Abstract
Background
Malaria is one of the tropical diseases of universal concern particularly with continuous appearance of resistant strains of P.falciparum. This calls for continous screening of traditional plants such that new and effective antimalarial agents will be developed. This study therefore explored the oral acute toxicity and antimalarial potentials of aqueous and methanolic extracts of roots, leaves and stem of Dictyandra arborescens on Plasmodium berghei infected mice.
Results
No mortality was recorded in any of the experimental animal groups even at the highest tested dose (5000 mg/kg b.wt) of the extract after monitoring them for 4hrs and subsequently for 7 days. Out of the six extracts, methanolic extracts of the roots and leaves exhibited more antimalarial activity than others. A significant difference (P < 0.05) was statistically observed in the parasite count of groups that received methanol extracts of roots and leaves of D. arborescens. This observation was made when these two extracts were compared with other groups as well as the negative control. However, activity of the standard antimalarial drug (artesunate) was higher (p˂0.05) than those of the extracts. Phytochemicals such as tannins, alkaloids, saponins, terpenoids, flavonoids etc. were present in the extracts in varying quantities. GC–MS analysis of methanol extract of the root of this plant showed different chemical compounds.
Conclusion
Administration of aqueous and methanol extracts of roots, leaves and stem of D. arborescens in mice is not harmful at any dose less than or equal to 5000 mg/kg. Methanol extracts exhibited more antimalarial activity than aqueous extracts suggesting that antimalarial activity of the plant parts could be affected by the solvent used for extraction and antimalarial activity may be more in a particular part of a plant. The presence of different bioactive compounds identified in phytochemical and GC–MS analysis could be the fundamental scientific evidence for the antimalarial activity exhibited by this plant especially in the root.
Similar content being viewed by others
Background
Malaria is one of the oldest diseases of man which causes more sufferings and deaths when compared to other infectious diseases. It is more prevalent in developing countries and is more severe in expectant mothers and young children (WHO 2017). Various studies reported that the disease causing agent transmitted by mosquitoes is a unicellular parasite called Plasmodium whose infective sporozoite stage is carried in the mosquito’s salivary glands (Kulihya et al. 2016, Udoh et al. 2016). There are four species of this disease causing agent in humans namely Plasmodium vivax, Plasmodium malariae, Plasmodium ovale and Plasmodium falciparum. Most incidence of malaria in the tropical regions are due to Plasmodium falciparum (Udoh et al. 2016). This specie is equally accountable for most fatal and severe cases of malaria in Africa (Ani 2004). Malaria has been implicated as a major cause of loss of many children per annum (Okafor and Oko-Ose 2012). A report by the Nigerian malaria fact sheet (2011) and World Health Organization (WHO 2016) stated that approximately 97% of Nigerians live in high malaria transmission areas, while the remaining few live in low malaria transmission areas. This makes her the highest bearer of the disease burden in the African region (WHO 2016). This high prevalence has been attributed to rainfall, numerous stagnant water, warm temperature, type and distribution of vegetation cover, poor sanitary conditions etc. which favour the breeding and multiplication of mosquitoes (Ukaegbu et al. 2014). Yet, (Patz and Olson 2006) and (Stresmann 2010) have projected an increase in this figure as a result of climate change. The disease is not only detrimental to the health and lives of Africans, and Nigerians in particular, it equally drains the economy. This is due to reduction in manpower as well as cost of treatment and high death rates related to the disease, which has made malaria a major setback in African development (Ekpenyong and Eyo 2008).
Presently treatment of this disease still relies on the administration of drugs whose effectiveness is always countered by the presence of stubborn strains of the parasite, high cost of orthodox drugs and unavailability of these drugs. Therefore, many caregivers have resorted to using available native plants in the management and treatment of malaria. Medicinal plants which are sources of many potent drugs have been continuously used by various countries. Researchers have developed great interest in screening medicinal plants for their therapeutic activities. These therapeutic activities are conferred on these plants by the active principles of many secondary metabolites found in these plants. Medicinal plants and herbs are used in treating many ailments and infections (Shrivastava et al. 2014; Tomar et al. 2014). Consequently, there is increased interest in research on traditional medicinal and aromatic plants because plant-derived drugs are safe without side effects (Ogidi et al. 2019).
Despite increased threat of malaria to life, particularly in Africa, there is still hope in curbing this disease (White et al. 2004). Such approaches include scientific investigation of traditionally used medicinal plants and herbs (Wright 2005). Dictyandra arborescens is a member of flowering plants called the rubiaceae. Rubiaceae derived its name from the madder genus Rubia. Other plants in this family include cinchona, gambier, ixora, nauclaceae, and theligonaceae. About 630 genera and more than 13,000 species make up the rubiaceae. This positioned rubiaceae as one of the six largest families of angiosperms. Species in this family are usually found in warm and tropical climates (Dalziel 1997). Members of this family are mainly shrubs, although trees and herbs are also present. Although plants in the rubiaceae family are pharmacologically important, very little is known about the medicinal properties of Dictyandra arborescens especially its toxic and antimalarial activities. The aim of this study is therefore to evaluate the acute toxicity and in vivo antimalarial potentials of aqueous and methanolic extracts of roots, leaves and stem of Dictyandra arborescens (a traditional plant used for the treatment of malaria in Ahiazu Mbaise L.G.A, Imo State, South-East, Nigeria) (Figs. 1, 2).
Methods
Gathering and identification of samples
Fresh roots, leaves and stems of Dictyandra arborescens were collected from a fallow farmland at Ahiazu Mbaise L.G.A. Samples were identified by a taxonomist-Dr. M.C Duru in the Department of Biological Sciences, Federal University of Technology, Owerri, Nigeria. They were sliced into pieces, dried at room temperature and ground using a laboratory milling machine. The coarse powder obtained was used to prepare the extracts.
Preparation of extracts
Exactly 200 g of the dry powdered plant samples was soaked in 1000 ml of distilled water (cold maceration) at room temperature with frequent shaking for fourty-eight (48) hours (Ene et al. 2013). Methanol extract was obtained as described by Dejen et al. (2018). Exactly 200 g of the plant parts were extracted with 1000 ml of 80%v/v methanol solvent using soxhlet extraction. Filtrates were obtained using a piece of clean muslin cloth, then with whatman number 1 filter paper. Extracts were dried using a rotary evaporator (RE- 52A, Searchtech Instruments) set at a temperature of 50 °C. Subsequently, extracts were reconstituted in distilled water to obtain the required volume given to the experimental animals.
Experimental animals
Only male Swiss albino mice, weighing 18–25 g (7 to 8 weeks old), were used in this study; they were obtained from animal house of Veterinary medicine, University of Nigeria, Nsukka and transferred to animal house of the Department of Biochemistry, University of Nigeria, Nsukka, where the study was carried out. These animals were acclamatized for 14 days with free access to feed (rat pellets) and water ad libitum and monitored under 12: 12hour, light:dark cycles, in well aerated cages. After acclamatization, the experimental animals were then separated into their respective groups according to their body weights and the cages were randomly placed in the room before treatment commenced. The cages were then randomized within the treatment group.
For antimalarial test, animals were included in the study if the percentage parasitemia before treatment was up to 60%. Animals were excluded if their percentage parasitemia before treatment was below 60%. Animals whose body weights were below 18 g or above 25 g were also excluded from the present study. This study was conducted after obtaining ethical approval from the Animal Research and Ethics committee of the Department of Veterinary Medicine, University of Nigeria, Nsukka, Enugu State, Nigeria. A written consent was obtained whereby all the participants/authors filled and signed an informed consent form. No reference number was issued.
Phytochemical screening
Crude aqueous and methanolic extracts of roots, leaves and stem of D. arborescens were screened for phytochemicals using standard methods (Trease and Evans 1989, Harborne 1973, 1998, Sofowora 1993, Raboy et al. 2000, Kgosana 2019) in order to identify biological components.
Oral Acute toxicity evaluation
Oral acute toxicity (LD50) of roots, leaves and stem of Dictyandra arborescens was determined using modified method of Lorke (1983) based on the description of Khan et al. (2015). This method involved two (2) phases with a total of 108 male Swiss albino mice. In the first phase, for each of the six extracts, nine male Swiss albino mice were randomly divided into 3 groups of 3 mice each (n = 3) and increasing doses of 10, 100 and 1000 mg/kg body weight of the extracts were administered orally to groups 1, 2 and 3 respectively. These were observed for the first 4 h and subsequently for 7 days for any signs of toxicity and mortality. These signs include pains, loss of appetite, difficulty in respiration, paw licking, weakness and death. In the second phase (which was carried out when no death was recorded in the first phase), for each of the six (6) extracts, high doses of 1600, 2900 and 5000 mg/kg body weight of the extracts were administered to another 3 random groups of 3 (n = 3) fresh male Swiss albino mice through the same route. They were observed for 4 h for signs of toxicity and mortality and subsequently for 7 days as in the first phase. This test was used to determine the safe dose of the extract that was administered to the experimental animals during the in vivo antimalarial test. Acute toxicity was calculated using the formula:
where, D0 = Highest dose that gave no mortality, D100 = Lowest dose that produced mortality
Animal grouping and administration of extracts and standard antimalarial drug for antimalarial test
Fifty-four (54) male Swiss albino mice weighing 18–25 g (7–8 weeks old) used for the study were sourced from animal house of Veterinary Medicine, University of Nigeria, Nsukka. The mice were accommodated in well aerated aluminum cages and acclimatized to laboratory conditions for a 2–week period under naturally illuminated environment with regular feeding (rat pellets) and water ad libitum, and monitored under 12:12 h dark/light cycle. These animals were used according to NIH guide for caring and usage of laboratory animals (NIH 1985) Standard operating manual of NIPRD. They were separated into 9 groups of 6 mice each (n = 6) according to their body weights. The sample size (n = 6) was selected based on information extracted from literature. A small sample size was selected because antimalarial potentials of aqueous and methanol extracts of D. arborescens roots, stem and leaves were tested in vivo for the first time in the present study. Therefore, the initial intention was to gather basic evidence regarding the use of these extracts in the treatment of malaria.
Therefore, the initial intention was to gather basic evidence regarding the use of these extracts in the treatment of malaria. On the basis of their position on the rack, the cages were given numerical designation. For each treatment group, a cage was selected randomly from the pool of cages. The cages were then randomized within the treatment group.
Grouping of animals were as follows:
Group 1—feed + water (normal control).
Group 2—infected with P.berghi + 200 mg/kg b.w of artesunate (standard control group).
Group 3—infected with P.berghei + no treatment (negative control).
Group 4—infected with P.berghei + 200 mg/kg b.w methanolic extract of D. arborescens root.
Group 5—infected with P.berghei + 200 mg/kg b.w methanolic extract of D. arborescens leaves.
Group 6—infected with P.berghei + 200 mg/kg b.w methanolic extract of D. arborescens stem.
Group 7—infected with P.berghei + 200 mg/kg b.w aqueous extract of D. arborescens root.
Group 8—infected with P.berghei + 200 mg/kg b.w aqueous extract of D. arborescens leaves.
Group 9—infected with P.berghei + 200 mg/kg b.w aqueous extract of D. arborescens stem.
In vivo culture of Plasmodium berghei
Plasmodium berghei infected blood from a donor mice was used to infect the animals used for experiment. Parasitized blood was first diluted with normal saline as described by Kabiru et al. (2012). This innoculum was prepared such that each 0.2 ml contained approximately 1 × 107 parasitized erythrocytes. The animals were then injected with 0.2 ml of this suspension on day 0 through intraperitoneal route and were allowed to stand for seventy-two hours (72 h) so that infection will be fully established. P. berghei was maintained by weekly serial blood passage from infected animals to non infected ones.
In vivo treatment of infected animals
A 4-day curative test described by David et al. (2004), Peter and Anatoli (1998) was used for the in vivo anitimalarial test. Only animals whose percentage parasitemia was up to 60% or more were included in the treatment groups. Animals whose percentage parasitemia was below 60% were excluded in the treatment groups. Treatment commenced 72 h (day 3) later, after establishment of infection. All animals in the treatment groups orally received 200 mg/kg body weight of a known antimalarial drug (artesunate) and 200 mg/kg body weight of extracts once a day for four consecutive days. A dose of 200 mg/kg body weight was administered based on earlier results from acute toxicity test which was up to 5000 mg/kg body weight for the aqueous and methanol extracts of the roots, leaves and stem of D. arborescens. Treatment was administered based on the average body weight of experimental animals.
Parasite count was observed in all the groups on day 7 (4 days after treatment) and on day 14 (7 days post treatment) as described by Arrey et al. (2014) using blood smears made from a cut on the tail tip of each mice. Blood smears were prepared by fixing with methanol, stained with 10% Geimsa for 25 min, washed with phosphate buffer (pH 7.2) and dried. Parasitemia was examined microscopically (magnification = X100) under a drop of immersion oil. Percentage parasitemia was calculated as outlined by Iwalewa et al. (1997):
Final body weight and percentage survival rates were also monitored after treatment with the extracts.
Euthanization of animals
At the end of the experiment, animals were euthanized as described by Moody et al. (2014). The animals were placed in a transparent perspex chamber and euthanized with slow rising concentrations of carbon dioxide using a gradual fill (30% chamber volume per minute) technique. Animals were monitored until they lost consciousness.
In this experiment, three different investigators were involved as follows: the first investigator administered the treatment based on the randomization table. This investigator was the only person aware of the treatment allocation. A second investigator unaware of the treatment was responsible for checking the toxicity and parasitemia level, while a third investigator was responsible for the euthanization procedure.
Gas chromatography-mass spectrometry (GC–MS) analysis of methanol extract of D. arborescens roots
The sample was analyzed as described by Pakkirisamy et al. (2017) using agilent technologies 7890A GC and 5977B MSD with experimental conditions of GC–MS system as follows: Hp 5-MS capillary standard non-polar column, dimension: 30 M, ID: 0.25 mm, Film thickness: 0.25 μm. Flow rate of mobile phase (carrier gas—Helium) was set at 1.0 ml/min. in the gas chromatography part, temperature programme (oven temperature) was 400C raised to 250 °C at 50C/min and injection volume was 1 μl. Samples dissolved in methanol were run fully scan at a range of 40–650 m/z and the results were compared and interpreted using National Institute Standard and Technology (NIST) Mass Spectral library database search programme with over 62,000 patterns for identifying chemical components.
Statistical analysis
Data obtained were presented as mean of three replicates ± standard error of mean (SEM). Analysis was done using one-way analysis of Variance (ANOVA) procedure using statistical analysis system (SAS) package version 20 software (Betty and Stern 2003). Mean differences were subjected to Duncan’s multiple range test (DMRT) and significance level was set at P < 0.05.
Results
Yield of extracts
Exactly 200 g of each plant part (roots, leaves and stem) of D. arborescens were used for extraction but different yield of extract was obtained from the two solvents. As seen on Table 1, the highest yield (24.9%) of extract was obtained from methanolic extract of D. arborescens leaves, followed by aqueous extract of the same plant part whose yield was 21.3%. The least yield (8.1%) was obtained from aqueous extract of the stem of this plant. Comparatively, the yield was higher in the methanolic extracts than in the aqueous extracts.
Acute toxicity test
No death was recorded in the first phase of the experiment where doses of 10, 100 and 1000 mg/kg body weight of the extracts were administered to the experimental animals for the different extracts (Tables 2). In the second phase, where high doses of the extracts (1600, 2900, and 5000 mg/kg body weight) were administered to the experimental animals, no sign of toxicity or death was also recorded in the albino mice for aqueous and methanolic extracts of roots, leaves and stem of Dictyandra arborescens. This indicated that these extracts were safe, even at a very high dose of 5000 mg/kg body weight (Table 3).
Effects of extracts on established infection
Aqueous and methanolic extracts of roots, leaves and stem of D. arborescens exhibited a significant (P < 0.05) decrease in parasitemia across the treatment groups unlike the infected and untreated group (group 3) where there was consistent increase in parasitemia. Table 4 showed the effects of crude aqueous and methanolic extracts of the roots, leaves and stem of D. arborescens on parasite count of Plasmodium berghi infected albino mice. Parasitemia was established in the groups infected with P. berghei after 72 h. Parasite count/percentage parasitemia in the group that received no treatment (Group 3) significantly (P < 0.05) increased on day 7 while it significantly (P < 0.05) reduced in all groups that received treatment. The experimental animals were further monitored till the 14th day (7 days post treatment); results obtained revealed that parasite count/percentage parasitemia in all treated animals further reduced significantly (p˂0.05) but increased in the negative control group (Tables 4 and 5). When compared with the known antimalarial drug (artesunate), results showed that the plant extracts recorded a significant difference (P < 0.05). Unlike the extracts, the standard antimalarial drug (artesunate) cleared all the parasites by the 14th day. The significant (P < 0.05) decrease in parasite count was more evident in groups 4 and 5 which received methanol extract of D. arborescens root and methanol extract of D. arborescens leaves, respectively. Increase in body weight as well as higher survival rate were also observed in animals across the experimental groups except the infected but untreated group (group 3) whose body weight and survival rate decreased (Table 6).
Discussion
Aqueous (water) and methanol were used as extracting solvents in this study is in line with folklore practice. Extraction yield (% w/w) measures the efficiency of a solvent to extract particular components from original plant materials. It refers to the amount of solid extract recovered in mass compared to the initial amount of plant material. Percentage yield of extract from methanol solvent was more than that of the aqueous solvent (Table 1). This result agrees with report by Wilcox et al. (2014) that phytochemicals are more soluble in organic solvents. Polarity of the various extracted compounds may be responsible for the difference in yield of extracts (Pareck et al. 2015). Low polarity of methanol could be accountable for the high yield of extracts because both polar and non polar compounds were extracted by the solvent. Methanol extracted more of the phytochemicals than the aqueous solvent implying that crude extracts obtained from D. arborescens could be affected by solvents used for extraction. This is in agreement with (Peace et al. 2011), who reported that aqueous methanol gave more yield of extractable solids in barley and flax seed respectively. Findings in this study suggested that most of the compounds in this plant are dissolveable in methanol and a mixture of 20% (v/v) water with methanol was a good choice for obtaining better extractable solids from this plant. Maceration used in this study has been reported to give low yield of extract when compared with other methods of extraction (Peace et al. 2011). It was selected as an extraction method in this study because heating is not required, thus preserving the phyto-constituents.
Africa is gifted with a myriad of medicinal plants. Indigenous people acquire this knowledge, preserve it and pass to their generations. Pharmacological activities of medicinal plants are attributed to their phytochemical contents (Ihekwereme et al. 2016). Results obtained in this study showed that D. arborescens is rich in various phytochemicals (Table 8). Tannins are major active components of medicinal plants (Haslam 2016). Tannins have antioxidant, anti-inflammatory, antibacterial, antiviral, antimicrobial, antimalarial properties (Vattern et al. 2015 and Mori et al. 2017). Terpenoids have biological activities and are used in fighting diseases such as malaria, inflammation, bacterial infections, cancer etc. (Ibrahim et al. 2005, Abdul-Fadl et al. 2011; Sunita et al. 2017). Alkaloids are significant biologically active compounds found in plants. The presence of this class of compounds brings about certain physiological changes in organisms such as antimalarial, antifungal, antibacterial (Stary 1998). Flavonoids have been reported to have significant in vitro antimalarial activity against P. falciparum (Chanphen et al. 2018). They also have effects against free radical, microbes, and tumour (Farquar 1996). Cardiac glycosides were present in the aqueous and methanol extracts of D. arborescens. This class of phytochemicals are referred to as ‘natural drugs’ because of their actions in the treatment of heart related problems (Radford et al. 2016). Activities of phenols also include antioxidant, anti-inflammatory, and antimalarial activities (Ovenden et al. 2011; Han et al. 2017). Steroids which were also present in the plant extracts have been reported to have anti-inflammation effects (Savithramma and Linga 2011). Saponins have anti-hyper cholesterol and haemolytic effects (Sodipo et al. 2010). Some medicinal herbs with high oxalate contents are used in the treatment of bronchitis, ringworm, skin diseases etc. (Huang and Liebman 2015). Consumption of phytate prevents formation of kidney stone, offers protection against atherosclerosis, cancer etc. (Navarro et al. 2016; Zheng et al. 2014). Since many of these phytochemicals have antimalarial potentials, their synergistic effect could have been responsible for the antimalarial properties exhibited by this plant.
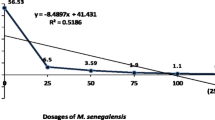
Results showed that aqueous and methanolic extracts of D. arborescens roots, leaves and stem have negligible toxicity as recorded in their LD50 value of 5000 mg/kg b.wt. These extracts did not produce any physical signs of toxicity such as paw licking, salivation, stretching, weakness or death. Their LD50 was thus estimated to be ≥ 5000 mg kg−1 because no mortality was recorded even at a high dose of 5000 mg kg−1 body weight. Absence of mortality following oral administration of extracts at 5000 mg kg-1 body weight observed in the experimental animals indicated that the extracts were not toxic acutely. These results are in consonance with the reports of Onwusonye et al. (2014) and Ma et al. (2006) who reported no mortality in Swiss albino mice treated with 10 to 5000 mg kg−1 body weights of leaf extract of Aspilia africana (Pers), methanol leaf extract of Annona senegalensis and hydroethanolic stem extract of Baphia pubescens respectively. Testing for antimalarial properties of extracts from a particular plant is a preliminary stage in isolating novel compounds with potent activities (Ma et al. 2006; Njoroge and Bussmann 2006). Crude aqueous and methanolic extracts of roots, leaves and stem of D. arborescens showed significant antimalarial activities against P. berghei infection in male Swiss albino mice which was evident in the reduction of parasite count and decrease in percentage parasitemia (Tables 4 and 5). Results from this study clearly showed that treatment with aqueous and methanol root, leaf and stem extracts of D. arborecsens, significantly (P < 0.05) reduced parasite count in infected mice and improved their survival and body weight. The differences between the antimalarial activities of methanol extracts of the roots and leaves of D. arborescens differed significantly (p˂0.05) from their aqueous extracts (Table 2). This gave an indication that the bioactive constituents of this plant were more soluble in methanol than the aqueous medium (Peace et al. 2011). Water is denser than methanol which may diffuse more in the same medium than water. Another possibility is that the bioactive compounds were more soluble in methanol than water which gave methanol extracts the benefit of having more of the bioactive compounds. This may be accountable for the higher antimalarial activity recorded for methanol extracts against the aqueous extracts. This corroborates the report of Ezeokeke et al. (2015). However, activity of the standard antimalarial drug (artesunate) was higher (P < 0.05) than activities of the extracts.
The antimalarial activities of D. arborescens could be credited to the synergy between many phytochemicals present in the extracts as seen in Tables 7 and 8. Although the exact phytochemical responsible for the antimalarial activity is not known, studies have shown that tannins, saponins, flavonoids, alkaloids etc. inhibit malaria parasites in infected animals (Igile et al. 1994; Udensi et al. 2002). Saponins have also been reported to have antiprotozoal activity (Wallace et al. 1994; Newbold et al. 1997).
Aqueous and methanol extracts of the stem of D. arborescens exhibited the least antimalarial activities on Plasmodium berghei infected mice. This could be attributed to the fact that the stem of this plant is not woody. This corroborates earlier reports by Mathaura et al. (2017), that plants whose stem extracts have significant antimalarial activities are trees with stem bark. Survival rate of mice treated with the various extracts supported the prolonged effects of the antimalarial compounds in these two plants. Although aqueous and methanol extracts of the stem exhibited post-treatment antimalarial activities, they had the least weight gain and survival rate. This may be attributed to the type of antimalarial compound present in the stems and their quantities. These results are in line with the report of Soniran et al. (2011), who evaluated in vivo antiplasmodial activities of extracts Morinda morindiodes (Bak.) in the treatment of malaria. The decrease in body weight observed in animals in the infected and untreated animals was attributed to parasite feeding on blood cells of the animals which reduced their body weight. This corroborates reports of Ene et al. (2013). Comparatively, survival rate was higher in animals in the treatment groups than the infected and untreated group, suggesting that extracts used in this study were not toxic to the animals at the administered doses (Table 6). Observed deaths may be due to effects of the parasites. This agreed with the report of Adeyemo-Salami et al. 2014.
Several chemical compounds were identified in the GC–MS analysis of methanol extract of D. arborescens root as seen in Table 9. 1-Octadecene has been reported to have therapeutic activities such as antioxidant, antibacterial (Mishra and Sree 2007). n-Hexadecanoic acid has anti-inflammatory, antioxidant, mosquito larvicide, nematicide effects (Rahuman et al. 2000; Aparna et al. 2012). The identified 9,17 Octadecadienal, (Z)-has antimicrobial activity (Rajeswari et al. 2013) while 6-Octadecenoic acid methyl ester (Z)- has been reported to have anti-inflammatory, antioxidant, anticancer activities (Geetha and Varalakshmi 2001). Hexadecanoic acid is a methyl ester with antioxidant, antimicrobial, hypocholesterolemic, nematicide, pesticide, antiandrogenic, insecticide effects (Akpuaka et al. 2013). Heptadecanoic acid, 16 methyl-, methyl ester has activities such as Protein, anticancer (Elaiyaraja and Chandramohan 2018). Bis (2-ethylhexyl) phthalate has been reported to have antimicrobial, antifungal (Habib and Karim 2009), and antitumor potentials (Habib and Karim 2012). Therefore, the occurrence of these compounds in D. arborescens root could be responsible for the afore-mentioned activity exhibited by this plant.
Conclusion
Results obtained in this study revealed that aqueous and methanol extracts of roots, leaves and stem of D. arborescens were not harmful even at a high dose of 5000 mg/kg b.wt. The extracts equally posses antimalarial activities. This justifies the belief and use of this plant in ethnomedicine for the treatment of malaria. The presence of different bioactive compounds identified in phytochemical and GC–MS analysis could be the fundamental scientific evidence for the antimalarial activity exhibited by this plant especially in the root.
Dictyandra arborescens could therefore represent a prospective source of antimalarial drug development. Since many existing antimalarial drugs are natural product-based, there is hope that perharps, new antimalarial drugs may emerge from our tropical plants if properly harnessed. Findings in this study therefore justified the rationale behind the pharmacological usage of this plant in traditional setting especially in the treatment of malaria.
Availability of data and materials
All the data generated or analyzed during the study are included in this published article.
Abbreviations
- mg/kg:
-
Milligram/kilogram
- b/wt:
-
Body weight
- LD :
-
Lethal dose
- NIH:
-
National institute of health
- P.berghei :
-
Plasmodium berghei
- GC–MS:
-
Gas chromatography-mass spectrometry
References
Adeyemo- Salami OA, Farombi EO, Ademowo OG (2014) An inestigation into the antimalarial effect of methanolic extract of Paullinia pinnata leaves in Plasmodium berghei infected mice and course of infection. Afr J Med Med Sci 43(1):93–100
Abul-Fadl MM, El-Badry N, Ammar MS (2011) Nutritional and chemical evaluation for two different varieties of mustard seeds. World Appl Sci J 15(19):1225–1233
Akpuaka A, Ekwenchi MM, Dashak DA, Dilda A (2013) Biological activities of characterized isolates of n-hexane extract of Azadiracter indica A.Juss (Neem) leaves. Natl Sci 11(5):141–147
Ani OC (2004) Endemicity of malaria among primary school children in Ebonyi state Nigeria. Anim Res Int 1(3):155–159
Aparna V, Dileep KV, Mandal PK, Karthe P, Sadasivan C, Haridas MC (2012) Anti-inflammatory and antioxidant properties of n-hexadecanoic acid: structural evidence and Kinetic assessment. Chem Biol Dug Des 80:434–439
Arrey TP, Okalebo FA, Ayong LS, Agbor GA, Guantai AN (2014) Antimalarial activity of a polyherbal product (Nefang) during early and established Plasmodium infection in rodent models. Malarial J 13:456
Betty K, Stern J (2003) Essentials of medical statistics, 2nd edn. Blackwell Science Ltd., Cambridge
Chanphen R, Thebtaranonth Y, Wanaupathamkul S, Yuthavong YJ (2018) Antimalarial principles from Artemisia indica. J Nat Prod 61:1146–1147
Dalziel IM (1997) The useful plants of West Tropical Africa, 2nd edn. Cress, London, p 196
David AF, Philip JR, Simon RC, Solomon N (2004) Antimalarial drug discovery: efficacy models for compound screening. Nature Revolution 3:509–520
Dejen N, Assefa S, Teshome N, Engidawork E (2018) In vivo antimalarial activity of the 80% methanolic root bark extract and solvent fractions of Gardenia ternifolia Schumac. & Thonn. (Rubiaceae) against Plasmodium berghei. Evid Based Complem Altern Med 11:1–10
Ekpenyong EA, Eyo JE (2008) Malaria control and treatment strategies among school children in semi- urban tropical communitie. West Indian Med J 57(5):456–461
Ejikeme CM, Ezeonu CS, Eboatu AN (2014) Determination of physical and phytochemical constituents of some tropical timbres indigenous to Niger Delta Area of Nigeria. Eur Sci J 10(18):247–270
Elaiyaraja A, Chandramohan C (2018) Comparative phytochemical profile of Crinum defixum Ker Gawler leaves using GC-MS. J Drug Deliv Therapeut 8(4):365–380
Ene AC, Obika CJ, Okwu GN, Alisi CS, Edeh NG (2013) In vivo anti-malarial effect of methanol and aqueous extracts of Picralima nitida plant parts. J Res Biochem 1(2):095–105
Ezeokeke EE, Ene AC, Igwe CU (2015) In Vivo Anti-Plasmodial effect of ethanol and aqueous extracts of Alchornea cordifolia. Biochem Anal Biochem 4(4):091–100
Ezeonu CS, Ejikeme CM (2016) Qualitative and quantitative determination of phytochemical contents of indigenous nigerian softwoods. New J Sci 9:9–20
Farquar JN (1996) Plant Sterols, their biological effects in human Handbook of Lipids in Nutrition. CRC Press, Boca Rotan, pp 101–105
Geetha T, Varalakshmi P (2001) Anti-inflammatory and antioxidant activities of lupeol and lupeol linoleate in rats. J Ethnopharmacol 76(1):77–80
Habib MR, Karim MR (2009) Antimicrobial and cytotoxic activity of Bis(2-ethylhexyl) phthalate. Mycobiology 6(3):21–29
Habib MR, Karim MR (2012) Antitumour evaluation of Di (2-ethylhexyl) phthalate (DEHP) isolated from Calotropis gigantean L. flower. Acta Pharmaceutica 62(4):607–615
Han X, Shen T, Lou H (2017) Dietary polyphenols and their Biological significance. Int J Mol Sci 8(9):950–988
Harborne JB (1973) Phytochemical methods: a guide to modern techniques on plant analysis. Chapman and Hall, London, pp 279–281
Harborne JB (1998) Phytochemical Methods, A guide to modern Techniques of plant analysis, 136–140.
Haslam E (2016) Ntural polyphenols (vegetable tannins) as drugs: possible modes of action. J Nat Prod 59(2):205–215
Huang J, Liebman M (2015) Oxalate contents of commonly used Chinese medicinal herbs. J Tradit Chin Med 33(5):594–599
Ibrahim H, Bolaji RO, Abdulrahman EM, Ilyas N, Habib AG (2005) Preliminary phytochemical and antimicrobial studies of the leaves of Carrissa edulis Vahl. Chem J 3:15–18
Igile GO, Oleszek W, Jurzysta M, Burda S, Fafunso F, Fasanmade AA (1994) Flavonoids from Vernonia amygdalina and their antioxidant activities. J Agric Food Chem 42(11):2445–2448
Ihekwereme CP, Agbata CA, Chukwueze KO (2016) In vivo evaluation of antiplasmodial activity of hydroethanolic stem extract of Baphia pubescens in Plasmodium berghei infected albino mice. J Herbmed Pharmacol 5(4):149–152
Iwalewa EO, Lege-Oguntuge L, Rai PP, Iyaniwura TT (1997) In vivo and in vitro anti malarial activity of two crude extracts of Cassia occidentalis leaf Nigerian. J Pharmaceut Sci 5:23–28
Kabiru YA, Okolie NL, Ogbadoyi MHL, EO, (2012) Preliminary studies on the antiplasmodial potential of aqueous and methanol extracts of Eucalyptus camadulensis leaf. Asian Pac J Trop Dis 2:S809–S814
Kgosana KG (2019) The effects of extraction techniques and quantitaive determination of oxalates in Nerium oleander and feeds. Onderstepoort J Vet Res 86(1):1611–1624
Khan ME, Amupitan JO, Oyewale AO, Ndukwe IG (2015) Evaluation of the in vivo anti malarial activity of the methanolic leaf extract of Nepata cateria. Res Pharmaceut Biotechnol 6(2):8–15
Kunihya IZ, Samaila AB, Pukuma MS, Qadeer MA (2016) Prevalence of malaria infection and malaria Anaemia among children attending federal medical centre Yola, Adamawa State, Nigeria. Int J Eng Sci 5(7):8–14
Lorke D (1983) A new approach to practical acute toxicity testing. Arch Toxicol 54:275–287
Ma C, Zhang HJ, Tan GT (2006) Antimalarial compounds from Grewia bilamellata. J Nat Prod 69(3):346–350
Mishra PM, Sree A (2007) Antibacterial activity and GC-MS analysis of the extract of leaves of Finlaysonia obovata (A mangrove plant). Asian J Plant Sci 6(1):168–172
Moody CM, Chua B, Weary DM (2014) The effect of carbon dioxide flow rate on the euthanasia of laboratory mice . Laboratory Animals 48(4): DOI: https://doi.org/10.1177/0023677214546509
Mori A, Nishino C, Enoki N, Tawata S (2017) Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry 26(8):2231–2234
Muthaura CN, Rukunga GM, Chhabra SC, Omar SA, Guantaiu AN, Hathirwa JW, Tola FM, Mwitari PG, Keter LK, Kirira PG, Kimani CW, Munga GW, Njagi ENM (2017) Antimalarial activity of some plants traditionally used in treatment of malaria in kwale district of Kenya. J Ethnopharmacol 112(3):545–551
Navarro S, Neuhouser ML, Chen DT, Tinker LF, Shinkany JM, Snetselaa L (2016) The interaction between dietary fibre and fat and risk of colorectal cancer in women’s health initiative. Nutrients 30(12):8–22
NIH (1985) Guide for the care and use of laboratory animals (Revised). NIH Publication, Bethesda, pp 15–23
Newbold CJ, El Hassan SM, Wang J, Ortega ME, Wallace RJ (1997) Influence of foliage from African multipurpose trees on activity of rumen protozoa and bacteria. Br J Nutr 78:237–249
Njoroge GN, Bussmann RW (2006) Diversity and utilization of antimalarial ethnophytotherapeutic remedies among the Kikuyus (Central Kenya). J Ethnobiol Ethnomed 2(8):112–118
Ogidi IO, Omu O, Ezeagba PA (2019) Ethnopharmacologically active components of Brassica juncea (Brown mustard) seeds. Int J Pharmaceut Res Dev 1(1):09–13
Okafor FU, Oko-Ose JN (2012) Prevalence of malaria infections among children aged six months to eleven years (6 months-11 years) in Benin City, Nigeria. Glob Adv Res J Med Med Sci 1(10):273–279
Onwusonye JC, Uwakwe AA, Iwuanyanwu P, Iheagwam U (2014) Oral acte toxicity (LD50) study of methanol extract of Annona senegalensis leaf in albino mice. Sky J Biochem Res 3(5):046–048
Osagie AU (1998) Antinutritional factors in nutritional quality of plant foods. Ambik Press Ltd Benin City, Nigeria, pp 1–40
Ovenden SP, Cobbe M, Kissell R, Birrell GW, Chavchich M, Edstein MD (2011) Phenolic glycosides with antimalarial activity from Grevillea poorinda Queen. J Nat Prod 28(1):74–78
Owiredu WKBA, Teye EK, Quaye L (2013) Proficiency testing of total serum cholesterol assay. J Med Biomed Sci 2(1):22–24
Pakkirisamy M, Kalakandan SK, Ravichandran K (2017) Phytochemical screening, GC-MS, FT-IR Analysis of Methanolic Extract of Curcuma caesia (Black turmeric). Pharmacognos J 9(6):952–956
Pareck J, Jadeja D, Chanda S (2015) Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turkey J Biol 29:203–210
Patz AJ, Olson HS (2006) Malaria risk and temperature: influence from global climate change and local land use practices. Proc Natl Acad Sci 103:5635–5638
Peace U, Ekaete A, Chinweizu EU, Ruth M (2011) Antifungal activity of aqueous and ethanolic extracts of Picralima nitida seed on Aspergillus flavus, Candida albicans and Microsporum canis. Res Pharm Biotech 3(5):57–60
Peter LT, Anatoli VK (1998) The current global malarial situation. Malarial parasite biology, pathogenesis and protection. ASM Press, Washington DC, pp 11–22
Raboy V, Gerbasi KA, Young KA, Stoneberg SD, Pickett SG, Bauman AT, Murthy PPN, SheridanWF EDS (2000) Origin and seed phenotype of maize low phytic acid 1–1 and low phytic acid 2–1. Plant Physiol 124:366–368
Radford DJ, Gillies AD, Hinds JA (2016) Naturally occurring cardiac glycosides. Med J Aust 144:540–544
Rahuman AA, Gopalakrishan G, Ghouse BS, Arumugan S, Himalayan B (2000) Effects of Feronia limonia on mosquito larvae. Fitoterapia 71:553–559
Rajeswari G, Murugan M, Mohan VR (2013) GC-MS analysis of bioactive components of Hugonia mystax L. (Linaceae). Res J Pharmaceut Biol Chem Sci 29(29):818–824
Savithramma N, Linga LM (2011) Screening of medicinal plants for secondary metabolites. Middle-East J Sci Res 8(3):579–584
Shrivastava VR, Tomar S, Mishra RK, Jyoti A, Kaushik S (2014) Medicinal potential of some mythologically important plants of India: a review. Int J Multidiscipl Curr Res 2(1):99–103
Sodipo OA, Akiniyi JA, Ogunbamosu JU (2010) Studies on certain characteristics of extracts of bark of Pausinystalia macroceras (K. Schemp) Pierre Exbeille. Glob J Pure Appl Sci 6:83–87
Sofowora A (1993) Medicinal plants and traditional medicines in Africa. Willey, New York, p 256
Soniran T, Idowu OA, Idowu AB (2011) Evaluation of in vivo antiplasmodial activities of extracts of Morinda morindiodes (Bak) in the treatment of malaria. Int J Biomed Health Sci 6(4):122–127
Stary F (1998) The natural guide to medicinal herbs and plants. Tiger Books International, London, pp 12–16
Stresman GH (2010) Beyond temperature and precipitation: ecological risk factors that modify malaria transmission. Acta Trop 116:167–172
Sunita S, Das SS, Singh G, Marina P, Carola S, Cesar CA (2017) Comparison of chemical composition, antioxidant and antimicrobial potentials of essential oils and Oleoresins obtained from seeds of Brassica juncea and Sinapis Alba. Medcrave Online J Food Process Technol 4(4):113–120
Tomar RS, Shrivastava V, Kaushik S (2014) In vitro efficacy of methanolic extract of Mimosa pudica against selected microorganisms for its broad spectrum antimicrobial activity. Int J Curr Microbiol App Sci 3(4):780–784
Trease GE, Evans WC (1989) Pharmacognosy, 13th edn. Bailliere Tindall Ltd, London, pp 132–133
Udensi E, Ijeh I, Ogbonna U (2002) Effect of traditional processing on the phytochemical and nutrient composition of some local Nigerian leafy vegetables. J Sci Technol 8:37–40
Udoh SB, Hamidu IM, Saleh AH (2016) Seasonal prevalence of malaria parasites infection in Maiduguri, Borno State, North East Nigeria. Scholar J Biol Sci 5(1):52–55
Ukaegbu CO, Nnachi AU, Mawak JD, Igwe CC (2014) Incidence of concurrent malaria and typhoid fever infections in febrile patients in jos, Plateau State Nigeria. Int J Sci Technol Res 3(4):157–161
Unuofin JO, Otunola GA, Afulayan JA (2017) Phytochemical screening and in-vitro evaluation of antioxidant and antimicrobial activities of Kedrostis africana (L) Cogn. Asian Pac J Trop Biomed 7(10):901–908
Uyotismita K, Arindan R (2015) Determination of tannin content by titrimetric method from different types of tea. J Chem Pharm Res 7(6):238–241
Vattern DA, Ghaedian R, Shetty K (2015) Benefits of berries through phenolics antioxidant enrichment focus on cranberry. Asia Pac J Clin Nutr 14(2):120–130
Wallace RJ, Arthaud L, Newbold CJ (1994) Influence of Yucca shidigera extract on riminal ammonia concentrations and ruminal microorganisms. Appl Environ Microbiol 60:1762–1767
White N, Nosten F, Bjorkman A, Marsh K, Snow RW (2004) WHO, the global fund and medical practice in malaria treatment. Lancet 563:1160
Willcox M, Graz B, Falquet J, Diakite C, Giani S, Diallo DA (2014) “Reverse pharmacology” approach for developing an anti-malarial phytomedicine. Malar J 10(1):8–12
World Health Organization (2016) Malaria Vaccine Development. www.who.int/malaria/ areas/vaccine/en/ Accessed 30th May 2018)
World Health Organization (2017) Severe falciparum malaria. Communicable Disease Cluster. Transaction Royal Society of Tropical Medicine and Hygiene, 94, 190.
Wright F (2005) Traditional antimalarials and the development of novel antimalarial drugs. J Ethnopharmacol 100:67–71
Zheng H, Lazarova DL, Bordonaro M (2014) Mechanism linking dietary fibre, gut microbiota and colon cancer prevention. World J Gastrointest Oncol 6(2):41–51
Acknowledgments
Authors are grateful to Dr. M.C Duru of the Department of Biological Sciences, Federal University of Technology, Owerri, Mr Rex Ezeh of Department of Biochemistry, University of Nigeria, Nsukka, Engr. Clement Obot of New Concepts Laboratories Ltd, Owerri, Nigeria, as well as staff of ChemSolvers Research and Computational Laboratories, Owerri, Nigeria for their technical assistance.
Funding
Authors received no funding for this study.
Author information
Authors and Affiliations
Contributions
Author UEE collected the plant samples, carried out the research work and wrote the manuscript, Author NCU, ICM, and HCN designed and supervised the laboratory work, Author CKE analyzed the data, Author CED interpreted the GC–MS result while Author OIO helped in the literature search. All authors read and approved the manuscript and gave permission to submit the manuscript for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted after obtaining ethical approval from the Animal Research and Ethics committee of the Department of Veterinary Medicine, University of Nigeria, Nsukka, Enugu State, Nigeria. A written consent was obtained whereby all the participants/authors filled and signed an informed consent form. No reference number was issued.
Competing interests
The authors declare that they have no competing interests.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Enenebeaku, U.E., Ukwandu, N.C., Mgbemena, I.C. et al. Oral acute toxicity and antimalarial potentials of aqueous and methanolic extracts of roots, leaves and stem of Dictyandra arborescens (Welw.) on Plasmodium berghei infected mice. Bull Natl Res Cent 45, 75 (2021). https://doi.org/10.1186/s42269-021-00530-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-021-00530-0