 30 min. An organic ligand is used to detect Cu from the color intensity change in a UV cell. It is noteworthy that pH value, scan rate, accumulation time, stripping potential, and reagent concentration are some influential factors that have a critical effect on the performance of the mentioned techniques in the voltammetry category (Mofidi et al. 2017; Romero-Cano et al. 2019).
Diagram shape of removal setup by utilizing supported liquid membrane (Romero-Cano et al. 2019)
Chromatography
Chromatography is an old but highly efficient method to separate the components in a mixture or purify a compound mixture. In the chromatography, the sample is introduced into the mobile phase, and its affinity towards the column stationary phase over the mobile phase controls the separation. In the standard liquid chromatography technique, the stationary phase is polar, and the eluent is nonpolar or less polar. However, in the reverse process, the stationary phase and eluent will be the opposite. Ali et al. (1998). The detection can be the absorption of light, changes in the refractive index, or conductivity. Various chromatographic techniques persist, but Reverse Process High-Pressure (or Performance) Liquid Chromatography- (RP-HPLC) and Ion Chromatography (IC) are the two techniques used for the detection and speciation of the Cu metal in the water sample. In HPLC, a high-pressure liquid carries the sample into the separation column and then to the detector. However, in IC, stationary phase interaction is through ion exchange.
Reverse process-high pressure liquid chromatography (RP-HPLC)
RP-HPLC is a technique for the simultaneous detection of heavy metals, including Cu(II) in the water sample utilizing the solid-phase extraction method to preconcentrate the minimal concentration of Cu in the water sample (Rekhi et al. 2017; Yang et al. 2004). The preconcentration is conducted using a precolumn derivatization agent for Cu is tetra (m-aminophenyl) porphyrin (Tm-App) (Daud and Alakili 2001; Hu et al. 2003). The HPLC apparatus mainly consists of a separation column, a solid-phase extraction cartridge, and detectors. A spectrometer is used for the absorbance measurement and an atmospheric absorption spectrometer for the atomic absorption spectrometry analysis. The standard solution of Cu mgmL−1 is collected and diluted to 0.2 µgmL−1. RP-HPLC has two mobile phases, A and B. Mobile phase A is methanol having 0.05 mol L−1PH 10 pyrrolidine-acetic buffer salt, and Mobile phase B is acetone having 0.05 molL−1 PH 10. The Cu-Tm-App chelates are steadier in a weakly alkaline medium. A mobile phase with a pH between 8.2 and 11.8 avoids decomposing of chelates and gets a good peak shape. Also, tetra (m- aminophenyl) porphyrin (Tm-App) was synthesized and dissolved in THF to make a 1.5 × 10–4 mol L−1solution. For the Cu-Tm-App, the proper eluent is needed. Different organic solvents were tested and found out that Tetra Hydro Furan (THF) efficiently elutes the reclaimed Cu-Tm-App chelates quantitatively from the cartridge THF containing 0.05molL−1 pyrrolidine-acetone acid buffer salt increases the stability of Cu-Tm-App chelates, and it was selected as eluent. pH was measured using the Beckman Ø-200PH meter. According to the concentration of Cu, the appropriate volume of standard solution was transferred to 100 ml volumetric flask and mix with 6 ml of 1.5 × 10−4molL−1Tm-App solution in THF,10 ml of 0.5 mol L−1pyrrolidine acetone acid buffer solution, and 4 ml of 2% Triton X-100 solution. Then the solution was diluted and makeup to 100 ml with water. Then it was kept in a boiling water bath and heated for 10 min. After cooling, it was passed through a C18 cartridge at a flow rate of 10 ml/min. After the enhancement, the cartridge was turned upward and separated the holding chelates from the cartridge with 2.5 ml of THF at a flow rate of 5mlmin−1in the opposite direction. Then the THF was filtered using 0.45 µm filters and volatilized to 0.6 ml and diluted to a volume of 1 ml with methanol.20µL of this solution is injected into HPLC analysis the photodiode array detector recorded 3-D chromatogram at 435 nm During the separation, the mobile phase component at 0 min is A-100% and B-0% and at 10 min is A-80% and B-20% using a linear gradient. The Cu-Tm-App chelate was detected at its maximum wavelength. It was a highly sensitive and selective method for determining Cu in water samples (Daud and Alakili 2001).
Ion chromatography
It is a type of liquid chromatography where the separation is based on the ion exchange. Depending on the ion exchange between the stationary and mobile phases, ions or charged species are separated. Different columns are using for separating the anions and cations (Kaiser 1974). Trace amounts of both hydrated and complex metal ions are determined by this method (Kulisa et al. 1999). The hydrated and complex metal ions carry a positive charge, and they are generally separated as cations in a cation exchange column. By adding carboxylic acid as a chelating agent, the net charge on the metal is reduced since carboxylic acid is anionic in the solution above their pKas. The separation depends on the net charge on the complex after associating with the chelating agent. Enough concentration of a strong chelating agent changes the charge of complex metal ions into a negative charge, and it is separated as anions in an anion exchange column. An Ionpac CS5A column is usually used in IC, which allows a mixed-mode of operation that includes both cation and anion exchange. After passing through the column, a metal complexing agent 4-(2-pyridylazo) resorcinol/PAR is added for forming a light-absorbing complex because UV-absorbance cannot determine transition metals like Cu (Kulisa et al. 1999). Conductivity detection is also impossible as all hydrated and complexed metal ions will precipitate in the suppressor, A 1000 mg L−1 standard transition metal dissolved in dilute acid can be used as IC standard. Figure 10 shows a schematic diagram of the Dionex DX-500 system Ion chromatography.
Schematic diagram of Dionex DX-500 system (Kulisa et al. 1999)
This system has GP 40 gradient pump, AD 20 absorbance detector, LC 20 chromatography disclosure, and PC 10 post-column pneumatic delivery package. The eluent for the IC contains 7.0Mmppca,66 Mm Koh,74 Mm Formic acid, and 5.6 mM potassium sulfate. 200 ml of eluent was taken and diluted into 1000 ml with deionized water and passed through the column. Reagents including 0.5 Mm par,1 M 2-Dimethyl aminoethanol,0.5 M Ammonium Hydroxide, and 0.3 M sodium bicarbonate were added to it, and o.12 g of PAR was dissolved in 1L of a post-column eluent. After that, it has been ultrasonicated for 5 min and stirred until the solid is completely dissolved. Columns used are Ionpac CS5A analytical and CG5A grade, and the eluent flow rate is 1.2 mL/min. A sample of 50µL is injected, and a post-column reagent is allowed to flow at a rate of 0.7 mL/min. The detector detects the chromatogram at a wavelength of 530 nm. The PDCA chelating agent added to the mobile phase reduced the charge of complex metal ions and separated them as anionic complexes. The metal Cu(II) is detected by measuring the absorbance at 530 nm of the complex formed with the post-column reagent. The prepared PAR reagent is easily oxidizable, thus should be stored under inert gas like nitrogen or helium (Haddad and Jackson 1990; Lu et al. 1998).
Advantages and drawbacks of the detection methods
With the availability of various Cu(II) detection methods as previously discussed and an in-depth evaluation for limited technique selection, the consideration of various critical criteria is required, including detection time, cost, analytical working range, data quality, and interferences. Generally, the selection can be made based on the capability of the company to have an expensive method that will result in high-resolution outcomes, as in the case of ICP-MS. Sensors are economically affordable and can produce measurements with high sensitivity but require highly skilled and qualified operators. AAS is a fast and straightforward method but requires high operating costs and is implemented in limited applications. Although the voltammetric method is cheap and rapid, it generates large volumes of chemicals, requiring further treatment later. Spectrophotometric is an inexpensive and straightforward method. However, it consumes much time for the analysis. Unlike spectrophotometric, chromatography needs a short time to perform the analysis, but it is expensive. Table 5 summarizes the advantages and drawbacks of the detection methods discussed in this review.
Conclusion
Several methods can be investigated to recover and detect the Cu(II) ion in water samples, such as atomic and molecular spectrophotometry, mass spectroscopy, sensors, voltammetry, and chromatography. Recent trends of Cu(II) detection in water samples, including their principle, definitions, experimental work, equipment, and devices used to run each technique, are represented in detail. Besides their advantages, the disadvantages of the detection methods were discussed. Hence, based on the water sample and its application, appropriate detection methods can be used.
Availability of data and materials
All data and materials are available.
Abbreviations
- AAS:
-
Atomic Absorption Spectrometry
- ASV:
-
Anodic Stripping Voltammetry
- CVAAS:
-
Cold Vapor Atomic Absorption Spectrometry
- DLLME-SQT:
-
Dispersive Liquid–Liquid Micro-Extraction-Slotted Quartz Tube
- EDX:
-
Energy Dispersive X-Ray Analysis
- ESR:
-
Erythrocyte Sedimentation Rate
- ETAAS:
-
Electro-Thermal Atomic Absorption Spectrometry
- FAAS:
-
Flame Atomic Absorption Spectrometry
- FTIR:
-
Fourier Transform Infrared
- GFAAS:
-
Graphite Furnace Atomic Absorption Spectrometry
- HAAS:
-
Hydride Atomic Absorption Spectrometry
- HR-ICP-MS:
-
High Resolution Inductively Coupled Plasma Mass Spectrometry
- IC:
-
Ion Chromatography
- ICP:
-
Inductively Coupled Plasma
- ICP-MS:
-
Inductively Coupled Plasma/Mass Spectrometry
- ICP-OES:
-
Inductively Coupled Plasma/Optical Emission Spectrometry
- MC-ICP-MS:
-
Multi-Collector Inductively Coupled Plasma Spectrometry
- NIR:
-
Near-Infrared
- PCVG–PN–PTSC:
-
Photochemical Vapor Generation Pneumatic Nebulization In The Programmable Temperature Spray Chamber
- PEI:
-
Poly Ethylene Imine
- POF:
-
D-Shaped Plastic Optical Fiber
- RF:
-
Radio Frequency
- RP-HPLC:
-
Reverse Process High-Pressure (Or Performance) Liquid Chromatography
- SEM:
-
Scanning Electron Microscope
- spICP-MS:
-
Single-Particle ICP-MS
- SPR:
-
Surface Plasmon Resonance
- TGA:
-
Thermo-Gravimetric Analysis
- UV:
-
Ultraviolet
References
Abdullah NA, Ramli S, Mamat NH, Khan S, Gomes C (2017) Chemical and biosensor technologies for wastewater quality management. Int J Adv Res Publ 1:10
Alharthi S, Al-Saidi H (2020) Spectrophotometric determination of trace Concentrations of copper in waters Using the chromogenic reagent 4-amino-3-mercapto-6-[2-(2-thienyl) vinyl]-1, 2, 4-triazin-5 (4H)-One: synthesis, characterization, and analytical applications. Appl Sci 10:3895
Ali A, Shen H, Yin X (1998) Simultaneous determination of trace amounts of nickel, copper and mercury by liquid chromatography coupled with flow-injection on-line derivatization and preconcentration. Anal Chim Acta 369:215–223
Awual MR, Hasan MM (2015) Colorimetric detection and removal of copper (II) ions from wastewater samples using tailor-made composite adsorbent. Sens Actuators B Chem 206:692–700
Bachmann LM, Miller WG (2020) Spectrophotometry. In: Clarke W, Marzinke MA (eds) Contemporary practice in clinical chemistry. Academic Press, New York, pp 119–133
Bagda E, Altundag H, Tuzen M, Soylak M (2017) A Novel selective deep eutectic solvent extraction method for versatile determination of copper in sediment samples by ICP-OES. Bull Environ Contam Toxicol 99:264–269
Bagherian G, Chamjangali MA, Evari HS, Ashrafi M (2019) Determination of copper (II) by flame atomic absorption spectrometry after its perconcentration by a highly selective and environmentally friendly dispersive liquid–liquid microextraction technique. J Anal Sci Technol 10:1–11
Balcaen L, Bolea-Fernandez E, Resano M, Vanhaecke F (2015) Inductively coupled plasma—tandem mass spectrometry (ICP-MS/MS): A powerful and universal tool for the interference-free determination of (ultra)trace elements—a tutorial review. Anal Chim Acta 894:7–19
Barshan-Tashnizi M, Ebrahimi-Hoseinzadeh B, Daneshgar P, Norouzi P (2013) Trace detection of thiols with Fourier transforms cyclic voltammetry technique in flowing solutions. J Nanostruct Chem 3:1–8
Baysal, A., Ozbek, N., and Akman, S., 2013. Determination of trace metals in waste water and their removal processes. In: Waste water-treatment technologies and recent analytical developments, pp 145–171
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6:e04691
Broekaert JAC (2006) Spectrometric instrumentation, analytical atomic spectrometry with flames and plasmas, pp. 34–93
Cennamo N, Massarotti D, Conte L, Zeni L (2011) Low cost sensors based on SPR in a plastic optical fiber for biosensor implementation. Sensors (basel) 11:11752–11760
Chen C-Y, Aggarwal SK, Chung C-H, You C-F (2020) Advanced mass spectrometry for beverage safety and forensic, safety issues in beverage production. Elsevier, New York, pp 223–269
Chen Z, Zhang C, Zhou T, Ma H (2015) Gold nanoparticle based colorimetric probe for dopamine detection based on the interaction between dopamine and melamine. Microchim Acta 182:1003–1008
Citak D, Tuzen M (2013) Separation and determination of copper in bottled water samples by combination of dispersive liquid–liquid microextraction and microsample introduction flame atomic absorption spectrometry. J AOAC Int 96:1435–1439
Council NR (2004) Forensic analysis: weighing bullet lead evidence. National Academies Press, Washington
Cui C, He M, Hu B (2011) Membrane solid phase microextraction with alumina hollow fiber on line coupled with ICP-OES for the determination of trace copper, manganese and nickel in environmental water samples. J Hazard Mater 187:379–385
da Costa Lopes AM, Bogel-Łukasik R (2015) Acidic ionic liquids as sustainable approach of cellulose and lignocellulosic biomass conversion without additional catalysts. Chemsuschem 8:947–965
Daud JM, Alakili IM (2001) High performance liquid chromatographic separations of metal pyrrolidine dithiocarbamate complexes. Malays J Anal Sci 7:113–120
Deng Y, Wu X, Tian Y, Zou Z, Hou X, Jiang X (2017) Sharing one ICP source for simultaneous elemental analysis by ICP-MS/OES: Some unique instrumental capabilities. Microchem J 132:401–405
Dimpe K, Ngila J, Mabuba N, Nomngongo P (2014) Evaluation of sample preparation methods for the detection of total metal content using inductively coupled plasma optical emission spectrometry (ICP-OES) in wastewater and sludge. Phys Chem Earth Parts a/b/c 76:42–48
Donati GL, Amais RS, Williams CB (2017) Recent advances in inductively coupled plasma optical emission spectrometry. J Anal at Spectrom 32:1283–1296
Einschlag FSG, Carlos L (2013) Waste water: treatment technologies and recent analytical developments. BoD–Books on Demand
Ejeian F, Etedali P, Mansouri-Tehrani HA, Soozanipour A, Low ZX, Asadnia M, Taheri-Kafrani A, Razmjou A (2018) Biosensors for wastewater monitoring: a review. Biosens Bioelectron 118:66–79
Fathi SAM, Rostamkhani S, Yaftian MR (2010) Determination of trace amounts of copper in water samples by flame atomic absorption spectrometry after preconcentration on octadecyl-bonded silica membranes modified by a Di-Schiff base ligand. J Anal Chem 65:614–619
Fattahi P, Yang G, Kim G, Abidian MR (2014) A review of organic and inorganic biomaterials for neural interfaces. Adv Mater 26:1846–1885
Fu D, Yuan D (2007) Spectrophotometric determination of trace copper in water samples with thiomichlersketone. Spectrochim Acta A Mol Biomol Spectrosc 66:434–437
Garbarino JR, Taylor HE, Batie WC (1989) Simultaneous determination of major and trace elements by inductively coupled plasma-mass spectrometry/optical emission spectrometry. Anal Chem 61:793–796
Giersz J, Bartosiak M, Jankowski K (2017) Sensitive determination of Hg together with Mn, Fe, Cu by combined photochemical vapor generation and pneumatic nebulization in the programmable temperature spray chamber and inductively coupled plasma optical emission spectrometry. Talanta 167:279–285
Gilstrap Jr RA (2009) A colloidal nanoparticle form of indium tin oxide: system development and characterization. Doctoral dissertation, Georgia Institute of Technology, Georgia. Retrieved from https://smartech.gatech.edu/handle/1853/33995.
Goudarzi N (2007) Determination of trace amounts of copper in river and sea water samples by flame atomic absorption spectrometry (FAAS) after cloud-point preconcentration. J Braz Chem Soc 18:1348–1352
Government of Western Australia, D.o.P.H.G.-D. (2016) Copper in drinking water. Retrieved from https://ww2.health.wa.gov.au/Articles/A_E/Copper-in-drinking-water
Gumpu MB, Sethuraman S, Krishnan UM, Rayappan JBB (2015) A review on detection of heavy metal ions in water–an electrochemical approach. Sens Actuators B Chem 213:515–533
Gupta S, Sarkar S, Katranidis A, Bhattacharya J (2019) Development of a cell-free optical biosensor for detection of a broad range of mercury contaminants in water: a plasmid DNA-based approach. ACS Omega 4:9480–9487
Haddad PR, Jackson PE (1990) Ion chromatography, vol 46, 1st ed. ISBN: 9780080858531. Elsevier, New York
Hasan T (2001) New development in ICP spectrometry: low flow torch for axial viewing ICP-AES & studies in semiconductor analysis by ICP-MS (Doctoral dissertation, Iowa State University, Iowa, US). Retrieved from chrome- https://lib.dr.iastate.edu/rtd/644/
Hashem E, Seleim M, El-Zohry AM (2011) Environmental method for spectrophotometric determination of copper (II). Green Chem Lett Rev 4:241–248
Holcombe JA, Borges DL (2006) Graphite furnace atomic absorption spectrometry. In: Encyclopedia of analytical chemistry: applications, theory and instrumentation
Hou X, Peters HL, Yang Z, Wagner KA, Batchelor JD, Daniel MM, Jones BT (2003) Determination of trace metals in drinking water using solid-phase extraction disks and X-ray fluorescence spectrometry. Appl Spectrosc 57:338–342
Hu Q, Yang G, Zhao Y, Yin J (2003) Determination of copper, nickel, cobalt, silver, lead, cadmium, and mercury ions in water by solid-phase extraction and the RP-HPLC with UV-Vis detection. Anal Bioanal Chem 375:831–835
Huang S-D, Shih K-Y (1993) Direct determination of copper in sea-water with a graphite furnace atomic absorption spectrometer. Spectrochim Acta Part B 48:1451–1460
Hung SC, Lu CC, Wu YT (2021) An investigation on design and characterization of a highly selective LED optical sensor for copper ions in aqueous solutions. Sensors (basel) 21:1099
Jackson PE (2006) Ion chromatography in environmental analysis. In: Encyclopedia of analytical chemistry: applications, theory and instrumentation
Javanbakht M, Divsar F, Badiei A, Fatollahi F, Khaniani Y, Ganjali MR, Norouzi P, Chaloosi M, Ziarani GM (2009) Determination of picomolar silver concentrations by differential pulse anodic stripping voltammetry at a carbon paste electrode modified with phenylthiourea-functionalized high ordered nanoporous silica gel. Electrochim Acta 54:5381–5386
Jung MY, Kang JH, Choi YS, Lee DY, Lee JY, Park JS (2019) Analytical features of microwave plasma-atomic emission spectrometry (MP-AES) for the quantitation of manganese (Mn) in wild grape (Vitis coignetiae) red wines: comparison with inductively coupled plasma-optical emission spectrometry (ICP-OES). Food Chem 274:20–25
Kaiser RE (1974) Gas chromatography in environmental analysis. J Chromatogr Sci 12:36–39
Kavitha C, Babu MS, Saraswathi K (2013) Spectrophotometric determination of copper as copper piperazine. Int Lett Chem Phys Astron 8:205–209
Khayatian G, Moradi M, Hassanpoor S (2018) MnO 2/3MgO Nanocomposite for preconcentration and determination of trace copper and lead in food and water by flame atomic absorption spectrometry. J Anal Chem 73:470–478
Kinuthia GK, Ngure V, Beti D, Lugalia R, Wangila A, Kamau L (2020) Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: community health implication. Sci Rep 10:1–13
Kulisa K, Polkowska-Motrenko I, Dybczynski R (1999) Determination of transition metals by ion chromatography. In: Smuiek W, Godlewska-Para E (eds) Radiochemistry, stable isotopes, nuclear analytical methods, chemistry in general in Institute of Nuclear Chemistry and Technology Annual Report. Institute of Nuclear Chemistry and Technology, Warszawa, pp 74–76
Lemos VA, Santos MS, dos Santos MJS, Vieira DR, Novaes CG (2007) Determination of copper in water samples by atomic absorption spectrometry after cloud point extraction. Microchim Acta 157:215–222
Li Y, Liu Y, Zhang Z, Tong J, Bian C, Dong H, Xia S (2019) A portable sensor system for detection of copper ions in water samples. In: 2019 IEEE 14th international confernece on nano/micro engineered and molecular systems (NEMS). IEEE, Bangkok, Thailand, pp 385–388
Lima RT, Raposo JL Jr, Virgílio A, Gomes Neto JA (2010) Determination of copper at wide range concentrations using instrumental features of high-resolution continuum source flame atomic absorption spectrometry. Eclética Química 35:87–92
Liu B (2017) Determination of copper in metal processing wastewater by stripping voltammetry, IOP Conference Series: Materials Science and Engineering. IOP Publishing, p 012060
Liu H-L, Meng Q, Zhao X, Ye Y-L, Tong H-R (2021) Inductively coupled plasma mass spectrometry (ICP-MS) and inductively coupled plasma optical emission spectrometer (ICP-OES)-based discrimination for the authentication of tea. Food Control 123:107735
Lu H, Mou S, Yan Y, Tong S, Riviello J (1998) On-line pretreatment and determination of Pb, Cu and Cd at the μg l− 1 level in drinking water by chelation ion chromatography. J Chromatogr A 800:247–255
Ma YR, Niu HY, Zhang XL, Cai YQ (2011) Colorimetric detection of copper ions in tap water during the synthesis of silver/dopamine nanoparticles. Chem Commun (camb) 47:12643–12645
Malik LA, Bashir A, Qureashi A, Pandith AH (2019) Detection and removal of heavy metal ions: a review. Environ Chem Lett 17:1495–1521
Michalke B, Nischwitz V (2013) Liquid Chromatography: Chapter 22. Speciation and Element-Specific Detection (Chapters). Elsevier Inc., New York
Mofidi Z, Norouzi P, Seidi S, Ganjali MR (2017) Determination of diclofenac using electromembrane extraction coupled with stripping FFT continuous cyclic voltammetry. Anal Chim Acta 972:38–45
O’Sullivan JE, Watson RJ, Butler EC (2013) An ICP-MS procedure to determine Cd Co, Cu, Ni, Pb and Zn in oceanic waters using in-line flow-injection with solid-phase extraction for preconcentration. Talanta 115:999–1010
Ouyang Z, Chen Z, Mou H (2010) Determination of copper by flame atomic absorption spectrometry using flow injection on-line preconcentration with double micro-columns. Rev Anal Chem 29:51–58
Özzeybek G, Erarpat S, Chormey DS, Fırat M, Büyükpınar Ç, Turak F, Bakırdere S (2017) Sensitive determination of copper in water samples using dispersive liquid-liquid microextraction-slotted quartz tube-flame atomic absorption spectrometry. Microchem J 132:406–410
Pesavento M, Profumo A, Merli D, Cucca L, Zeni L, Cennamo N (2019) An optical fiber chemical sensor for the detection of copper(II) in drinking water. Sensors (basel) 19:5246
Poirier L, Nelson J, Leong D, Berhane L, Hajdu P, Lopez-Linares F (2016) Application of ICP-MS and ICP-OES on the determination of nickel, vanadium, iron, and calcium in petroleum crude oils via direct dilution. Energy Fuels 30:3783–3790
Pourabdollah K, Mokhtari B (2014) Spectroscopy Letters: An International Journal for Rapid Communication (Retraction of vol 541, 2012). Spectrosc Lett 47:76–76
Pujol L, Evrard D, Groenen-Serrano K, Freyssinier M, Ruffien-Cizsak A, Gros P (2014) Electrochemical sensors and devices for heavy metals assay in water: the French groups’ contribution. Front Chem 2:19
Raafid E, Al-Da’amy MA, Kadhim SH (2020) Spectrophotometric determination of Cu(II) in analytical sample using a new chromogenic reagent (HPEDN). Indonesian J Chem 20
Ranjbar L, Yamini Y, Saleh A, Seidi S, Faraji M (2012) Ionic liquid based dispersive liquid-liquid microextraction combined with ICP-OES for the determination of trace quantities of cobalt, copper, manganese, nickel and zinc in environmental water samples. Microchim Acta 177:119–127
Rehacek V, Hotovy I, Vojs M, Mika F (2008) Bismuth film electrodes for heavy metals determination. Microsyst Technol 14:491–498
Rekhi H, Rani S, Sharma N, Malik AK (2017) A review on recent applications of high-performance liquid chromatography in metal determination and speciation analysis. Crit Rev Anal Chem 47:524–537
Romero-Cano LA, Zárate-Guzmán AI, Carrasco-Marín F, González-Gutiérrez LV (2019) Electrochemical detection of copper in water using carbon paste electrodes prepared from bio-template (grapefruit peels) functionalized with carboxyl groups. J Electroanal Chem 837:22–29
Rutzke M (2018) Atomic absorption, inductively coupled plasma optical emission spectroscopy, and infrared spectroscopy. Encyclopedia of Geochemistry. Springer, Cham
Saber Tehrani M, Rastegar F, Parchehbaf A, Rezvani Z (2005) Determination of copper by flame atomic absorption spectrometry after preconcentration with activated carbon impregnated with a new schiff base. Chin J Chem 23:1437–1442
Sarker KC (2010) Determination of trace amount of copper (Cu) using UV-VIS spectrophotometric method
Satheesh K, Rao VS (2016) A Study on spectrophotometric determination of copper from wastewater and its removal using magnatite nanoparticles. Arch Appl Sci Res 8:31–36
Sawatari H, Fujimori E, Itoh A, Aikawa M, Haraguchi H (1995) Versatile simultaneous multielement measurement system with combination of ICP-MS and ICP-AES through optical fiber. Bull Chem Soc Jpn 68:1635–1640
Seher S, Shah A, Iftikhar FJ, Nisar J, Ashiq MN, Aljar MA, Akhter MS (2020) Detection of copper ions by a simple, greener and cost effective sensor with GCE modified with L-tryptophan. J Electrochem Soc 167:027506
Sharma S, Rahman N, Azmi SNH, Iqbal B, Amburk MIBB, Al Barwani ZMH (2010) UV spectrophotometric determination of Cu (II) in synthetic mixture and water samples. J Chin Chem Soc 57:622–631
Sobhanardakani S, Tayebi L, Farmany A (2011) Toxic metal (Pb, Hg and As) contamination of muscle, gill and liver tissues of Otolithes rubber, Pampus argenteus, Parastromateus niger, Scomberomorus commerson and Onchorynchus mykiss. World Appl Sci J 14:1453–1456
Švehla J, Žídek R, Ružovič T, Svoboda K, Kratzer J (2019) Simple approaches to on-line and off-line speciation analysis of mercury in flue gases with detection by atomic absorption spectrometry: a pilot study. Spectrochim Acta, Part B 156:51–58
Taylor AA, Tsuji JS, Garry MR, McArdle ME, Goodfellow WL Jr, Adams WJ, Menzie CA (2020) Critical review of exposure and effects: implications for setting regulatory health criteria for ingested copper. Environ Manag 65:131–159
Thermofisher, Comparison of ICP-OES and ICP-MS for Trace Element Analysis: Choosing the right instrument for environmental sample analysis. Retrieved from https://www.thermofisher.com/qa/en/home/industrial/environmental/environmental-learning-center/contaminant-analysis-information/metal-analysis/comparison-icp-oes-icp-ms-trace-element-analysis.html#%3A~%3Atext%3DICP-OES%20is%20mainly%20used%2C%2C%20soil%2C%20and%20solid%20waste.%26text%3DICP-MS%2C%20on%20the%20other%2Csamples%20with%20low%20regulatory%20limits
Thomas SN (2019) Mass spectrometry. In: Clarke W, Marzinke MA (eds) Contemporary practice in clinical chemistry. Academic Press, New York, pp 171–185
Timoshenko R, Vaneev A, Savin N, Klyachko N, Parkhomenko YN, Salikhov S, Majouga A, Gorelkin P, Erofeev A (2020) Promising approaches for determination of copper ions in biological systems. Nanotechnol Russ 15:121–134
Tomic S, Nagj M, Raos D (1985) Determination of heavy metal in drinking waters by X-ray spectroscopy. Int J Environ Anal Chem 23:87–95
Turkoglu O, Soylak M (2005) Spectrophotometric determination of copper in natural waters and pharmaceutical samples with chloro (phenyl) glyoxime. J Chin Chem Soc 52:575–579
US-EPA, U.S.-E.P.A. (2009) National Primary Drinking Water Regulations. US-EPA
Venkatesan AK, Rodríguez BT, Marcotte AR, Bi X, Schoepf J, Ranville JF, Herckes P, Westerhoff P (2018) Using single-particle ICP-MS for monitoring metal-containing particles in tap water. Environ Sci Water Res Technol 4:1923–1932
Wang R, Wang W, Ren H, Chae J (2014a) Detection of copper ions in drinking water using the competitive adsorption of proteins. Biosens Bioelectron 57:179–185
Wang Z, Wang M, Wu G, Wu D, Wu A (2014b) Colorimetric detection of copper and efficient removal of heavy metal ions from water by diamine-functionalized SBA-15. Dalton Trans 43:8461–8468
Wang Y, Kang J, Chen Y, Li R (2019) Sensitive analysis of copper in water by LIBS–LIF assisted by simple sample pretreatment. J Appl Spectrosc 86:353–359
Wen T, Qu F, Li NB, Luo HQ (2017) A facile, sensitive, and rapid spectrophotometric method for copper (II) ion detection in aqueous media using polyethyleneimine. Arab J Chem 10:S1680–S1685
WHO, W.H.O. (2004) Copper in Drinking-water: Background document for development of WHO Guidelines for Drinking-water Quality, WHO/SDE/WSH/03.04/88 ed
Wilschefski SC, Baxter MR (2019) Inductively coupled plasma mass spectrometry: introduction to analytical aspects. Clin Biochem Rev 40:115–133
Wołowiec M, Komorowska-Kaufman M, Pruss A, Rzepa G, Bajda T (2019) Removal of heavy metals and metalloids from water using drinking water treatment residuals as adsorbents: a review. Minerals 9:487
Xing G, Sardar MR, Lin B, Lin J-M (2019) Analysis of trace metals in water samples using NOBIAS chelate resins by HPLC and ICP-MS. Talanta 204:50–56
Yang Y, Guangyu Y, Lin Q (2004) Determination of heavy metal ions in Chinese herbal medicine by microwave digestion and RP-HPLC with UV-Vis detection. Microchim Acta 144:297–302
Yetimoğlu EK, Urucu OA, Gündüz ZY, Filik H (2010) Selective determination of copper in water samples by atomic absorption spectrometry after cloud point extraction. Anal Lett 43:1846–1856
Zagurskaya-Sharaevskaya O, Povar I (2015) Determination of Cu (II) ions using sodium salt of 4-phenylsemicarbazone 1, 2-naphthoquinone-4-sulfonic acid in natural and industrial environments. Ecol Process 4:1–5
Zamani HA, Rajabzadeh G, Firouz A, Ganjali MR (2007) Determination of copper (II) in wastewater by electroplating samples using a PVC-membrane copper (II)-selective electrode. J Anal Chem 62:1080–1087
Zhang Y, Qiao R, Sheng C, Zhao H (2020) Technologies for detection of HRPs in wastewater, High-risk pollutants in wastewater. Elsevier, New York, pp 79–100
Zhuang J, Zhang L, Lu W, Shen D, Zhu R, Pan D (2011) Determination of trace copper in water samples by anodic stripping voltammetry at gold microelectrode. Int J Electrochem Sci 6:4690–4699
Acknowledgements
Not applicable
Funding
Not applicable. The authors declare that this research work does not receive any funding.
Author information
Authors and Affiliations
Contributions
AE led the research team, authored the atomic spectrometric methods section, X-ray spectroscopy, contributed to other sections, copy-edited, peer-reviewed, and proofread the entire article. MS collected, reviewed, and authored the introduction and AAs sections. RI collected, reviewed, and authored the abstract and ICP sections. SA collected, reviewed, and authored the sensors section. NA collected, reviewed, and authored the spectrophotometric method section. SM collected, reviewed, and authored the chromatography section. SF collected, reviewed, and authored the voltammetric method and conclusion sections. MK supervised and proofread the article. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors declare that the manuscript complies with the research publication ethics. It does not report on or involve the use of any animal or human data and does not contain data from any person.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elkhatat, A.M., Soliman, M., Ismail, R. et al. Recent trends of copper detection in water samples. Bull Natl Res Cent 45, 218 (2021). https://doi.org/10.1186/s42269-021-00677-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-021-00677-w