Abstract
Objective
Was to evaluate effect of clove, turmeric and garlic nano-herbal extracts on surface roughness and microhardness of demineralized dentin, and their bactericidal effect on Streptococcus mutans and Streptococcus sobrinus with or without diode laser irradiation.
Methods
Three 5% nano-formulas were prepared and characterized using transmission electron microscope. MI paste Plus™ was used as control. A total of 100 specimens of demineralized dentin were prepared and treated with 3 W-power diode laser; then, the different tested materials for 10-min before the surface roughness and Vickers microhardness tests were conducted. Eighty coronal cavities were prepared (1-mm diameter × 2-mm depth). Cavities were inoculated with the tested materials with S. mutans or S. sobrinus bacteria, with or without diode laser irradiation for 20-s. Colony-forming unit method was used for counting the viable bacteria. Data were explored for normality using Kolmogorov–Smirnov and Shapiro–Wilk tests and showed parametric distribution for the surface roughness and microhardness tests, and non-parametric distribution for the bactericidal activity test.
Results
The herbal formulas had a significant surface roughness and microhardness mean values. It showed a significant antimicrobial effect on the tested bacteria. When they were combined with diode laser, they showed a significantly higher antimicrobial effect.
Conclusions
The tested herbal formulas represent potent topical remineralizing and antibacterial agents especially when they are used in conjunction with diode laser irradiation.
Similar content being viewed by others
Background
Dental caries is a multifactorial disease, responsible for demineralization of the tooth structure and increasing the surface roughness of the tooth structures with a subsequent decrease in their microhardness due to partial removal of the mineral content (Marsh 2003).
One of the main etiologic factors of dental caries is oral bacteria. S. mutans and S. sobrinus cariogenic bacteria are found abundantly in dental plaque. Their adherence to tooth surface is evident, and their pathogenic characteristics make them initiating agents of dental caries (Forssten et al. 2010).
Dentin demineralization occurs in a faster rate than enamel due to the lower inorganic content of the dentin and higher organic content which magnify the development of caries. Moreover, the size of the hydroxyapatite crystals in dentin is considerably lesser than that in enamel, so the dentin-matrix is more prone to acidic attacks. Thereby, dentin remineralization is more complex than enamel that might be due to the presence of more pronounced remaining hydroxyapatite crystals in enamel (Xu et al. 2011).
Several preventive strategies for dental caries have been advocated to counteract the demineralization process triggered by the bacterial acids. Lately, Recaldent™ “Casein-phospho-peptide-amorphous calcium phosphate” (CPP-ACP) has been developed. It is a nanocomplex derivative from milk natural protein “casein”, and it was proven to promote dental hard tissues remineralization and prevent their demineralization as well as maintaining enamel minerals at a state of supersaturation at the tooth surface (Grychtol et al. 2014).
On the other hand, different herbal extracts were demonstrated to have a natural remineralizing and antibacterial effect and they are now being widely used as an alternate therapy for dental caries (Kshirsagar et al. 2018). Clove is a powerful antioxidant that has been used for different types of medicinal purposes. Its essential oil is effectively used as a painkiller in different fields of dentistry. Turmeric is a proven antioxidant, analgesic, anti-inflammatory, antiseptic and a rich source of calcium and phosphorus (Nagpal and Sood 2013). Garlic is a well-known antioxidant that has an antimicrobial action against oral bacteria. It is a rich source of many minerals including calcium which aid in the remineralization process. Moreover, nanoparticles were found to be more efficient than the bulk substances. As they have significantly increased the antioxidant and antibacterial activities indicating improved application prospects (Lou et al. 2017).
Laser treatments were demonstrated to enhance the teeth remineralization process especially once combined with fluoride application (Kumar et al. 2016), besides their ability to eliminate oral bacteria due to their thermal and photo-disruptive effects (Lee et al. 2006).
This in vitro study investigated the effect of nano-herbal extracts of clove, turmeric, and garlic on surface roughness, microhardness of demineralized dentin and their bactericidal efficacy against S. mutans and S. sobrinus using diode laser.
The null hypotheses inspected was that the three tested nano-herbal extracts have no effect on surface roughness, microhardness of demineralized dentin as well as no bactericidal efficacy against the two tested bacteria with and without diode laser irradiation.
Methods
Selected materials
One commercial remineralizing agent was used in this study: (CPP-ACP) with fluoride (ACP-F) [GC MI paste Plus, GC America Inc., IL, USA]. Three 5% concentration of clove, turmeric and garlic nano-herbal extracts were prepared for the study.
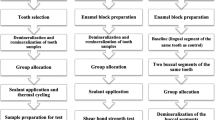
Study design and specimen grouping
A total of 180 specimens were prepared for the study. One hundred specimens were prepared and randomly divided into 10 groups (n = 10/group) according to the tested materials [GC MI paste Plus, and three nano-herbal extracts], that each one was evaluated for its effect on surface roughness and Vickers microhardness of demineralized dentin with and without diode laser irradiation. Eighty specimens were randomly divided into 16 groups (n = 5/group) representing the different tested materials, that each one was further evaluated for its bactericidal efficacy against S. mutans and S. sobrinus with and without diode laser irradiation.
Preparation of the nano-herbal extracts
Clove, turmeric and garlic essential oils loaded with solid-lipid nanoparticles were prepared by ultrasonic-solvent emulsification technique as described by Asnawi et al. (2008); using 1% (w/w) stearic acid, 2.5% (w/w) Tween-80, 2.5% (w/w) Soybean lecithin and 50-ml of dichloromethane. An oil phase of 1% (w/w) stearic acid and 5% of each tested essential oil was mixed with 50-ml of dichloromethane and heated to 50 °C. A water phase consisted of 2.5% (w/w) of both Soybean lecithin and Tween-80 as an emulsifier. They were dispersed in 50-ml of distilled water with magnetic stirring. After evaporation of solvents, the water phase was added to oil phase drop-by-drop at 50 °C followed by magnetic stirring for 10-min.
The coarse extract was ultrasonically treated at 55-W for 5-min using high-power ultrasonication probe (Sonics Vibra Cell, Ningbo Haishu Kesheng Co. Ltd, China) with water bath at 0 °C. The cold nano-extract was then dispersed into cold water using homogenizer (Unidrive X1000 homogenizer, CAT Scientific Co, Germany) to prevent lipid aggregation, then followed by magnetic stirring to remove any traces of organic solvents and stored at 4 °C till use.
Transmission electron microscopy (TEM) characterization of the nano-herbal extracts
The three prepared nano-extracts were placed on carbon-coated copper slide, and a drop of 2% phosphotungstic acid was added. Excess liquid was blotted with filter paper for 2-min. Specimens were left to dry for 10-min at room temperature before observation using TEM (JEM-2100 Electron Microscope, JEOL Ltd, Japan).
Teeth selection and specimen’s preparation for surface roughness and Vickers microhardness assessments
Fifty sound upper human premolars were extracted for orthodontic purpose and stored at 4 °C in 0.1% thymol solution. The teeth were ultrasonically cleaned and scaled to remove any debris or calculus deposits. The coronal middle and cervical 1/3 of the buccal and palatal surfaces of each tooth were ground using 600–1200 grit silicon carbide (SiC) abrasive paper to remove the enamel and expose the underlying flat dentin surface under running water. A low speed, double-side cutting diamond was equipped to remove teeth roots 2-mm below the enamel-cementum junction. Pulp tissues were removed from the pulp chambers with barded broaches, and then each crown was bisected into buccal and palatal halves. Specimens were placed in polyvinyl cylindrical tubes with their ground surfaces facing upwards. Clear auto-cured acrylic resin was mixed according to manufacturer instructions then poured into the tubes and left to set completely for 1-h.
Demineralization of the dentin surface of the prepared specimens
Specimens were coated with an acid-resistant varnish excluding a 3-mm × 3-mm window at the middle 1/3 of the crown (Lee et al. 2006), then immersed at room temperature for 72-h in 20-ml of demineralizing solution; 1.35-mM KH2PO4, 50-mM acetic acid derivation, 130-mm KCl, 2.25-mM CaCl2 2H2O of pH = 5.0 according to Rahiotis and Vougiouklakis (2007), then washed thoroughly with distilled water.
Diode laser irradiation
A 970-nm wavelength Gallium-Aluminium-Arsenide (Ga AlAs) diode laser [Siro-Laser Advance class III b, SIRONA Dental Company, Germany] with 3-W power in continuous mode was equipped for 20-s using 220-μm fiberoptic conductor. The assigned marked windows on the ground surfaces of the specimens were irradiated with the diode laser prior to the application of the tested materials. Thereby, the fiberoptic tip was placed perpendicularly at a non-contact method and it was moved longitudinally in a uniform way (Gera et al. 2017). After the irradiation of each assigned group, 2-mm were cut off the tip of the fiberoptic to evade loss of the laser energy.
Application protocols of tested remineralizing agent and nano-herbal extracts
MI paste Plus remineralizing agent as well as turmeric, garlic, and clove nano-herbal extracts were applied to the demineralized dentin surfaces of the assigned groups using micro brushes in a generous amount and left for 10-min (Cheng et al. 2015), then rinsed off the teeth surfaces with distilled water for 1-min to remove any remnants. The specimens were kept in distilled water, in tight-seal poly-ethylene vessels at 37 °C for 24-h before the different tests were performed.
Surface roughness assessment
The lens of a digital camera (C 5060, Olympus, Japan) was adjusted at the middle 1/3 of the specimens and images were taken at magnification 9X. The digital camera was mounted on a stereomicroscope (Olympus® BX 60, Olympus Optical Co. LTD, Tokyo, Japan). Ra factor was calculated using an image analysis software (Image J, 1.4 1a, NIH, USA). Arithmetic means of elevations and depressions (Ra) factor calculations were performed for analysis regarding the reflection of light from the surface.
Vickers microhardness assessment
Using a load of 200-g for 15-s dwell time (Prabhakar et al. 2013) at magnification 20X, the demineralized dentin surface microhardness was assessed using Digital Vickers hardness tester (Nexus 4000TM, INNOVTEST, model number 4503, The Netherlands). Three random indentations were made at the center of the marked window of each specimen using Vickers’s pyramidal diamond indenter. Calculations of the surface micro-hardness were evaluated using computer software (Hardness-Course Brinell/Vickers/Rockwell, copy right IBS 2012, version 10.4.4).
Specimens’ preparation for the bactericidal efficacy assessment
Forty sound premolars were collected, and their roots were removed using a low-speed double-sided diamond disc. Each crown was bisected into two halves. One cavity was prepared per each buccal, palatal, or lingual surfaces using a high speed, regular length, carbide, crosscut fissure bur (U.S. No. 557) with profuse water coolant. Cavity preparation was centred at the middle 1/3 of the surface. Each cavity was 1-mm in diameter and 2-mm in depth to avoid unnecessary pulp exposures (Prabhakar et al. 2013). The prepared cavities dimensions were measured using a periodontal probe (UNC#12 HDL#6, Hu Friedy, Tuttilnger, Germany). Specimens were autoclaved for 15-min at 121 °C then dried using sterile paper points.
Bacterial suspensions and culture media preparation
A single colony of S. mutans and S. sobrinus bacteria was separately inoculated in 5-ml brain–heart infusion broth (BHI broth) in two vials then incubated at 37 °C for 24-h. A bacterial suspension of 0.5-ml was added to 0.5-ml BHI-broth to obtain 4 × 107 colony forming unit (CFU)/ml. A nutrient broth was added to both bacteria then sub-cultured on nutrient-agar plates and anaerobically incubated at 37 °C for 24-h. The inoculum was prepared by adjusting bacterial density to nearly 108 CFU/ml using 0.5 Mcfarland opacity standards.
Inoculation of the prepared cavities and diode laser irradiation
Using micropipette, the prepared cavities were inoculated with 10-μl of the bacterial suspensions containing 4 × 105 CFU. Another 10-µl of the three prepared 5% nano-herbal extracts and MI paste Plus were added to the assigned cavities then incubated for another 24-h at 37 °C. The assigned inoculated cavities were irradiated with diode laser using the previously mentioned parameters. The optic fiber was thoroughly disinfected after each use (with 70% ethyl alcohol) and re-inserted inside the cavity to a depth of 2-mm with a helicoidal continuous movements, clockwise from the top (inwards) to the floor and anti-clockwise in the opposite direction (outwards). This way, laser-light distribution inside the cavities was enhanced, and carbonization as well as unnecessary heat generation of the internal cavity surfaces were evaded.
Bacterial colony counting
Dentin chips (25 ± 5 mg) were collected from circumferential cavity walls using a sterile, new carbide crosscut small fissure bur (U.S. No. 556). The collected chips were placed into sterile plastic tubes with 0.5-ml normal saline (Arslan et al. 2019). A 200-µl of saline was dispensed on separate MSA Petri-dishes for S. mutans and mitis-salivarius for S. sobrinus, then incubated for 24-h at 37 °C. CFU method was used to assess the viable bacterial cells number.
Statistical analysis
Means and standard deviation values were premeditated for each group in each test. For the surface roughness and Vickers microhardness tests, Kolmogorov–Smirnov and Shapiro–Wilk tests were used to investigate the data for normality, which showed parametric (normal) distribution. For comparison between more than two groups in non-related samples, one-way ANOVA followed by Tukey post hoc test was utilized. For comparison between two groups in non-related samples, independent sample t-test was performed. For testing the interaction effect between various variables, two-way ANOVA test was done.
Viable counts of the tested bacteria were transformed to their LOG10 values. Data were explored for normality using Kolmogorov–Smirnov and Shapiro–Wilk tests and showed non-parametric distribution. Kruskal Wallis test was used to compare between more than two groups in non-related specimens. Mann Whitney test was used to compare between two groups in non-related specimens. Wilcoxon test was used to compare between two groups in related specimens. Significance level was set at p ≤ 0.05 and IBM® SPSS® Statistics Version 20 for Windows was used to perform the statistical analysis.
Results
The main investigated groups for the surface roughness and Vickers microhardness tests were presented as follows: group A; control group (demineralized dentin), group B; MI paste Plus, group C; 5% clove, group D; 5% turmeric and group E; 5% garlic nano-herbal extracts. These groups were either did not receive diode laser irradiation (0) or were subjected to diode laser irradiation (1).
The effect of diode laser irradiation on the surface roughness of the demineralized dentin specimens and treatment with MI paste Plus remineralizing agent and the three nano-herbal extracts tested in the study is investigated in Table 1. The data showed that the highest mean value was recorded for group B1, while the least mean value was recorded for group A0. Regarding the different tested materials, there was a statistically significant difference between all groups at p < 0.001, and the highest mean value was recorded for group B0 followed by C0, D0, E0 and A0 correspondingly. Regarding diode laser irradiation within each material, there was a statistically significant difference between all groups at p < 0.001, and the highest mean value was recorded for group B1 followed by C1, D1, E1 and A1, respectively, at p < 0.001, p < 0.001, p < 0.001, p = 0.014 and p = 0.007 correspondingly.
The effect of diode laser irradiation on the Vickers microhardness of demineralized dentin treatment with MI paste Plus remineralizing agent and the three nano-herbal extracts tested in the study is investigated in Table 2. The data revealed that the highest mean value was recorded for group B1, while the least mean value was recorded for group A0. Regarding the different tested materials, there was a statistically significant difference between all groups at p < 0.001, and the highest mean value was recorded for group B0 followed by C0, D0, E0 and A0, respectively. Regarding diode laser irradiation within each material, there was a statistically significant difference between all groups at p < 0.001, and the highest mean value was recorded for group B1 followed by C1, D1, E1 and A1, respectively, at p < 0.001, p < 0.001, p < 0.001, p < 0.001and p = 0.009, respectively.
The main tested groups for the bactericidal efficacy against S. mutans and S. sobrinus bacteria were presented as follows: Group I: MI paste Plus (control), Group II: 5% clove, Group III: 5% turmeric and Group IV: 5% garlic nano-herbal extracts. These groups were either did not receive laser irradiation or were subjected to diode laser irradiation.
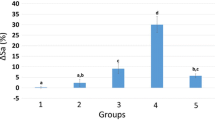
Bactericidal efficacy of different tested nano-herbal extracts and MI paste Plus against S. mutans and S. sobrinus with and without diode laser irradiation is demonstrated in Table 3. The least mean value was recorded for group IV with S. mutans after diode laser irradiation. For S. mutans bacteria regarding the tested materials, there was no statistically significant difference between the four groups within (No diode laser) group where p = 1. There was a statistically significant difference between (No diode laser) and (With diode laser) groups where p = 0.005.
For the bactericidal efficacy of diode laser irradiation against S. sobrinus bacteria regarding the tested materials, there was a statistically significant difference between (No diode laser) and (With diode laser) groups where p = 0.005. Within (With diode laser) group, there was a statistically significant difference between groups I, II, III and group IV where p = 0.024.
Discussion
In the current study, TEM scanning revealed that the average particles size of the three prepared nano-herbal extracts ranged from 40- to 50-nm, which lies within the nanoscale.
MI Paste Plus™ contains Recaldent™ (CPP-ACP) in addition to 900 ppm of fluoride in form of NaF, according to its manufacturer. Such amount of fluoride is almost equivalent to the fluoride amount found in over the counter different toothpastes.
The present study evaluated a 970-nm diode laser efficacy accompanied by application of CPP-ACP-fluoride and 5% nano-extracts of clove, turmeric and garlic on surface roughness and microhardness of demineralized dentin and their bactericidal effect against S. mutans and S. sobrinus cariogenic bacteria. The results displayed a significant difference in surface roughness, microhardness, and the viable bacterial counts of the tested groups. Therefore, the null hypothesis was rejected.
In the current study, surface roughness of the demineralized dentin group (control) showed the highest significant mean values. This could be owed to removal of the constituent minerals by the action of the demineralizing solution, in which the prepared dentin specimens were kept for 72-h. Moreover, the least significant surface roughness values were recorded for MI paste Plus groups. This finding could be related to its elevated levels of Ca, P, and F ions which might have precipitated upon the demineralized dentin surface forming a CaF2 layer that could partially block the demineralized surface porosities and the patent dentinal tubules leading to decreased surface roughness. Yamazaki and Margolis (2008) concluded that a CaF2 layer was formed at the enamel surface following topical fluoride application which has reacted with Ca and P ions leading to inhibition of the demineralization and enhancement of the remineralization of the crystals. Moreover, CPP-ACP has a unique nature as an amorphous electroneutral nanocomplexes. This nano-scale complex could facilitate the diffusion of the Ca and P ions released from MI paste Plus into the porosities of the demineralized tooth structure. These released ions were found to have a significant binding-affinity to the apatite of the tooth (Reynolds et al. 2008).
On the other hand, the nano-extract of clove recorded the least significant surface roughness compared to those of turmeric and garlic, respectively. These findings could be attributed to the amount of minerals in the investigated nano-herbal extracts. According to the agricultural research services of the US department of agriculture, clove was reported to have the highest Ca and P content, followed by turmeric then garlic (Food Central Data, Agricultural Research Services, US Department of Agriculture USDA, USA). Thus, Ca and P ions might have precipitated on the demineralized dentin surface blocking the demineralized surface porosities and patent dentinal tubules and decreasing the surface roughness. Nonetheless, the influence of the nano-herbal extracts on the surface topography of the dental hard tissues is less well investigated and requires further research.
Our results disagreed with Gungor and Donmez (2020). This could be related to the difference in the control groups used in the studies. They used sound dentin specimens as control in contrast to demineralized dentin specimens that were used as control in our study. Moreover, they have equipped AFM to assess the surface roughness instead of the stereomicroscope that was employed in our study.
Data obtained from the present study showed that diode laser irradiation had significantly increased the surface roughness of the tested specimens. This could be owed to the thermal effect of the laser energy, which might lead to dentin structure alterations (Alfredo et al. 2009). These findings agreed with those of Viapiana et al. (2012), and they concluded that near-infrared wavelength range of 980-nm diode laser allowed the dentin to uptake some of the emitted laser energy by its carbonate and phosphate mineral content leading to crystalline arrangement variation and melting of the irradiated dentin surface.
Vickers microhardness results revealed that MI paste Plus showed the highest significant mean values which could be accredited to CPP, which was proven to stabilizes the levels of Ca and P ions and develops a state of supersaturation of these ions around the teeth resulting in enhancement of teeth remineralization due to increased surrounding pH and ions levels (Jayarajan et al. 2011). This finding was approved by Behrouzi et al. (2020), and they concluded that hydroxyapatite crystals density was increased by F, Ca and P ions penetration into the crystals enhancing the remineralization process.
The significance of interaction between laser irradiation and the hard tooth structures is not fully documented, and it can differ in relation to the designated parameters. Therefore, dentin inherent properties can be influenced by laser irradiation.
Diode laser irradiation prior to MI paste Plus application showed a significant increase in the present study. This could be explained by increased dental structure uptake of F, Ca and P ions when combined with laser application. The combined treatment of laser irradiation and fluoride application was proved to increase the acid resistance of hard tooth structures than either of the previously mentioned treatments alone (Kumar et al. 2016).
Our findings were in accordance with Wiegand et al. (2010), and they disclosed that a proper remineralization of the dental structures necessitates the availability of materials that could successfully deliver Ca and P ions to the tooth structure with the aid of laser irradiation.
The investigated nano-herbal extracts showed a comparable microhardness values to MI paste Plus. Clove nano-extract showed the highest significant Vickers microhardness, followed by turmeric then garlic. This might be owed to the discrepancies in their Ca and P content, which might have been directly related to their remineralization potential. As Ca and P ions are the main keys in balance between de- and re-mineralization processes and they could alter teeth vulnerability to caries development (Hara and Zero 2010).
Furthermore, it was determined that the decreased pH values in the demineralized dentin will activate the MMPs (Matrix-Metallo-Proteinases) types 2, 8, and 9 causing hydrolysis of the dentin organic matrix (collagen), which can be prevented by using MMPs inhibitors (Chaussain-Miller et al. 2006). MMPs inhibitors include many natural herbs which usually comprise active biological compounds with powerful antioxidant properties such as phenolic and polyphenolic compounds.
Our results agreed with Al-lami and Al-Alousi (2011), who concluded that clove extract could efficiently rise the demineralized enamel surface microhardness. They owed their findings to the Ca and P ions content of clove extract. Moreover, Gungor and Domenz 2020 investigated different herbal teas for their effect on erosive dentin microhardness and found that the highest values were recorded for clove due to its high Ca and P ions content. Additionally, tannin is one of the constituent polyphenolics compounds of clove and an in vitro study demonstrated its ability to increase the enamel acid resistance, thereby increasing the remineralization capacity of clove extract (Yu et al. 1995).
On the other hand, Basir et al. (2018) investigated the anti-caries effect and microhardness of different curcumin concentrations on enamel. They declared that curcumin showed an anti-caries effect and increased enamel microhardness with all tested concentrations. Furthermore, Prabhakar et al. (2013) compared the effect of 2% chlorhexidine and turmeric extract on root dentin microhardness, and they found out that turmeric extract had significantly increased dentin microhardness. Nevertheless, curcumin (an antioxidant polyphenolic principal compound of turmeric) could have impeded the action of MMP-9 responsible for the degradation of the collagen matrix of the demineralized dentin. As curcumin can chelate the catalytic Zinc ions that are vital for the activity of MMPs, thus inhibiting their action (Zhang et al. 2012).Furthermore, Słowianek and Leszczyńska (2016) showed that clove had the highest antioxidant activity by far followed by turmeric then garlic and this was owed to the differences in their phenolic compounds types which have variable antioxidant properties with variable effect on MMPs inhibition, which agrees with our results.
On the other hand, our results showed that diode laser irradiation combined with MI paste Plus and nano-extracts had an increased significant antibacterial effect against S. mutans and S. sobrinus than the tested materials alone. This could be attributed to combining the antibacterial efficiency of the diode laser on one hand with that of the tested materials on the other hand which might have an intense significant effect on the overall viable bacterial count for both strains. These results were in accordance with Lee et al. (2006), who reported that the antibacterial efficacy of diode laser against S. mutans is obviously elevated in relation to the antibacterial mouthwash used in their study.
The antibacterial efficiency of diode laser irradiation could be explained through its photo-disruptive and thermal effects. It is not necessary for an immediate bacterial cell death to happen during lasing, but a sub-lethal cell damage repressing its growth, is more likely to occur. Such damage might possibly accumulate denatured proteins and destroy cell wall integrity. Thus, terminating the cell growth and causing cell lysis. Furthermore, thermal changes greatly affect cellular protein. Laser irradiation might lead to denaturation of cell proteins inducing the cell to produce new proteins. Cells will prevent building-up of debris from denatured protein causing cell death (Lee et al. 2006).
In agreement with our study, Patel et al. (2017) reported that fluoride-containing CPP-ACP varnish was effective in decreasing S. mutans count in saliva which could be owed to CPP-ACP anticariogenic effect in addition to its F ions, which might lead to ACP-F localization at the tooth surface by the action of casein protein. It was determined that CPP-ACP binds successfully to S. mutans bacteria which was mediated by cell moieties of surface phosphate through cross-linking of calcium and by the hydrophobic and hydrogen bond-mediated cellular interactions (Memarpour et al. 2015).
The chief component of clove essential oil is eugenol, which has a powerful antibacterial effect on different oral bacteria. Its mechanism of action is related to interaction with cell membrane of bacteria (Oyedemi et al. 2009). Our results agreed with Mirpour et al. (2015), who concluded that clove showed an antibacterial activity against S. mutans and S. aures. They owed their results to the antibacterial effect of saponins and flavonoids components of the investigated plant extract. Furthermore, Lapinska et al. (2020) concluded that clove essential oil had distinct antibacterial activity on S. mutans even when it was incorporated in a flowable composite.
On the other hand, “curcumin”, the exclusive bioactive agent of turmeric, was proven to have an adequate antibacterial influence. Javid et al. (2017) suggested that curcumin could enhance prevention of caries through decreasing the amounts of produced acids from S. mutans, proteins, and polysaccharides.
It was demonstrated that turmeric induced membrane permeabilization of bacterial cells, which causes cell damage (Gera et al. 2017). Furthermore, when curcumin was used in different surfactants formulas, it showed a photo-sensitizing efficacy during antibacterial photo-dynamic treatments. The mechanism by which curcumin resulted in photo-induced bacterial cell death has not yet been fully recognized. However, it is believed that binding of photosensitizer to the outer membrane of microbial cells is an essential requirement for microbial photosensitization (Jori and Coppellotti 2007). This might explain the interaction between the turmeric and diode laser which has a significant bactericidal effect against both tested bacteria in our study.
Whereas garlic is a powerful antibiotic. It is a reliable antimicrobial agent, and it can inhibit gram-positive and gram-negative bacteria (Kshirsagar et al. 2018). The main antibacterial mechanism of garlic is through interaction with the enzymes responsible for bacterial metabolism and nutrition (Bakri and Douglas 2005).
Padiyar et al. (2018) demonstrated the effectiveness of garlic mouthwash against S. mutans bacteria which could be due to its antioxidant sulfur compound “allicin” which can easily diffuse inside the bacterial cells through their membranes and bind the sulfur groups to the bacterial enzymes and proteins, causing modification and inhibition of the bacterial activities.
Nonetheless, a former study inspected the antibacterial and antioxidant properties of ginger, turmeric and garlic spice extracts and it was concluded that turmeric recorded higher antioxidant and antibacterial activity than garlic (Panpatil et al. 2013). These results agreed with our study. They owed their results to the higher amounts of polyphenolic antioxidant compounds of turmeric than garlic. Moreover, Sofia et al. (2007) investigated the antibacterial effect of different Indian spices including garlic and clove. They revealed that 3% clove extract displayed a broad antibacterial activity against all investigated bacteria than garlic, as it can be concluded from our results.
It worth mentioning that the mechanism of antibacterial activity of clove, turmeric and garlic nano-herbal extracts combined with lasers irradiation is not entirely fulfilled and require further research. The therapeutic potential of different herbal formulas is now justified. Therefore, the urge of using natural plants as an alternative medicine will not only decrease clinical implications of bacterial drug resistance but also total cost and unwanted drugs side-effects. However, more studies to evaluate different clinical effects of herbal extracts are recommended for teeth remineralization and prevention of different oral diseases.
Conclusions
Regarding the limitations of this in vitro study, it can be concluded that diode laser irradiation had a positive significant effect on the surface roughness and Vickers microhardness of the demineralized dentin. Moreover, diode laser irradiation may present a potential antibacterial agent against S. mutans and S. sobrinus. Nevertheless, the nano-herbal extracts of clove, turmeric and garlic can be considered as a potential remineralizing and antibacterial agents especially upon combining with diode laser irradiation.
Availability of data and materials
The authors declare that the data supporting the findings of this study are available within the article.
Abbreviations
- S. mutans :
-
Streptococcus mutans
- S. sobrinus :
-
Streptococcus sobrinus
- CPP-ACP:
-
Casein-phospho-peptide-amorphous calcium phosphate
- MMPs:
-
Matrix-metallo-proteinases
- TEM:
-
Transmission electron microscopy
References
Alfredo E, Souza-Gabriel AE, Silva SR, Sousa-Neto MD, Brugnera A Jr, Silva-Sousa YTC (2009) Morphological alterations of radicular dentine pretreated with different irrigations solutions and irradiated with 980 nm diode laser. Microsc Res Tech 72:22–27
Al-lami AK, Al-Alousi WS (2011) Effect of water clove extract on the microhardness and microscopic features of initial caries-like lesion of permanent teeth, compared to fluoridated agent. J Baghdad Coll Dent 23(2):110–113
Arslan I, Baygin O, Bayram G, Akyol R, Tuzuner T (2019) Effects of various agents and laser systems on antibacterial activity and microtensile bond strength when used for cavity disinfection. J Dent Lasers 13:12–18
Asnawi S, Abd Aziz A, Abd Aziz R, Khamis AK (2008) Formulation of geranium oil loaded solid lipid nano particles for mosquito repellent application. J Chem Natl Resour Eng 2:90–99
Bakri IM, Douglas CWI (2005) Inhibitory effect of garlic extract on oral bacteria. Arch Oral Biol 50:645–651
Basir L, Kalhori S, Javid AZ, Masjedi MK (2018) Anticaries activity of curcumin on decay process in human tooth enamel samples (in vitro study). J Natl Med Assoc 110(5):486–490
Behrouzi P, Heshmat H, Ganjkar MH, Tabatabaei SF, Kharazifard MJ (2020) Effect of two methods of remineralization and resin infiltration on surface hardness of artificially induced enamel lesions. J Dent Shiraz Univ Med Sci 21(1):12–17
Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S (2006) The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res 85:22–32
Cheng L, Li J, He L, Zhou X (2015) Natural products and caries prevention. Caries Res 49(1):38–45
Food Central Data, Agricultural Research Services, US Department of Agriculture USDA, USA. https://fdc.nal.usda.gov/fdc-app.html#/
Forssten SD, Björklund M, Ouwehand AC (2010) Streptococcus mutans, caries and simulation models. Nutrients 2(3):290–298
Gera M, Sharma N, Ghosh M, Huynh DL, Lee SJ, Min T et al (2017) Nanoformulations of curcumin: an emerging paradigm for improved remedial application. Oncotarget 8(39):66680–66698
Grychtol S, Basche S, Hannig M, Hannig C (2014) Effect of CPP/ACP on initial bioadhesion to enamel and dentin in situ. Sci World J 2014:512682
Gungor AS, Donmez N (2020) Dentin erosion preventive effects of various plant extracts: an in vitro atomic force microscopy, scanning electronmicroscopy, and nanoindentation study. Microsc Res Tech 84:1–11
Hara A, Zero D (2010) The caries environment: saliva, pellicle, diet, and hard tissue ultrastructure. Dent Clin North Am 54(3):455–467
Javid AZ, Ardekani MTF, Basir L, Ekrami AM, Motamedifar M, Haghighizadeh MH, Dehghan P (2017) Effect of curcumin on acidogenicity, viable bacteria and biomass in experimental biofilm model on human tooth. Int J Adv Biotechnol Res 1(8):e77–e82
Jayarajan J, Janardhanam P, Jayakumar P, Deepika (2011) Efficacy of CPP-ACP and CPP-ACPF on enamel remineralization—an in vitro study using scanning electron microscope and DIAGNOdent. Indian J Dent Res 22:77–82
Jori G, Coppellotti O (2007) Inactivation of pathogenic microorganisms by photodynamic techniques: mechanistic aspects and perspective applications. Antiinfect Agents Med Chem 6:119–123
Kshirsagar MM, Dodamani AS, Karibasappa GN, Vishwakarma PK, Vathar JB, Sonawane KR et al (2018) Antibacterial activity of garlic extract on cariogenic bacteria: an in vitro study. AYU 39:165–168
Kumar P, Goswami M, Dhillon JK, Rehman F, Thakkar D, Bharti K (2016) Comparative evaluation of microhardness and morphology of permanent tooth enamel surface after laser irradiation and fluoride treatment—an in vitro study. Laser Ther 25(3):201–208
Lapinska B, Szram A, Zarzycka B, Grzegorczyk J, Hardan L, Sokolowski J, Lukomska-Szymanska M (2020) An in vitro study on the antimicrobial properties of essential oil modified resin composite against oral pathogens. Materials 13:4383
Lee BS, Lin YW, Chia JS, Hsieh TT, Chen MH, Lin CP et al (2006) Bactericidal effects of diode laser on streptococcus mutans after irradiation through different thickness of dentin. Lasers Surg Med 38:62–69
Lou Z, Chen J, Yu F, Wang H, Kou X, Ma C et al (2017) The antioxidant, antibacterial, antibiofilm activity of essential oil from Citrus medica L. var. sarcodactylis and its nanoemulsion. LWT 80:371–377
Marsh PD (2003) Are dental diseases examples of ecological catastrophes? Microbiology-SGM 149:279–294
Memarpour M, Fakhraei E, Dadaein S, Vossoughi M (2015) Efficacy of fluoride varnish and casein phosphopeptide-amorphous calcium phosphate for remineralization of primary teeth: a randomized clinical trial. Med Princ Pract 24:231–237
Mirpour M, Siahmazgi ZG, Kiasaraie MS (2015) Antibacterial activity of clove, gall nut methanolic and ethanolic extracts on Streptococcus mutans PTCC 1683 and Streptococcus salivarius PTCC 1448. J Oral Biol Craniofac Res 5:7–10
Nagpal M, Sood S (2013) Role of curcumin in systemic and oral health: an overview. J Natl Sci Biol Med 4(1):1–7
Oyedemi SO, Okoh AI, Mabinya LV, Pirochenva G, Afolayan AJ (2009) The proposed mechanism of bactericidal action of eugenol, α-terpineol and γ-terpinene against Listeria monocytogenes, Streptococcus pyogenes, Proteus vulgaris and Escherichia coli. Afr J Biotechnol 68(7):1280–1286
Padiyar B, Marwah N, Gupta S, Padiyar N (2018) Comparative evaluation of effects of triphala, garlic extracts, and chlorhexidine mouthwashes on salivary Streptococcus mutans counts and oral hygiene status. Int J Clin Pediatr Dent 11(4):299–306
Panpatil VV, Tattari S, Kota N, Nimgulkar C, Polasa K (2013) In vitro evaluation on antioxidant and antimicrobial activity of spice extracts of ginger, turmeric and garlic. J Pharmacog Phytochem 2(3):143–148
Patel PM, Hugar SM, Halikerimath S, Badakar CM, Gokhale NS, Thakkar PJ et al (2017) Comparison of the effect of fluoride varnish, chlorhexidine varnish and casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) varnish on salivary Streptococcus mutans level: a six-month clinical study. J Clin Diagn Res 11:53–59
Prabhakar AR, Taur S, Hadakar S, Sugandhan S (2013) Comparison of antibacterial efficacy of calcium hydroxide paste, 2% chlorhexidine gel and turmeric extract as an intracanal medicament and their effect on microhardness of root dentin: an in vitro study. Int J Clin Pediatr Dent 6(3):171–177
Rahiotis C, Vougiouklakis G (2007) Effect of CPP-ACP agent on the demineralization and remineralization of dentine in vitro. J Dent 35(8):695–698
Reynolds EC, Cai F, Cochrane NJ, Shen P, Walker GD, Morgan MV et al (2008) Fluoride and casein phophopeptideamorphous calcium phosphate. J Dent Res 87:344–348
Słowianek M, Leszczyńska J (2016) Antioxidant properties of selected culinary spices. Herba Pol 62(1):29–41
Sofia PK, Prasad R, Vijay VK, Srivastava AK (2007) Evaluation of antibacterial activity of Indian spices against common foodborne pathogens. Int J Food Sci Technol 42(8):910–915
Viapiana R, Sousa-Neto MD, Souza-Gabriel AE, Alfredo E, Silva-Sousa YTC (2012) Microhardness of radicular dentin treated with 980-nm diode laser and different irrigant solutions. Photomed Laser Surg 30(2):102–106
Wiegand A, Magalhães AC, Navarro RS, Schmidlin PR, Rios D, Buzalaf MA, Attin T (2010) Effect of titanium tetrafluoride and amine fluoride treatment combined with carbon dioxide laser irradiation on enamel and dentin erosion. Photomed Laser Surg 28(2):219–226
Xu HH, Moreau JL, Sun L, Chow LC (2011) Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mater 27:762–769
Yamazaki H, Margolis HC (2008) Enhanced enamel remineralization under acidic conditions in vitro. J Dent Res 87:569–574
Yu H, Oho T, Xu X (1995) Effects of several tea components on acid resistance of human tooth enamel. J Dent 23:101–105
Zhang Y, Gu Y, Lee HM, Hambardjieva E, Vranková K, Golub LM, Johnson F (2012) Design, synthesis and biological activity of new polyenolic inhibitors of matrix metalloproteinases: a focus on chemically-modified curcumins. Curr Med Chem 19:4348–4358
Acknowledgements
Not applicable
Funding
The authors revealed receiving the following funding for the study, authorship, and/or publication of this article: The authors acknowledge National Research Centre in Egypt for funding the published work through project no. AR 1111404.
Author information
Authors and Affiliations
Contributions
DM and LM developed the original idea, main conception and constructed the study design. LM executed the study methodology and conducted the mechanical tests of the study, interpreted the results, wrote the manuscript, and revised the final presented manuscript. DM was responsible for LASER application and revised the part of the methodology related to LASER application. MM developed the nano-herbal extracts, wrote the part of the methodology related to extraction of the herbal extracts in the nano-form. KM developed the antibacterial tests methodology and wrote this part of the methodology. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moharam, L.M., Sadony, D.M., Adel, M.M. et al. Evaluation of surface roughness and Vickers microhardness of various nano-herbal extracts on demineralized dentin and their bactericidal efficacy with 970-nm wavelength diode laser irradiation. Bull Natl Res Cent 45, 178 (2021). https://doi.org/10.1186/s42269-021-00638-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-021-00638-3