Abstract
Background
The increase in multidrug resistance (MDR) among pathogenic bacteria responsible for infectious diseases has led to lack of effectiveness of some antibiotics. The ability of Escherichia coli to harbor resistant genes has made the treatment of infections a major challenge. This study was carried out to assess antibiotic resistance and extended-spectrum beta-lactamase (ESBL) production of E. coli from various sources in Aba metropolis, Nigeria.
Results
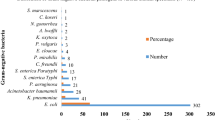
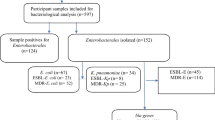
From a total of 350 samples collected from clinical and non-clinical sources, 137 were presumptively identified as E. coli by standard phenotypic methods and 83 were confirmed as E. coli by the detection of E. coli specific 16S rRNA gene fragments. The majority of these isolates (52, 62.7%) were from non-clinical sources. The clinical isolates, however, exhibited a higher level of resistance against 62.5% of tested antibiotics. Both group of isolates exhibited similar levels (58.1% vs 53.9%) of MDR, though. A low rate of ESBL production was observed (1.2%) following phenotypic detection of ESBL-producing abilities using the double-disc synergy test. An assessment of the presence of three beta-lactamase gene genotypes (blaTEM, blaSHV and blaCTX-M) revealed that none of the three predominant ESBL genotypes was identified in this study.
Conclusions
This study reports high levels of antibiotic resistance in both clinical and non-clinical E. coli isolates. Though higher rates of resistance were observed among the non-clinical isolates, both group of organisms had similar levels of MDR. Strikingly, however, was the low level of ESBL producers detected in this study and the absence of the three main genotypes associated with ESBL production in this study.
Similar content being viewed by others
Background
Extended-spectrum beta-lactamase (ESBL)-producing bacteria are one of the more recent bacterial evolutions observed in the ongoing antibiotic-resistant pandemic. Since the first detection of these organisms, an increasing prevalence of ESBL-producing bacteria has been described worldwide (Denis et al. 2015). These particular groups of resistant bacteria are important because the extended-spectrum beta-lactamase enzymes confer on the producing organism resistance to a wider spectrum of beta-lactam antibiotics, key of which are the third-generation cephalosporins. These were at the forefront in treating beta-lactamase drug-resistant bacteria (Paterson and Bonomo 2005). ESBL production has also been associated with increasing levels of drug failure, longer hospital stays, cost, prognosis and mortality in some cases (Nivesvivat et al. 2018; Sakellariou et al. 2016; Wilson and Torok 2018; Mita et al. 2019).
Escherichia coli and Klebsiella pneumoniae have been described as the two predominant groups of bacteria associated with ESBL production. Initial reports of ESBL-producing bacteria were associated with nosocomial outbreaks, but over time spread to the community (Wilson and Torok 2018). The ESBLs themselves are generally of different types. The less commonly reported ESBLs include the OXA type, PER type and GES type (Shaikh et al. 2015). Those belonging to the SHV, TEM and CTX-M families have, however, been described as the most commonly encountered among clinical isolates (Bradford 2001; Mahamat et al. 2019; Malande et al. 2019). A recent review study (Tanko et al. 2020) on ESBL-producing Gram-negative bacteria in Nigeria similarly reports on the detection of these predominant ESBL genes. The study carried out between January 2004 and November 2019 retrieved 217 articles published within that time period, but only 60 reported on both the phenotypic and genotypic detection of ESBL production in human isolates. More research related to this is therefore essential. Studies exploring this phenomenon will result in more robust local epidemiological data which is key in a proper characterization of contribution of this antimicrobial resistance and hence in proffering a solution.
Considering the pivotal role E. coli plays as a human pathogen and the numerous non-clinical reservoirs associated with it, this study therefore set out to explore the antibiotic resistance and ESBL production of E. coli from various sources in Aba metropolis of Abia State, Nigeria.
Methods
Study area and sample collection
The work was carried out within Aba metropolis in Abia State, Nigeria. Aba is a commercial city which is located at latitude of 5.1216° N and 7.3733° E longitude. Isolates were obtained from various clinical and non-clinical sources including urine, stool, wound, soil, water, abattoir effluents, animal litter and ready-to-eat food. Ethical approval was obtained from the hospital board.
Isolation and identification of Escherichia coli
Clinical isolates were inoculated directly on blood agar and eosin methylene blue agar plates and incubated at 37 °C for 24 h. Non–clinical samples were serially diluted appropriately and inoculated into eosin methylene blue agar and MacConkey agar and incubated at 37 °C for 24 h. They were further sub-cultured and identified phenotypically using standard biochemical methods (Cheesbrough 2000).
Molecular Identification of E. coli
Isolates were further identified using molecular methods adopted from previous studies with slight modifications (Islam et al. 2010; Rahman et al. 2017; Otokunefor et al. 2019a). In brief, the standard boiling method was employed for the isolation of the bacteria DNA. The pure isolates were boiled in 100 µL of molecular grade water for 5 min. This was followed by centrifugation at 10,000g/min for 5 min, and bacterial DNA which was suspended in the supernatant was separated from the sediment and was used as DNA template. E. coli isolates were confirmed by specific 16S rRNA gene fragments detection using the Ec16 primer pair (F 5′-GACCTCGGTTTAGTTCACAGA-3′ and R 5′-CACACGCTGACGCTGACCA-3′). The reaction mixture was prepared by adding 3 µl of the genomic DNA, 10 µl of PCR master mixtures, 1 µl of each of the primers and 5 µl of nuclease-free H2O to adjust the volume to 20 µl. Initial denaturation was done at 95 °C to apply the primers, which was followed by denaturation at 94 °C for 45 s. The primers were annealed at 55 °C for 45 s with extension at 72 °C for 1 min. Final extension was done at 72 °C for 5 min. Thirty cycles were completed for the total reaction. Using 2% agarose gel, the amplified PCR was resolved for 30 min by electrophoresis, and the gel was stained with ethidium bromide and visualized using a UV transilluminator.
Antibiotic susceptibility testing
Antibiotic susceptibility testing of the identified E. coli was carried out by Kirby–Bauer disc diffusion method (Bauer et al. 1966). After standardization of the bacterial isolates to a turbidity level equivalent to 0.5 McFarland standard, Mueller–Hinton agar plates were inoculated with the organisms using sterile swab sticks. Following a 5-min pre-incubation, the test antibiotic multidisc was placed at the center of the petri dish. The plates were inverted and were incubated at 37 °C for 24 h. Susceptibility was then determined by comparing the zones of inhibition against a standard (CLSI 2018).
Additionally, MAR index and multidrug-resistant status of isolates were also determined from the results of the antibiotic susceptibility testing. The MAR index was calculated using the formula a/b, where "a" is the total number of antibiotics to which the organism was resistant and "b" is the total number of antibiotics against which the organisms were tested. The multidrug resistance was defined as resistance to three or more classes of antibiotics.
Phenotypic detection of ESBL
The presence of ESBL was determined phenotypically by the double-disc synergy test. In brief, a standard suspension of the isolate was spread evenly on the surface of Mueller–Hinton agar plates. Discs of cefotaxime, ceftazidime, ceftriaxone and cefpodoxime (30 µg each) were placed at a distance of 15 mm edge to edge from a centrally placed amoxicillin–clavulanate disc containing 20 µg of amoxicillin and 10 µg of clavulanic acid. The plates were incubated at 35 °C for 24 h. The pattern of zone of inhibition was noted. Isolates that exhibited a distinct shape/size (keyhole effect) with potentiation toward amoxicillin + clavulanate disc were confirmed as ESBL producers (CLSI 2018).
Detection of blaTEM, blaSHV and blaCTX-M genes in E. coli
The presence of three beta-lactamase gene genotypes (blaTEM, blaSHV and blaCTX-M) in the E. coli isolates was assessed as previously described (Goudarzi et al. 2013). The primers were amplified with thermal cycling conditions for 5 min at 94 °C and 36 cycles of amplification consisting of 1 min at 94 °C, 1 min at 55 °C and 1 min at 72 °C, with 5 min at 72 °C for the final extension. Analysis of the PCR band was carried out using electrophoresis in a 1% agarose gel at 95 V for 45 min using ethidium bromide under UV irradiation.
Results
From a total of 350 samples collected, 137 were phenotypically identified as Escherichia coli. Of these, only 83 isolates were confirmed to be E. coli using genotypic typing based on the presence of E. coli specific 16S rRNA fragments. Majority (52, 62.7%) of the 83 confirmed E. coli isolates were from non-clinical sources.
An analysis of the antibiotic susceptibility testing showed that in general, isolates exhibited mid-level resistance against the different antibiotics with resistance rates ranging from 22.9% to 53.0% (Fig. 1). For majority of the antibiotics (7/8, 87.5%), a less than 50% resistance was observed. A comparison of resistance exhibited by the different isolates based on source, however, showed clinical isolates exhibited higher levels of resistance than non-clinical isolates against majority (5/8, 62.5%) of antibiotics. Clinical isolates had resistance rates ranging from 16.1% to 61.3% with rates above 50% observed against 50% of antibiotics. The non-clinical isolates on the other hand had rates ranging from 26.9 to 55.8% with rates above 50% observed against only one antibiotic (87.5%).
An assessment of the resistance pattern of individual isolates revealed that 11 of the 83 isolates (13.3%) were fully susceptible to all antibiotics (Table 1). Surprisingly, a higher percentage of clinical isolates (16.1%) were fully susceptible as opposed to the non-clinical isolates (11.5%). A total of 51 antibiogram patterns were detected among the isolates. For the clinical isolates, 20 different patterns were identified, while 37 different patterns were identified from the non-clinical isolates. Only five of these patterns were common to the two groups of isolates.
Isolates in this study showed similar levels of distribution across the different MAR indices (Fig. 2). One difference between clinical and non-clinical isolates was in the representation among the higher MAR indices (0.75–1). A total of 20.4% of isolates were found within this range, but for the non-clinical isolates, this comprised only 13.4%, while for the clinical, it comprised 32.5% of isolates.
Majority of isolates tested were multidrug resistant (55.4%), exhibiting resistance to three or more drug classes (Fig. 3). Similar levels of MDR were observed from both clinical and non-clinical isolates (58.1% and 53.9%, respectively). More of the clinical isolates, however, were resistant to all five classes of antibiotics than the non-clinical isolates (19.4% versus 5.8%).
Phenotypic detection of ESBL showed a low occurrence of ESBL among the test isolates. Only one isolate (1.2%) showed ESBL production as detected by the double-disc synergy test (Fig. 4). This isolate was a clinical rather than a non-clinical isolate (Fig. 5). Genotypic detection of three ESBL-related genes in all the test isolates was similar to the results of the phenotypic detection. None of the three ESBL-related genes was detected among all the test isolates.
Discussion
Escherichia coli have currently been classified as one of the antibiotic-resistant organisms which are of serious clinical concern (Goudarzi et al. 2013). In addition to being one of the two predominant organisms isolated from the clinical microbiology laboratory, these isolates have been readily described in food, water and soil (Haberecht et al. 2019; Sabala et al. 2021). These could therefore serve as reservoirs which encourage the spread of resistance determinants to man. This was buttressed by results of this study reporting resistance rates of 26.9% to 55.8% among non-clinical isolates. Similar moderate rates in non-clinical E. coli isolates have been described by other studies (Haberecht et al. 2019; Sabala et al. 2021; Adelowo et al. 2014; Odonkor and Addo 2018). Though, in some instances, much higher rates of resistance were described either in general or to specific antibiotics (Adelowo et al. 2014; Sarker et al. 2019; Otokunefor et al. 2019b). Some of these were linked with poultry environment and thought to be a reflection of antibiotic use in these environments.
Clinical environments are generally related to higher levels of antibiotic exposure, and this is often thought to lead to the selection of resistant isolates in these environments. Quite often, resistance rates described in clinical environments are relatively high, with rates over 50% observed against a significant number of antibiotics (Kibret and Abera 2011; Igwe et al. 2016; Monira et al. 2017; Tuem et al. 2018; Pormohammad et al. 2019). This similar trend was observed in this present study.
However, unlike some previous reports from within and outside Nigeria which observed MDR rates ranging from 52 to 100% (Igwe et al. 2016; Monira et al. 2017; Makanjuola et al. 2018; Ramírez-Castillo et al. 2018; Onyeadi and Agbagwa 2019), the rate of MDR observed in this study was much lower. It was, however, similar to a 2019 study (Pormohammad et al. 2019) reporting MDR prevalence rates of 22% and 31.3% in E. coli from human and animal sources, respectively.
The low prevalence of ESBL production detected in this study is quite dissimilar to majority of current reports. As ESBL production has been noted to be on the rise worldwide (Lob et al. 2016; Alqasim et al. 2018), recent studies from around the globe report rates ranging from 33 to 91% (Monira et al. 2017; Alqasim et al. 2018; Hassuna et al. 2020; Pandit et al. 2020; Tufa et al. 2020). This could be a reflection of both an increase in awareness and detection practices and a genuine increase in the development of such strains. In Nigeria, rates as low as 24% and as high as 100% have been reported (Igwe et al. 2016; Agbagwa and Aminofifori 2017; Nwafia et al. 2019; Onanuga et al. 2019; Ugwu et al. 2020). A 2020 review on ESBL in Nigeria reported rates ranging from 7.5% to 82.3% with rates as low as 8.1% observed from the same region as this present study (Tanko et al. 2020). Low rates of prevalence have been linked with several regions worldwide, especially the north/eastern European countries. This low prevalence has been thought to be possibly affected by variations in specific ESBL detection methodologies, in addition to low antibiotic use (Sepp et al. 2019).
Quite often, lower ESBL rates have been observed using molecular techniques, as opposed to the phenotypic techniques. A 2019 study reported the detection of ESBL genes in only 94.7% of phenotypically identified ESBL isolates (Sepp et al. 2019). While three main ESBL genes exist, numerous genes have been linked with ESBL production. The inability therefore to detect any of the three main ESBL genes in this study points to the presence of another ESBL gene in the test isolates in this study.
Conclusions
This study reports high levels of antibiotic resistance in both clinical and non-clinical E. coli isolates. Though higher rates of resistance were found among the non-clinical isolates, both group of organisms had similar levels of MDR. Strikingly, however, was the low level of ESBL producers detected in this study and the absence of the three main genotypes associated with ESBL production in this study.
Abbreviations
- ESBL:
-
Extended-spectrum beta-lactamase
- RNA:
-
Ribonucleic acid
- rRNA:
-
Ribosomal RNA
- MDR:
-
Multidrug resistance
- DNA:
-
Deoxyribonucleic acid
- PCR:
-
Polymerase chain reaction
- UV:
-
Ultraviolet
- MAR:
-
Multiple antibiotic resistance
References
Agbagwa OE, Aminofifori J (2017) Extended-spectrum Beta-Lactamase and AmpC Beta-lactamase mediated resistance in Escherichia coli from clinical sources. Am J Microbiol Res 5:107–112
Adelowo OO, Fagade OE, Agerso YY (2014) Antibiotic resistance and resistance genes in Escherichia coli from poultry farms, Southwest Nigeria. J Infect Dev Count 8(9):1103–1112
Alqasim A, Abu Jaffal A, Alyousef AA (2018) Prevalence of multidrug resistance and extended-spectrum β-lactamase carriage of clinical uropathogenic Escherichia coli isolates in Riyadh, Saudi Arabia. Int J Microbiol 2018:3026851
Bauer AW, Kirby WMM, Strerris JC, Turk M (1966) Antibiotic susceptibility testing by a standard single disk method. Am J Clin Pathol 45:493–496
Bradford PA (2001) Extended-spectrum beta-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev 14(4):933–951
Cheesbrough M (2000) District laboratory practice in tropical countries Part II. Cambridge University Press, Cambridge
Clinical and Laboratory Standards Institute. 2018 Performance Standards for Antimicrobial Susceptibility Testing. 20th Information Supplement. CLSI document M100-S20. Clinical and Laboratory Standards Wayne, PA
Denis B, Lafaurie M, Donay JL, Fontaine JP, Oksenhendler E, Raffoux E, Hennequin C, Allez M, Socie G, Maziers N, Porcher R (2015) Prevalence, risk factors, and impact on clinical outcome of extended-spectrum beta-lactamase-producing Escherichia coli bacteraemia: a five-year study. Int J Infect Dis 39:1–6
Goudarzi H, Aghamohammad S, Hashemi A, Nikmanesh B, Noori M (2013) Distribution of blaTEM, blaSHV and blaCTX-M genes among Escherichia coli isolates causing urinary tract infection in children. Arch Clin Infect Dis 8(3):e16207
Haberecht HB, Nealon NJ, Gilliland JR, Holder AV, Runyan C, Oppel RC, Ibrahim HM, Mueller L, Schrupp F, Vilchez S, Antony L (2019) Antimicrobial-resistant Escherichia coli from environmental waters in northern Colorado. J Environ Public Health. 2019:3862949
Hassuna NA, Khairalla AS, Farahat EM, Hammad AM, Abdel-Fattah M (2020) Molecular characterization of Extended-spectrum β lactamase-producing E. coli recovered from community-acquired urinary tract infections in Upper Egypt. Sci Rep 10(1):1–8
Igwe J, Olayinka B, Ehnimidu J, Onaolapo J (2016) Virulent characteristics of multidrug resistant E. coli from Zaria, Nigeria. Clin Microbiol 5:268
Islam MA, Mondol AS, Azmi IJ, de Boer E, Beumer RR, Zwietering MH, Heuvelink AE, Talukder KA (2010) Occurrence and characterization of Shiga-toxin producing Escherichia coli in raw meat, raw milk, and street vended juices in Bangladesh. Foodborne Pathog Dis 7:1381–1385
Kibret M, Abera B (2011) Antimicrobial susceptibility patterns of E. coli from clinical sources in northeast Ethiopia. Afr Health Sci Suppl 1:S40-45
Lob SH, Nicolle LE, Hoban DJ, Kazmierczak KM, Badal RE, Sahm DF (2016) Susceptibility patterns and ESBL rates of Escherichia coli from urinary tract infections in Canada and the United States, SMART 2010–2014. Diagn Microbiol Infect Dis 85(4):459–465
Mahamat OO, Lounnas M, Hide M, Dumont Y, Tidjani A, Kamougam K, Abderrahmane M, Benavides J, Solassol J, Bañuls A, Carrière C (2019) High prevalence and characterization of extended-spectrum ß-lactamase producing Enterobacteriaceae in Chadian hospitals. BMC Infect Dis 19(1):1–7
Makanjuola OB, Fayemiwo SA, Okesola AO, Gbaja A, Ogunleye VA, Kehinde AO, Bakare RA (2018) Pattern of multidrug resistant bacteria associated with intensive care unit infections in Ibadan, Nigeria. Ann Ibadan Postgraduate Med 16(2):162–169
Malande OO, Nuttall J, Pillay V, Bamford C, Eley B (2019) A ten-year review of ESBL and non-ESBL Escherichia coli bloodstream infections among children at a tertiary referral hospital in South Africa. PLoS ONE 14(9):e0222675
Mita Y, Shigemura K, Osawa K, Kitagawa K, Kotaki T, Shirakawa T, Miyara T, Fujisawa M (2019) Clinical risk factors for death caused by extended-spectrum beta-lactamase: producing bacteria. Urol Int 102(2):205–211
Monira S, Shabnam SA, Ali SI, Sadique A, Johura FT, Rahman KZ, Alam NH, Watanabe H, Alam M (2017) Multi-drug resistant pathogenic bacteria in the gut of young children in Bangladesh. Gut Pathog 9(1):1–8
Nivesvivat T, Piyaraj P, Thunyaharn S, Watanaveeradej V, Suwanpakdee D (2018) Clinical epidemiology, risk factors and treatment outcomes of extended-spectrum beta-lactamase producing Enterobacteriaceae bacteremia among children in a Tertiary Care Hospital, Bangkok, Thailand. BMC Res Notes 11(1):624
Nwafia IN, Ohanu ME, Ebede SO, Ozumba UC (2019) Molecular detection and antibiotic resistance pattern of extended-spectrum beta-lactamase producing Escherichia coli in a Tertiary Hospital in Enugu, Nigeria. Ann Clin Microbiol Antimicrob 18(1):41
Odonkor ST, Addo KK (2018) Prevalence of multidrug–resistant Escherichia coli isolated from drinking water sources. Int J Microbiol. https://doi.org/10.1155/2018/724013
Onanuga A, Mahindroo J, Singh S, Taneja N (2019) Phenotypic and molecular characterization of antimicrobial resistant Escherichia coli from urinary tract infections in Port-Harcourt. Nigeria. Pan Afr Med J 34:144
Onyeadi DJ, Agbagwa OE (2019) Plasmid curing in multi-drug resistant hospital and community uropathogenic Escherichia coli. J Appl Sci Environ Manag 23(1):29–34
Otokunefor K, Tamunokuro E, Amadi A (2019a) Molecular detection of mobilised colistin resistance (mcr-1) gene in Escherichia coli isolates from Port Harcourt, Nigeria. J Appl Sci Environ Manag 23:401–405
Otokunefor K, Osogho VO, Nwankwo CP (2019b) Escherichia coli as possible agents of spread of multidrug resistance in Port Harcourt, Rivers State. Ann Sci Technol 4(1):16–21
Pandit R, Awal B, Shrestha SS, Joshi G, Rijal BP, Parajuli NP (2020) Extended-Spectrum β-Lactamase (ESBL) Genotypes among multidrug-resistant uropathogenic Escherichia coli clinical isolates from a teaching hospital of Nepal. Interdiscip Perspect Infect Dis 2020:6525826
Paterson DL, Bonomo RA (2005) Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev 18(4):657–686
Pormohammad A, Nasiri MJ, Azimi T (2019) Prevalence of antibiotic resistance in Escherichia coli strains simultaneously isolated from humans, animals, food, and the environment: A systematic review and meta-analysis. Infect Drug Resist 12:1181–1197
Rahman MA, Rahman AK, Islam MA, Alam MM (2017) Antimicrobial resistance of Escherichia coli isolated from milk, beef and chicken meat in Bangladesh. Bangladesh J Vet Med 15(2):141–146
Ramírez-Castillo FY, Moreno-Flores AC, Avelar-González FJ, Márquez-Díaz F, Harel J, Guerrero-Barrera AL (2018) An evaluation of multidrug-resistant Escherichia coli isolates in urinary tract infections from Aguascalientes, Mexico: cross-sectional study. Ann Clin Microbiol Antimicrob 17(1):34
Sabala RF, Usui M, Tamura Y, Abd-Elghany SM, Sallam KI, Elgazzar MM (2021) Prevalence of colistin-resistant Escherichia coli harbouring mcr-1 in raw beef and ready-to-eat beef products in Egypt. Food Control 119:107436
Sakellariou C, Gürntke S, Steinmetz I, Kohler C, Pfeifer Y, Gastmeier P, Schwab F, Kola A, Deja M, Leistner R (2016) Sepsis caused by extended-spectrum beta-lactamase (ESBL)-positive K. pneumoniae and E. coli: Comparison of severity of sepsis, delay of anti-infective therapy and ESBL genotype. PLoS ONE 11(7):e0158039
Sarker MS, Mannan MS, Ali MY, Bayzid M, Ahad A, Bupasha ZB (2019) Antibiotic resistance of Escherichia coli isolated from broilers sold at live bird markets in Chattogram, Bangladesh. J Adv Vet Animal Res 6(3):272–277
Sepp E, Andreson R, Balode A, Bilozor A, Egorova S, Huik K, Ivanova M, Kaftyreva L, Kõljalg S, Kõressaar T, Makarova M (2019) Phenotypic and molecular epidemiology of ESBL-, AmpC-, and carbapenemase-producing Escherichia coli in Northern and Eastern Europe. Front Microbiol 10:2465
Shaikh S, Fatima J, Shakil S, Rizvi SM, Kamal MA (2015) Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J Biol Sci 22(1):90–101
Tanko N, Bolaji RO, Olayinka AT, Olayinka BO (2020) A systematic review on the prevalence of extended spectrum beta lactamase producing Gram-negative bacteria in Nigeria. J Global Antimicrob Resist 22:488–496
Tuem KB, Gebre AK, Atey TM, Bitew H, Yimer EM, Berhe DF (2018) Drug resistance patterns of Escherichia coli in Ethiopia: a meta-analysis. BioMed Res Int 2018:4536905
Tufa TB, Fuchs A, Tufa TB, Stötter L, Kaasch AJ, Feld T, Häussinger D, Mackenzie CR (2020) High rate of extended-spectrum beta-lactamase-producing Gram-negative infections and associated mortality in Ethiopia: a systematic review and meta-analysis. Antimicrob Resist Infect Control 9(1):1–10
Ugwu MC, Shariff M, Nnajide CM, Beri K, Okezie UM, Iroha IR, Esimone CO (2020) Phenotypic and molecular characterization of β-lactamases among enterobacterial uropathogens in Southeastern Nigeria. Can J Infect Dis Med Microbiol 25:2020
Wilson H, Török ME (2018) Extended-spectrum β-lactamase-producing and carbapenemase-producing Enterobacteriaceae. Microb Genom 4(7):e000197
Acknowledgements
The authors would like to acknowledge the assistance of Dr. B.O. Agaviezor for the molecular aspect of the work.
Funding
This research did not receive any funding; the research was funded by authors.
Author information
Authors and Affiliations
Contributions
OEA, KO and MUA conceptualized the work. MUA carried out most of the phenotypic analysis. OEA and KO carried out the molecular analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Ethical approval was obtained from the hospital board.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ajuga, M.U., Otokunefor, K. & Agbagwa, O.E. Antibiotic resistance and ESBL production in Escherichia coli from various sources in Aba metropolis, Nigeria. Bull Natl Res Cent 45, 173 (2021). https://doi.org/10.1186/s42269-021-00628-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-021-00628-5