Abstract
Background
Africa is blessed with rich floral biodiversity which are harnessed as herbal medicines and remedies for several ailments. Since there has been evidence of organ toxicity following prolonged ingestion of medicinal plant concotions, this study investigated the biochemical and hematological enhancement activities of aqueous and methanolic leaves, stem and roots extracts of Chasmanthera dependens (Hochst) and Dictyandra arborescens (Welw) in adult male albino rats.
Results
Leaves, stem and roots extracts of D. arborescens, as well as extracts of leaves and roots of C. dependens did not record any mice mortality even at 5000 mg/kg b.wt but extracts of C. dependens stem in both medium recorded death at 2900 mg/kg b. wt and 5000 mg/kg b.wt. Ingestion of the extracts by rats over a 14-day period increased (p < 0.05) the body weights of the experimental animals in the C. dependens and D. arborescens treated groups. The relative organ weights of rats that received the extracts did not differ (p < 0.05) significantly from those of the standard and normal control. Administration of the extracts revealed significant (p < 0.05) increase in haematological parameters (PCV, Hb, RBCs, WBCs, MCH, Neutrophils, Lymphocytes and MCHC) at 200 mg/kg−1 body weight. For biochemical parameters, levels of ALT, AST, ALP, total protein, and albumin were not significantly (p < 0.05) elevated following administration of the extracts.
Conclusion
These parameters did not differ significantly from the normal and standard control. Since these extracts did not exhibit any chronic toxicity on experimental animals, suggesting no harmful effects following their use, their continuous use in ethno-medicine is therefore justified.
Similar content being viewed by others
Background
Plants have been a source of medicine for many years and medicinal plants play a crucial role in the primary health care of more than 80% of the population in developing countries (Ezeigwe et al. 2020). Plants, in their diversities have been the basic sources human needs such as food, clothing and shelter, as well as natural remedies for the well-being of man (Gurib-Fakim 2006). Several conventional drugs owe their origin to plants, such drugs include aspirin (Salix alba), quinine (Cinchona officinalis), digoxin (Digitalis purpurea) and morphine (Papaver somniferum) (Gurib-Fakim 2006). Globally, the use of herbal medicines in the treatment of various disease conditions has increased rapidly. Medicinal plants, therefore, remain a priceless gift of nature to man. It is believed that traditional medicinal plants and natural products are important sources of novel chemical substances with possible therapeutic values (Enenebeaku et al. 2021a). Although there has been evidence of organ toxicity following prolonged ingestion of medicinal plant concotions (Ahmad et al. 2006), the patronage of medicinal plants and related products is still on the increase, due largely to the supposed availability, safety, and affordability (Bandaranayake 2006). This increasing patronage also comes with rising tendencies of abuse, from indiscriminate uses of these plant- derived drugs. This therefore necessitates the need to establish the safety of medicinal plants. Additionally, following a resounding call for the integration of traditional medicine into the conventional modern medicine (Hosseinzadeh et al. 2015), the determination and documentation of the safety/toxic risk potentials of these medicinal plants become a necessity.
Dictyandra arborescens, a flowering plant that belongs to the Rubiaceae family which derived their name from the madder genus Rubia. It is called 'edo' by the Igbos (South East Nigeria). Species in the Rubiaceae family are usually found in tropical climates (Dalziel 1997). They are mostly herbs and shrubs. A lot of plants in this family are known for their important medicinal values. The ethanolic extract of the wild plant Canthium coromandelicum showed a broad spectrum of antimicrobial activity against Salmonella typhi and antifungal activity against Candida albicans (Sathish et al. 2018). Borreria spermacoce species of rubiaceae, as well as their isolated compounds, exhibit biological activities such as anti-inflammatory, antimicrobial, antioxidant, anti-ulcer and hepatoprotective activities (Conserva and Ferreira 2012; Simplice et al. 2011). Phytochemical evaluation and molecular docking of compounds in D. arborescens roots, as well as antimalarial potentials of its leaf, stem and root aqueous and methanolic extracts have earlier been reported by Enenebeaku et al. (2021a, b). However, information on its biochemical and hematological protective potentials is still lacking in literature. The plant is widely used by natives of Ahiazu Mbaise, Imo State, South Eastern Nigeria for the treatment of malaria and other ailments.
Chasmanthera dependens (Hochst) is an angiosperm which belongs to the Menispermaceae family. Its common is Chasmanthera. The plant is a woody climber found in forest margins and secondary forests which traditional healers use in the management of malaria cases. Due to its medicinal values, it is usually cultivated in homes or gardens. The plant is called ‘aguru’ in Igbo (South East Nigeria), and ‘atoo’ in Yoruba (South West Nigeria). It is commonly used by natives of Ahiazu Mbaise (South East Nigeria) in the management of malaria and other ailments.
Available information shows it has been used in the treatment of red-eye infections, snakebites, convulsions, epilepsy, and in management of fractures (Ogunlesi et al. 2008). Iwu et al. (1999) reported its effect in controlling physical and nervous weaknesses. Methanol extract of the dried leaves have anti-inflammatory and analgesic properties (Morebise et al. 2001). Adekunle and Okoli (2002) reported antifungal properties of the ethanolic and aqueous leaf extracts. Antimicrobial activities of C. dependens stem on fungal yeast, Gram + and Gram – bacteria were reported by Githinji et al. (2010). Aqueous extract of the roots demonstrated pro-spermatogenic, fertility enhancing, and androgenic activities in male rats (Quadri and Yakubu 2017).
The present study was therefore undertaken to provide information on the biochemical and hematological enhancement activities of aqueous and methanol leaves, stem and roots extracts of Chasmanthera dependens (Hochst) and Dictyandra arborescens (Welw.).
Methods
Collection and authentication of plant materials
The roots, leaves and stems of Chasmanthera dependens and Dictyandra arborescens were collected from Mbaise, Imo State, Nigeria. It is located between latitude 5° 19′ and 5° 32′ N and longitude 7° 12′ and 7° 20′ E (Ume et al. 2016). They were verified and identified by Dr. C.M Duru of Biological Sciences Department, Federal University of Technology, Owerri, where the specimen were deposited with voucher numbers—FUTOH-2021/005 and FUTOH-2021/006.
Preparation of extracts of C. dependens and D. arborescens
Fresh leaves, stems and roots of Chasmanthera dependens and Dictyandra arborescens were cleaned and air-dried at room temperature (30 ± 0.5 °C) for 2 weeks to a constant weight. They were pulverized to coarse powder using electric blender (Kenwood-BL440A-UK). The coarse powder was then sieved, using a 2 mm mesh sieve, to obtain smooth samples. Aqueous extracts were prepared using cold maceration method (Ene et al. 2013). The samples were macerated for a period of 48 h. They were filtered and filtrates were dried and concentrated using a rotary evaporator (RE-52A, Searchtech Instruments) set at 50 °C to remove the solvent. Methanol extraction was obtained as described by Dejen et al. (2018). Two hundred grams (200 g) of various parts of the two study plants were subjected to extraction using soxhlet extractor with 1000 ml of 80% v/v methanol. This extraction was allowed for 6 h to ensure maximum extraction. The filtrate were dried using a rotary evaporator. Extracts were stored in properly labeled bijou bottles kept in the refrigerator at 4 °C before use, and were reconstituted with distilled water prior to administration.
Experimental animals
Animals used for the study, 84 male albino rats (85–100 g) were sourced from Department of Veterinary medicine, University of Nigeria, Nsukka. They were taken to the Department of Biochemistry, University of Nigeria, Nsukka, where the research was carried out. The animals were acclimatized under laboratory conditions of 12 h light/dark cycle in well aerated cages for 14 days with free access to feed (rat pellets) and water ad libitum before the experiment commenced. Care and use of animals were according to NIH guide for care and use of laboratory animals (NIH 1985). Ethical approval to carry out the study was obtained from Department of Biochemistry, Federal University of Technology, Owerri, with reference number—FUTO/BCH/DEC/XXI/03/01.
Study design
They animals were separated into 14 groups of six (6) rats each based on their body weights. Group 1 received only feed and water (normal control), Group 2 was given 200 mg/kg b. wt of artesunate while groups 3–14 received 200 mg/kg b. wt of the various extracts of C. dependens and D. arborescens. These groups were labeled 1–14 as outlined below:
-
Group 1 feed and water (normal control group).
-
Group 2 artesunate (standard control group).
-
Group 3 methanolic extract of Dictyandra arborescens root.
-
Group 4 methanolic extract of Dictyandra arborescens leaves.
-
Group 5 methanolic extract of Dictyandra arborescens stem.
-
Group 6 aqueous extract of Dictyandra arborescens root.
-
Group 7 aqueous extract of Dictyandra arborescens leaves.
-
Group 8 aqueous extract of Dictyandra arborescens stem.
-
Group 9 methanolic extract of Chasmanthera dependens root.
-
Group 10 methanolic extract of Chasmanthera dependens leaves.
-
Group 11 methanolic extract of Chasmanthera dependens stem..
-
Group 12 aqueous extract of Chasmanthera dependens root.
-
Group 13 aqueous extract of Chasmanthera dependens leaves.
-
Group 14 aqueous extract of Chasmanthera dependens stem.
On the 15th day, the rats were sacrificed by cervical decapitation and blood was collected by cardiac and ocular puncture using syringe and needle, into plane and ethylene diamine tetra-acetic (EDTA) bottles for biochemical and hematological analyses. Each animal was laid on a dissecting board and a pair of scissors was used to open the animal by cutting through vertical mid-line from neck to peritoneum (Osaro et al. 2016). The kidney, liver, spleen, heart and lungs were excised and weighed.
Determination of weight
The weight of the animals were checked using an electronic weighing balance. The weights were monitored before and after the experiment to know the effect of the extracts on their body weights.
Acute toxicity (LD50) test
The oral and median lethal doses (LD50) of the extracts in mice were determined using modified method of Lorke (1983) as described by Khan et al. (2015a). This method involved two phases where 216 male Swiss albino mice were used. In the first phase of each of the twelve (12) extracts, nine (9) male Swiss albino mice were divided into 3 groups of 3 mice each (n = 3) and increasing doses of 10, 100 and 1000 mg/kg body weight of the extract were administered orally to groups 1, 2 and 3 respectively. They were observed for the first 4 h and subsequently for 7 days for any signs of toxicity and mortality. These signs include pains, loss of appetite, difficulty in respiration, paw licking, weakness and death.
In the second phase (which was carried out when no death was recorded in the first phase), another one hundred and eight mice were used for the experiment as in phase one. For each of the twelve (12) extracts, high doses of 1600, 2900 and 5000 mg/kg body weight of the extracts were administered to another 3 groups of 3 fresh male Swiss albino mice (for each extract) through the same route. They were observed for 4 h for signs of toxicity and mortality, and subsequently for 7 days as in the first phase. Acute toxicity was calculated using the formula:
where D0 Highest dose that gave no mortality, D100 Lowest dose that produced mortality.
Determination of hematological parameters
Blood samples used for analysis of hematological parameters were collected in EDTA bottles and were analyzed using a haematology analyzer (Mindray Auto Hematology Analyzer, BC-5200, USA) following the manufacturer’s instructions. Parameters analyzed were red blood cell count (RBC), packed cell volume (PCV), white blood cell count (WBC), haemoglobin (Hb), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), lymphocytes and neutrophils.
Determination of biochemical parameters
Blood samples collected in plain bottles were centrifuged at 3000 revolution per minute (rpm) for 10 min and the resultant serum was analyzed using diagnostic kits from Randox laboratory UK. Liver function marker enzymes such as alkaline phosphatase (ALP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were analyzed using method of Yakubu et al. (2017). Serum protein and albumin were also determined using method of Oyinloye et al. (2016).
Change in body weight of experimental animals
Weight of the rats in all the groups were determined before and after administration of the extracts.
Determination of relative organ weight
The heart, liver, spleen, kidney and lungs of the experimental animals were removed and weighed. The relative organ body weight (ROW) was calculated as follows: ROW = Absolute organ weight (g)/Body weight of rats on the final day.
Statistical analysis
One Way Analysis of Variance (ANOVA) (SPSS Version 20.0 software USA) was used to analyze data obtained and results expressed as mean ± SEM. Duncan multiple range test was used to separate differences between means at significance level p < 0.05.
Results
Acute toxicity test
The oral LD50 of leaves, stem and roots aqueous and methanolic extracts of Dictyandra arborescens as well as leaves and roots of C. dependens in mice was ≥ 5000 mg/kg b. wt, while that of the stem of C. dependens was estimated to be ≤ 3808 mg/kg b. wt.
Administration of aqueous and methanol leaves, stem and roots extracts of Chasmanthera dependens and Dictyandra arborescens on body weight of albino rats after 14 days
Oral administration of extracts of the roots, leaves and stem of Chasmanthera dependens and D. arborescens did not induce any signs of adverse reactions and there were no changes in the general behaviour of the experimental animals during the 14-day period of administration of 200 mg/kg b. wt of extracts. Increase in body weight of the animals was recorded in all the groups, as well as the normal and standard control groups (Table 1). No mortality was recorded throughout the period of testing.
Effects of the extracts on relative organ weights of albino rats
The relative organ weights of the rats that received the various extracts of methanol and aqueous and extracts of C. dependens and D. arborescens did not significantly (p ˂ 0.05) differ from those of the control and standard groups (Table 2).
Effects of methanol and aqueous roots, leaves and stem extracts of C. dependens and D. arborescens on heamatological indices
Results obtained in this study shows that hematological parameters in the albino rats (PCV, RBC, WBC, Hb, MCH, Neutrophils, Lymphocytes, and MCHC) significantly increased (p ˂ 0.05) on administration of 200 mg/kg b. wt of methanol and aqueous extracts of roots, leaves and stem of Chasmanthera dependens and Dictyandra arborescens (Figs. 1, 2, 3, 4, 5, 6, 7, 8). For packed cell volume (PCV), results for the standard drug (artesunate) and DARME did not differ significantly (p ˂ 0.05) from the normal control (Fig. 1). For red blood cells (RBCs), values obtained from groups that received the extracts did not differ significantly (p ˂ 0.05) from the control. This also applied for the white blood cells (WBCs) and hemoglobin (Hb) (Figs. 2, 3, 4). For MCH, data obtained for aqueous and methanol extracts of C. dependens root as well as aqueous extract of C. dependens stem differed from the normal and standard control (p ˂ 0.05) (Fig. 5). Although results obtained for some extracts differed from the normal and standard control for neutrophils, lymphocytes and mean corpuscular hemoglobin concentration, they did not reduce these parameters (Figs. 6, 7, 8). This gives an indication that these extracts are not hepatotoxic.
Effects of methanolic and aqueous extracts of roots, leaves and stem of D. arborescens and C. dependens on Packed cell volume (PCV) of albino rats. Bars with similar alphabets are not statistically different (p < 0.05). NC normal control, ST standard control, DARM D. arborescens root methanol, DALM D. arborescens leaves methanol, DASM D. arborescens stem methanol, DARA D. arborescens root aqueous, DALA D. arborescens leaves aqueous, DASA D. arborescens stem aqueous, CDRM C. dependens root methanol, CDLM C. dependens leaves methanol, CDSM C. dependens stem methanol, CDRA C. dependens root aqueous, CDLA C. dependens leaves aqueous, CDSA C. dependens stem aqueous
Biochemical parameters
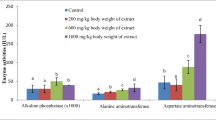
Results of the biochemical parameters show that administration of 200 mg/kg b. wt. of methanolic and aqueous leaves, stem and roots extracts of the two plants under study did not increase levels of the liver function enzymes (AST, ALT and ALP), serum total protein nor albumin (Figs. 9, 10, 11, 12, 13).
For Aspartate transaminase (AST), values obtained for methanol root extract of C. dependens (CDRM) and aqueous leaves extract of C. dependens (CDLA) differed (p ˂ 0.05) from the normal and standard control (Fig. 9). Other extracts did not differ significantly (p ˂ 0.05) from the normal and standard controls. For Alanine transaminase (ALT), all the extracts did not differ significantly (p ˂ 0.05) from the normal and standard control (Fig. 10). For alkaline phosphatase (ALP), results obtained for aqueous leaves extract of D. arborescens (DALA) and methanol leaves extract of C. dependens (CDLM) significantly differed (p ˂ 0.05) from the normal and standard control (Fig. 11). Other extracts did not differ from the normal and standard controls. For serum albumin and total protein, some of the extracts differed significantly (p ˂ 0.05) from the normal and standard control (Figs. 12, 13). However, they did not elevate these biochemical parameters. Results obtained therefore indicate that methanolic and aqueous and extracts of the roots, leaves and stem of these two plants had no hepatotoxic effects on the experimental animals.
Discussion
The need to evaluate the toxicity profile of Chasmanthera dependens and Dictyandra arborescens leaves, stem and root extracts was necessitated by their extensive use in the management of malaria by natives of Ahiazu Mbaise, Imo State Nigeria.
Toxicity is an expression of the poisonous potential of any extract. It reveals the state of adverse effects which is caused by interaction between the toxicants and cells. For the evaluation and assessment of toxicity of medicinal plants, determination of acute oral toxicity (LD50) is usually a preliminary step. Testing for toxicity is very crucial in the screening of new drugs or compounds prior to its use on humans (Enenebeaku et al. 2021a). Toxicity tests are usually carried out on experimental animals (Cunny et al. 2009). Acute toxicity test (dose which can kill 50% of experimental animals) is a short term evaluation of potential hazards by a test substance (Monosson 2013). Results obtained from acute toxicity studies pave way for dose determination in animal studies, aid in the determination of LD50 values which provide many indices of potential types of drug activity.
The experimental mice were orally treated with single dose each of 10–5000 mg/kg b. wt. of the extracts. Toxicity and mortality were not recorded within 24 h and subsequently for 7 days in both phases of the study for methanolic and aqueous extracts of the roots, leaves and stem of D. arborescens. Similar findings were recorded for the methanolic and aqueous extracts of roots and leaves of C. dependens. Physical signs of toxicity, such as paw licking, salivation, stretching, and weakness were not recorded thus the LD50 was estimated to be ≥ 5000 mg kg−1 b.wt. Absence of mortality in the test animals indicated that the extracts were not toxic acutely (Salawu et al. 2009). Several authors, Christian et al. (2012), Onwusonye et al. (2014) and Ihekwereme et al. (2016) had similar reports for Aspilia africana (Pers), Annona senegalensis and Baphia pubescens respectively.
The LD50 of aqueous and methanolic extract of C. dependens stem was estimated to be ≤ 3808 mg kg−1 b. wt. of the extract. Khan et al. (2015b), reported an LD50 of 3808 mg/kg b. wt. for leaf extracts of N. cateria in albino mice.
D. arborescens and C. dependens are some of the plants which are currently being exploited to mitigate against diseases and ailments. Various biochemical and hematological parameters investigated in this study serve as useful indices in the evaluation of toxicity of plant extract in animals. Evaluation of hematological parameters is not only used to determine the extent of harmful effects of extracts on the blood of an animal, it is also used to elucidate blood relating functions of a plant extract or its products (Yakubu et al. 2017). Analysis of blood parameters is very important in risk assessment because changes in the hematological indices predicts human toxicity when the data are translated from animal studies (Olson et al. 2010).
Haematological parameters are equally very important for assay because the hematopoetic system is a major target for toxic compounds. These parameters are also used to monitor the pathological and physiological status of animals and humans (Olson et al. 2010). Blood is the major transportation channel for food and foreign bodies, therefore, its components, such as hemoglobin, platelets, RBCs, and WBCs, are usually exposed to higher doses of toxic compounds thereby exposing these parameters to danger. From results obtained, most of the extracts significantly increased (p ˂ 0.05) these haematological parameters compared to the normal and standard control. Increase in the RBC and its indices following administration of the extracts is an indication of normal erythropoiesis. Increase in the number of WBC is a normal reaction of rats to foreign substances as recorded in this study, and this alters their normal physiological processes. This is consistent with earlier reports by Adebayo et al. (2010). Increased WBC also suggests that the extracts may have immunological properties, which stimulated increased production of white blood cells, thus boosting the defence system of the animals. These results are consistent with the findings of Dioka et al. (2012) who reported that aqueous dried leaf extract of Catharanthus roseus improved heamatological indices in male albino rats.
Hematotoxicity occurs when these blood parameters are elevated beyond their normal ranges (Dioka et al. 2012). Red blood cells are highly susceptible to lipid peroxidation because they have unsaturated membrane lipids. Increase in packed cell volume, heamoglobin, white blood cells, red blood cells, mean corposcular heamoglobin, mean corposcular heamoglobin concentration, neutrophils, and lymphocytes in the experimental animals suggests that leaves, roots and stem extracts of D. arbrescens and C. dependens may have haematopoietic properties which will promote erythropoiesis in animals and are therefore nontoxic. This justifies the use of these plants in traditional medicine. Observations in this study are in line with previous study by Nwinuka et al. (2008) that M. indica stem bark extract had the ability to boost haematopoietic system of rats. Ogbe et al. (2010) also reported that M. indica stem bark extract possesses antianaemic properties. Increased levels of MCH and MCHC indicates that phytoconstituents in the extract stimulated the secretion of erythropoietin which stimulates the stem cells in the bone marrow to produce RBC (Yang et al. 2014). The difference between the results obtained from some of the extracts and the controls could be attributed to difference in body weights of the experimental animals.
Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) have been identified as indicators of hepatocellular injury, while Alkaline phosphatase (ALP) is a marker of cholestasis (Yang et al. 2014). Results obtained show that methanolic and aqueous extracts of the roots, leaves and stem of C. dependens and D. arborescens did not significantly elevate the activities of liver function enzymes AST, ALT and ALP in the serum of test animals. This suggests that these extracts did not induce hepatocellular injury in animals. Decrease in the level of these enzymes in the serum of test animals may be due to non- leakage of hepatocytes which normally occurs from peroxidative damage of their membranes leading to increased membrane permeability (Abu and Uchendu 2010). The significant reductions recorded in the activities of these enzymes equally indicate that extracts of the different parts of the two plants were not harmful to the liver. This agrees with the findings of Adebayo et al. (2010) who reported that ethanolic leaf extract of Chrysophyllum albidum reduced levels of ALT, AST, ALP, total protein, albumin and other biochemical parameters.
Serum albumin and total protein are some of the markers of liver dysfunction. Albumin transports bilirubin and other substances in blood (Ogbe et al. 2010). Serum total protein is a marker of the synthetic function of the liver and serves as a guide in accessing the severity of liver damage (Iweala et al. 2011). Significant increase in serum total protein is an indication of tissue damage while a significant decrease in total protein of the liver contents suggests hepatic toxicity (Gatsing et al. 2015). The non significant difference in the levels of these biochemical parameters when compared to the normal and standard controls suggests that extracts used in this study may not interfere significantly with the metabolism of these biochemical parameters. This supports earlier report by Unuofin and Otunola (2018) who studied acute and subacute toxicity of aqueous extract of the tuber of Kedrostis africana (L.) in Wistar rats. Results equally agree with the findings of Uboh et al. (2010) who evaluated effects of aqueous extract of Psidium guajava leaves on liver enzymes, histological intergrity and heamatological indices in rats. Their results revealed that the liver function enzymes, ALT, AST and ALP, as well as total protein and albumin in male and female rats were not significantly (p ˃ 0.05) affected by oral administration of the extract. Prasad and Venugopal (2016) similarly reported that extract of Argemone mexicana root did not record any evident clinical signs of toxicity and mortality during a 14-day test period, even at a high dose of 5000 mg/kg b. wt.
These findings suggest that methanolic and aqueous extracts of roots, leaves and stem of the two plants did not have any negative impact, they rather seemed to have a protective effect on hematological and biochemical parameters.
Conclusions
The extracts of C. dependens and D. arborescens were not toxic judging from the lack of serious alteration in behavioural observations and lack of mortality following administration of the stated doses in sub-acute toxicity study. Therefore, this study has provided evidence of unaltered hematological and biochemical indices following the use of these extracts, thus justifying the continuous use of these plants in the management of malaria and other ailments.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- mg/kg:
-
Milligram/kilogram
- b.wt.:
-
Body weight
- LD :
-
Lethal dose
- NIH:
-
National Institute of Health
- NIPRD:
-
National Institute for Pharmaceutical Research and Development
- EDTA:
-
Ethylene diamine tetra-acetic
- PCV:
-
Packed cell volume
- RBC:
-
Red blood cells
- WBC:
-
White blood cells
- Hb:
-
Hemoglobin
- MCH:
-
Mean corpuscular haemoglobin
- MCHC:
-
Mean corpuscular hemoglobin concentration
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine transaminase
- ALP:
-
Alkaline phosphatase
References
Abu AH, Uchendu CN (2010) Antispermatogenic effects of aqueous ethanolic extract of Hymennocardia acida stem bark in Wistar rats. J Med Plants Res 4(23):2495–2502
Adebayo AJ, Abolaji AO, Opata TK, Adegbenro IK (2010) Effects of ethanolic leaf extract of Chrysophyllum albidum G. on biochemical and haematological parameters of albino Wistar rats. Afr J Biotechnol 9(14):2145–2150
Adekunle AA, Okoli SO (2002) Antifungal activities of the crude extracts of Alafia barteri Oliver (Apocynaceae) and Chasmanthera dependens (Hochst). Menispermaceae Hamdard Medicus Pakistan 45(3):52–56
Ahmad IA, Farrukh F, Owais M (2006) Herbal medicines: prospects and constraints. In: Ahmad IF, Aqil F, Owais M (eds) Modern phytomedicine: turning medicinal plants into drugs. VCH Verlag GmbH & Co, Munich, pp 59–78
Bandaranayake WM (2006) Quality control, screening, toxicity, and regulation of herbal drugs. In: Ahmad IAF, Owais M (eds) Modern phytomedicine: turning medicinal plants into drugs. VCH Verlag GmbH & Co., Munich, pp 25–57
Christian AG, Mfon AG, Dick EA, David-Oku E, Linus AJ, Chukwuma EB (2012) Antimalarial potency of the leaf extract Aspilia Africana (Pers) C.D Adams. Asian Pac J Trop Med 2:126–129
Conserva LM, Ferreira FC (2012) Borreria and Spermacoce species (Rubiaceae): a review of their ethnomedicinal properties, chemical constituents and biological activities. Pharmacogn Rev 6(11):46–55
Cunny H, Hodgson E (2009) Toxicity testing. In: Hodgson E (ed) A textbook on modern toxicology, 3rd edn. Wiley, Hoboken, pp 353–384
Dalziel IM (1997) The useful plants of West Tropical Africa, 2nd edn. Cress, London, p 196
Dejen N, Assefa S, Teshome N, Engidawork E (2018) In vivo antimalarial activity of the 80% methanolic root bark extract and solvent fractions of Gardenia ternifolia Schumac. & Thonn. (Rubiaceae) against Plasmodium berghei. Evid Based Complem Altern Med 11:1–10
Dioka C, Orisakwe OE, Afonne J, Agbasi P, Akumka DD, Okonkwo CJ, Ilondub N (2012) Investigation into the heamatologic and hepatotoxic effects of Ribacin in rats. J Health Sci 48(5):393–398
Ene AC, Obika CJ, Okwu GN, Alisi CS, Edeh NG (2013) In vivo anti-malarial effect of methanol and aqueous extracts of Picralima nitida plant parts. J Res Biochem 1(2):095–105
Enenebeaku UE, Ukwandu NC, Mgbemena IC, Nwigwe HC, Enenebeaku CK, Duru CE, Ogidi OI (2021a) Oral acute toxicity and antimalarial potentials of aqueous and methanolic extracts of roots, leaves and stem of Dictyandra arborescens (Welw.) on Plasmodium berghei infected mice. Bull Natl Res Centre 45(75):1–13
Enenebeaku UE, Duru CE, Mgbemena IC, Ukwandu NCD, Nwigwe HC, Enenebeaku CK, Okotcha EN (2021b) Phytochemical evaluation and molecular docking of bioactive compounds from the roots of Dictyandra arborescens (Welw.) against Plasmodium berghei targets. Trop J Nat Prod Res 5(2):370–381
Ezeigwe OC, Nzekwe FA, Nworji FO, Ezennaya FC, Iloanya LE, Asogwa KK (2020) J Exp Pharmacol 1:191–201
Gatsing D, Aliyu R, Kuiate JR, Garba IH, Jaryum KH, Tedongma N, Tchouanguep MF, Adoga GI (2015) Toxicological evaluation of the aqueous extract of Allium sativum bulbs on laboratory mice and rats. Cameroon J Exp Biol 1:39–45
Githinji EK, Irungu LW, Tonia WK, Rukuga GM, Mutai C, Mathaura CN, Wanjoya A (2010) In vitro effects of Warburgia ugandensis, Psidia puntulata and Chasmanthera dependens on leishmania major promastigotes. Afr J Tradit Compliment Altern Med 7(3):264–275
Gurib-Fakim A (2006) Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Asp Med 27(1):1–93
Hosseinzadeh S, Jafarikukhdan A, Hosseini A, Armand R (2015) The application of medicinal plants in traditional and modern medicine: a review of Thymus vulgaris. Int J Clin Med 06(09):635–642
Ihekwereme CP, Agbata CP, Agbata CA, Chukwueze KO, Agu SC (2016) In vivo evaluation of antiplasmodial activity of hydroethanolic stem extract of Baphia pubescens Plasmodium infected albino mice. J Herbmed Pharmacol 5(4):149–152
Iweala EEJ, Obichi IC, Omotosho OE (2011) Biochemical and histological responses of hepatotoxic rats fed Musa paradisiaca L. supplemented diet. Int J Pharmacolol 4:471–477
Iwu MD, Duncan AR, Okunji CO (1999) New antimicrobials of plant origin. In: Janick J (ed) Perspectives on new crops and new uses. ASHS press, Alexandria, pp 457–462
Khan ME, Amupitan JO, Oyewale AO, Ndukwe IG (2015a) Evaluation of the in vivo anti malarial activity of the methanolic leaf extract of Nepata cateria. Res Pharm Biotechnol 6(2):8–15
Khan ME, Amupitan JO, Oyewale AO, Ndukwe IG (2015b) Evaluation of the in vivo anti malarial activity of the methanolic leaf extract of Nepata cateria. Res Pharm Biotechnol 6(2):8–15
Lorke D (1983) A new approach to practical acute toxicity testing. Arch Toxicol 54:275–287
Monosson E (2013) Toxicity testing methods; encyclopedia of earth topics. http://www.eoearth.org/view/article/1566.73. Accessed 13 Mar 2020
Morebise O, Awe EO, Makinde JM, Olajide AO (2001) Evaluation of the anti inflammatory and analgesic properties of Chasmanthera dependens leaf methanol extract. Fitoterapia 72:497–502
Nwinuka NM, Monanu MO, Nwiloh BI (2008) Effects of aqueous extract of Mangifera indica L. (Mango) stem bark on heamatological parameters of normal albino rats. Pak J Nutr 7(5):663–666
Ogbe RJ, Adoga GI, Abu AH (2010) Anti anaemic potentials of some extracts on phenyl hydrazine- induced anaemia in rabbits. J Med Plants Res 4(8):680–684
Ogunlesi M, Okiei W, Ademoye M (2008) Medical plants used in treating eye infection in Niogeria. In: Odugbemi T (ed) A textbook of medicinal plants from Nigeria. University of Lagos press, Lagos, p 304
Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, Lilly P, Sanders J, Sipes G, Bracken W, Dorato M, Deun KV, Smith P, Berger B, Heller A (2010) Concordance of toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol 32:56–67
Onwusonye JC, Uwakwe AA, Iwuanyanwu P, Iheagwam U (2014) Oral acte toxicity (LD50) study of methanol extract of Annona senegalensis leaf in albino mice. Sky J Biochem Res 3(5):046–048
Osaro E, Udomah FA, Jobbi YD, Isah IZ, Abdulrahaman Y, Onuigwe F, Egenti NB, Musa B, Erhabo RO (2016) Cytomegalovirus infection among Blood Donors in Usmanu Danfodiyo University Teaching Hospital Sokoto, Nigeria. Am J Pharmacol Technol Resour 6(2):321–334
Oyinloye BE, Adenowo AF, Kappo AP (2016) Aqueous extract of Monodora myristica ameliorates cadnium-induced heptatotoxity in male rats. Springerplus 5:641–647
Prasad M, Venugopal SP (2016) Preliminary phytochemical analysis and oral acute toxicity study of the root of Argemone mexicana (Linn). Int J Res Dev Pharmacy Life Sci 5(2):2010–2017
Quadri AL, Yakubu MT (2017) Fertility enhancing activity and toxicity profile of aqueous extract of Chasmanthera dependens roots in male rats. Andrologia 1:4–9
Salawu OA, Chindo B, Tijani Obidike I, Salawu TA, Akingbasa J (2009) Acute and sub-acute toxicological evaluation of the methanolic stem bark extract of Crossopteryx febrifuga in rats. Afr J Pharm Pharmacol 3(12):621–626
Sathish K, Shanmugam T, Palvannan S, Kumar VM (2018) Evaluation of antioxidant properties of Canthium parviflorum Lam leaves. Nat Prod Radiance 7(2):122–126
Simplice DK, Tchadjobo T, Ilboudo DP, Jacques S (2011) Sub-saharan rubiaceae. A review of their traditional uses, phytochemistry and biological activities. Pak J Biol Sci 14:149–169
Uboh FE, Okon IE, Ekong MB (2010) Effect of aqueous extract of Psidium guajava leaves on liver enzymes, histological integrity and hematological indices in rats. Gastroenterol Res 3(1):32–38
Ume SI, Jiwuba PC, Obi JI, Dauda E (2016) Economics of broiler production among rural women in Ahiazu Mbaise L.G.A of Imo State, Nigeria. Asian Res J Agric 1(2):1–8
Unuofin JO, Otunola GA (2018) Acute and subacute toxicity of aqueous extract of the tuber of Kedrostis africana (L.) Cogn in Wistar rats. J Complement Integr Med 3(5):17–24
Yakubu MT, Akanji MA, Oladiji AT (2017) Haematological evaluation in male albino rats following chronic administration of aqueous extract of Fadogia agrestis stem. Pharmacogn Mag 3:34
Yang X, Schnackenberg LK, Shi Q, Salminen WF (2014) Hepatic toxicity biomarkers. In: Biomakers in Toxicology, pp 241–259
Acknowledgements
We thank Dr. M.C Duru of the Department of Biology, Federal University of Technology, Owerri, Nigeria, for his assistance in the identification of the plant materials. W also thank Mrs P. Azode who assisted us in collecting the plant materials. We equally acknowledge the Department of Biochemistry, University of Nigeria, Nsukka for their facilities and assistance in carrying out this study.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
Author ENO designed the study, Author UEE gathered the plant materials, conducted the experiment and prepared the manuscript, Author ICM supervised the research work, Author CKE carried out data analysis the data, Authors LMO and CAO helped with literature search. All authors read, corrected and approved the manuscript and gave consent to submit the manuscript for publication in Bulletin of the National Research Centre. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experimental animals were used following NIH guide for care and use of laboratory animals Standard operating manual of NIPRD and approved by the Research Ethics Committee of Biochemistry, Federal University of Technology, Owerri, Nigeria with reference number- FUTO/BCH/DEC/XXI/03/01.
Consent for publication
Not applicable.
Competing interests
Authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Enenebeaku, U.E., Okotcha, E.N., Oguoma, L.M.O. et al. Biochemical and haematological enhancement activities of aqueous and methanol leaves, stem and roots extracts of Chasmanthera dependens (Hochst) and Dictyandra arborescens (Welw.). Bull Natl Res Cent 45, 186 (2021). https://doi.org/10.1186/s42269-021-00642-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-021-00642-7