Abstract
Background
Ecological risk assessment of the levels of polycyclic aromatic hydrocarbons in soils of the abandoned sections of Orji Mechanic Village, Owerri, Imo State, Nigeria. The main objective of the study was to determine the levels of soil contamination from polycyclic aromatic compounds (PAHs) due to anthropogenic events in the mechanic village before reclamation.
Results
Analysis of soil samples revealed mean PAH levels ranging from 1.22E−02 ± 0.00 to 5.60E−02 ± 0.01 mg/kg, with higher PAH concentrations observed at 0–10 cm compared to other depths. The sum of PAHs (∑PAHs) ranged from 1.58E−01 to 6.71E−01 mg/kg, as the sum of low molecular weight PAHs (∑LPAHs) varied from 7.16E−03 to 1–1.60E−01 mg/kg, while the sum of high molecular weight PAHs (∑HPAHs) ranged from 1.81E−02 to 5.42E−01 mg/kg. The sum of carcinogenic PAHs (∑Carcino-PAHs) varied from 5.39E−01 to 9.74E−02 mg/kg, indicating elevated levels of carcinogens in the sampled soils. Diagnostic ratio, principal component, and correlation analysis of the results revealed that pyrogenic sources were primarily responsible for the observed PAHs owing to the activities of automobile mechanics in the Orji Mechanic Village. Benzo(a)pyrene equivalent (BePeq) of the PAHs indicated high carcinogenic potency for sample A. The total cancer risk was above 1 × 10–6, indicating a greater risk of cancer due to contact with the contaminated soil samples; hence, the result of this study is a serious ecological concern.
Conclusion
There may be accumulation of these contaminants in the investigated area, which will eventually contaminate the groundwater as well as surface water resources. Remedial measures to prevent possible health problems due to the PAHs which results from the activities of automobile mechanics are therefore recommended.
Similar content being viewed by others
Background
One of the most remarkable ecological contaminants considered nearly as extensive as crude oil pollution in most parts of Nigeria today is the unguarded disposal of waste or used engine oil in the surroundings (Odjegba and Sadiq 2002; Emoyan et al. 2020). Petroleum products like gasoline, lubricating oil, diesel, and others used frequently in most automobile workshops may result in extensive and inadvertent spills of these products in the surroundings (Kidman and Boehlecke 2011; Muze et al. 2020). This may therefore lead to contamination of the surroundings of most mechanic workshops by petroleum hydrocarbons as well as polycyclic aromatic hydrocarbons. Some mechanics in automobile workshops discard waste engine oils resulting from automobile maintenance services within the surrounding environment (Ololade 2014). Besides indiscriminate spilling of used engine oil around most automobile workshops, additional contamination may be due to mishandling of hydrocarbon products. This often happens when used engine oil is being removed from vehicle engines during servicing and some other repair works (Sharifi et al. 2007).
The Orji Mechanic Village, Owerri, accommodated hundreds of mechanic and automobile workshops scattered over the entire area before their eviction by the Imo State Government, Nigeria, in 2017. However, some of the automobile mechanics and other artisans are still in some parts of the area to date. The mechanics and other artisans engaged in automobile repairs frequently dispose of or unintentionally discharged waste engine oils, lubricants, as well as organic solvents which may enrich their workshops and surroundings with petroleum hydrocarbon compounds (Obini et al. 2013). It has been reported that waste oils and organic solvents used in most workshops constitute a major part of the hazardous contaminants usually released from automobile mechanic workshops in most Nigerian cities (Iwegbue 2007; Ipeaiyeda and Dawodu 2008). Spent or waste engine oil contains a combination of diverse chemicals which include petroleum hydrocarbon compounds, chlorodibenzofurans, chlorinated biphenyls, additives, decomposed products, and metallic elements due to wearing of engine parts (ATSDR 1997; Wang et al. 2018).
Spent engine oils migrate with ease into the soil, and filtration of leachates from materials contained in waste engine oil may pose serious threats to groundwater quality and the ecosystem (Olugboji and Ogunwole 2008). The significance of polycyclic aromatic hydrocarbons (PAHs) in the ecosystem is gradually attracting global interest, more especially as soil constitutes an essential part of the surroundings (Wcisło 1998; Adeniyi and Afolabi 2002; Dong and Lee 2009; Kwon and Choi 2014).
It is a well-documented fact that PAHs ranked 7th in 2005, in the biennial ranking of chemicals deemed harmful which may pose the greatest possible risk to human health (Christopher 2008). The activities of most automobile mechanic workshops therefore pose a serious threat to man and his environment (Duru et al., 2019; Ali et al. 2017). This could be due to the co-occurrence of hydrocarbons and toxic heavy metals in most automechanic contaminated sites (Duru et al., 2017; Muze et al. 2020).
Previous publications have reported the presence of PAHs in mechanic villages and automobile workshops (Ogoko 2014; Ekanem et al. 2019; Muze et al. 2020). The six USEPA target PAHs including phenanthrene, fluorene, benzo[α]anthracene, benzo[β]fluoranthene, and benzo[γ]fluoranthene, with concentrations ranging from 0.0184 ± 0.02 to 0.1385 ± 0.2 mg/kg were observed in Abakaliki automechanic village (Obini et al. 2013). Result of PAHs and TPH ranging from 6.30 to 7.40 mg/kg and 5120.50 to 24,902.23 mg/kg, respectively, with elevated Pb, Hg, and Cd levels has also been reported (Ogoko 2014). A related study noted that the major source of the PAHs was due to pyrogenic sources resulting from vehicular emissions, fossil fuel combustion, as well as incineration of diesel oil, wood, and coal (Emoyan et al. 2020). The effects of the activities of automobile mechanics in the Niger Delta area of Nigeria revealed elevated levels of Pb, Cd, TPH, and some PAHs (Muze et al.2020). Also, the total PAH concentrations ranging from 0.47 to 14.85 mg/kg in soil samples collected from some automobile workshops in Akwa Ibom State, Nigeria, have been reported (Ekanem et al. 2019).
The need for this study became necessary owning to the paucity of PAH data in the study area. There are few documented studies on soil of this area, and these studies reported heavy metals on the surface soil and in groundwater sources (Duru et al. 2017; Duru 2019). There was no reference to other contaminants such as PAHs, considered in the present study. Also, recycling of electronic waste was a serious business in the area before the automobile mechanics were evacuated. Often the dismantled components are burnt, which could lead to severe contamination of the surrounding air, surface water, groundwater, soil, and river sediments by organic contaminants, such as PAHs, polychlorinated biphenyls (PCBs), and polybrominated diphenyls ethers (PBDEs) (Yu et al. 2006; Li et al. 2007; Opara et al. 2016; Njoku et al. 2016). Besides, there is an urgent need to regularly assess the level of contaminants in the area due to the activities of the automobile mechanics and other artisans in the area. Long-term exposure to these pollutants could lead to severe problems such as brain dysfunction, cancer, tumors, nervous system, and reproductive disorders in humans and animals (Obidike et al. 2007; Kinawy 2009).
The ecological as well as human health risks of PAHs are attracting serious concern due to their carcinogenic, mutagenic, as well as its persistent characteristics (Peng et al. 2011; Abdel-Shafy and Mansour 2016; Jia et al. 2017; Lasota and Błońska 2018; Cao et al. 2019). The importance of this study arises from the urgency to regularly monitor PAH levels in work environments like the mechanic villages and automobile workshops, where it is believed that the mechanics come in contact with hydrocarbon products as well as accidental spilling of these products in the environment.
In 2017, the State Government ordered the automechanics and others involved in automobile repairs within the Orji Mechanic Village to vacate the area after almost thirty years of occupation of this area. Also, government agencies have started erecting buildings within the environs of the evacuated portions of the mechanic workshops with the intention of setting up schools and other residential houses in the area.
The dearth of data is on the impact of the activities of automobile mechanic workshops in this area, before the intended actions of the State Government call for serious concern. Therefore, there is exigent need to carry out assessment of the ecological impact of the activities of auto mechanics in the mechanic village after their exit from this area. This move is very urgent, most especially now that the area is being conceived to become a residential and primary school environment. Therefore, the present study is aimed at assessing the levels of polycyclic aromatic hydrocarbons (PAHs) from the soils of the evacuated sections of Orji Mechanic Village, Owerri, Southeastern, Nigeria.
Methods
Study location
The reclaimed Orji automechanic village is in Owerri North Local Government Area, which is among the Local Government Areas, that makeup the capital of Imo State. The area lies within longitude 7° 3′ 50" and latitude 5° 31′ 39", at about 125.8 m above sea level, covering an area of about 0.41 km2. Activities in the reclaimed automechanic village commenced in 1987, with over 300 occupants, including automechanics and other artisans engaged in automobile repairs and maintenance.
Collection of soil samples
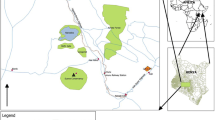
Soil samples were taken from the evacuated sections of the mechanic village to have an overview of the level of contamination by PAHs resulting from the activities of automechanics in the study area before reclamation. Soil samples were collected from two other locations (more than 1 km away) considered free of activities that could introduce hydrocarbon contaminants in the surroundings to serve as control. The samples were collected at three distinct depths: 0–10 cm, 10–20 cm, and 20–30 cm represented by 1, 2, and 3 for samples A–E and 1.1–2.3 for the control samples (CN). All the samples were collected using stainless steel auger. Duplicate soil samples were collected from each point and homogenized to form representative samples. Aluminum foil was used to wrap the soil samples and labeled as A1, A2, A3, B1, B2, B3, C1, C2, C3, D1, D2, D3, E1, E2, E3, CN1.1, CN1.2, CN1.3, CN2.1, CN2.2, and CN2.3. A total of 21 soil samples were collected in October 2019, and sample points were referenced using Garmin GPSmap 76CSx as indicated in Fig. 1.
Preparation of soil sample for PAH extraction and separation
Irrelevant materials were separated from the soil samples and then air-dried at room temperature in an environment devoid of contaminants. Pulverization of the soil samples was achieved with mortar and pestle made of porcelain and further sieved with 2-mm mesh. The level of PAHs contained in the soil samples was extracted according to standard procedures as described by previous publications (Paíga et al. 2012; Jiao et al. 2017; Ekanem et al. 2019). In other to remove moisture in the soil samples, 10 g of each sample was weighed and mixed with another 10 g of dried sodium sulfate. The blend was mixed with surrogate standards containing 1.0 mL of 1-chloro-octadecane, 2-fluorobiphenyl, and 2,4,6-triborophenol and extracted with a mixture (1:1) of n-hexane and dichloromethane (v/v) in a Soxhlet extractor for 17 h. A rotary evaporator was used to concentrate the extract to 25 mL.
Purification of the extract was achieved by passing it through a 10-mm internal diameter and 250-mm-long chromatographic column packed with glass wool. Two grams of activated silica slurry in 10 mL dichloromethane was added into the column and little sodium sulfate placed on top of the column and then rinsed with more 10 mL of dichloromethane. In total, 20 mL of hexane was used to previously elute the column and allowed to drift through the column until it is above the sodium sulfate layer. Subsequently, 1 mL of the extracted sample was introduced into the column from the extraction vessel and rinsed again with 1 mL hexane poured into the column. A 10-mL cylinder was used to collect the eluates when the stop cork was opened. About 1–2 mL of more hexane was gradually added into the column until the sodium sulfate layer was exposed to air as the fractions exited the column. The collected eluates were concentrated using a rotary evaporator for PAH determination (USEPA 1996a).
Determination of PAHs
The PAHs were determined in the extracts using gas chromatography (GC) with mass spectrometer detector according to USEPA (United State Environmental Protection Agency) method (USEPA 1996b; Sadler and Connell 2003; Okparanma and Mouazen 2013; Hu et al. 2014). The PAHs contained in the extracts were quantified and identified by a Hewlett Packard (HP) 6890 GC with HP 5MS. The instrument has a HP 5973 mass selective detector (MSD) and a fused silica capillary column of film thickness 0.25 μm, 30 m and internal diameter of 0.25 mm. The sample was introduced into the instrument by splitless injection of 1μL of the extract observing 5-min solvent delay, maintaining the initial column temperature of 50 °C for 4 min. Then, the temperature was increased to 320 °C at a rate of 8 °C/min and held for 3 min, maintaining a temperature of 230 °C and 250 °C, respectively, for injector and interface. Helium gas was used as the carrier gas at a flow rate of 1 mL/min. Quantification of the PAH levels in the sample was achieved by the internal calibration method using a five-point calibration curve of the individual constituents.
Quality control
High-quality PAH standards (> 97% purity) were sourced from Sigma-Aldrich Chemie GmbH, Steinheim, Germany, through Fin lab, Owerri. High-purity n-hexane, dichloromethane, and analytical grade anhydrous sodium sulfate (dried at 430 °C for 8 h using DHG–9023A, B. Brans Scientific and Instrument Company, England) were purchased from Chemisciences Nig. Ltd, Owerri. Double-distilled water was produced with Eco-Still Mark, BSIC/ECO-4 (Bhanu Scientific Instruments Company, India). The glassware and sample containers were rinsed and dried at 430 °C for 4 h in DHG–9023A (B. Brans Scientific and Instrument Company, England). The soil samples and other reagents were weighed with Shimadzu AW 320 analytical balance (Shimadzu, Japan). The extracts were concentrated using a rotary evaporator (RE201) (Yamato Scientific, Japan). Hewlett Packard (HP) 6890 GC (Agilent technologies, USA) used for the determination of the PAH content of the samples was installed with chemstation software for integration of the chromatogram.
Laboratory quality assurance was maintained through standard laboratory procedures to ensure the integrity of the analytical results. The laboratory instruments used for the analysis were properly calibrated before being used for the determinations. Accuracy and precision of the analysis were ensured through duplicate analysis of the samples against standard reference materials and blanks. The quality control of each sample type contains over 9.8% of the recovery matrix spikes with a relative deviation matrix spike replicate ranging from 0.0 to 26.9%. The limit of detection (LOD) was determined using a relative value of three times the standard deviation of five duplicate determinations. The LOD of the PAHs ranged from 2.0 \(\times\) 10–6–3.4 \(\times\) 10–5 2.0 × 10–6–3.4 × 10–5 mg/kg. The chromatographic instrument used for the analysis has a high instrumental detection limit with a minimum detection limit of 0.04 μg/L. Its accuracy is over 99.0% with a precision of less than 8.0% (RSD).
Data analysis
The mean and the standard deviation of the analytical data were computed using Microsoft Excel 2010. One-way analysis of variance (ANOVA), Pearson correlation, and principal component analysis (PCA) were carried out with IBM SPSS version 20.0. One-way ANOVA at p < 0.05 was conducted in order to establish the level of variation in the measured PAH concentrations in the soil samples. Pearson correlation and PCA were used to further determine the similarities or the dissimilarities in the source of occurrence of the PAHs. The level of variation within each sample point was determined using Eq. 1. Variation was categorized according to previous publications (Njoku et al. 2016; Opara et al. 2016). Health risk assessment was also carried out to determine the tendency of cancer risk due to the PAHs. Furthermore, the diagnostic ratio of the PAHs was determined to characterize the source of contamination.
where CV is the coefficient of variation, STD is the standard deviation, while \(\stackrel{-}{x}\) is the mean value.
Risk assessment
Carcinogenic potency of the study area
The PAHs are considered among the severe environmental contaminants and have been classified into two groups of carcinogenic compounds. According to the International Agency for Research on Cancer (IARC), the PAHs could be likely human carcinogens (2A) and a potential source of human carcinogens (2B) (IARC 2002). Therefore, the carcinogenic effect linked to contact with PAH may be estimated by determining the benzo(a) pyrene equivalent (BePeq) of the individual PAH using Eq. 2.
where BePeq is benzo(a) pyrene equivalent. TEQF is the toxic equivalent factor of the individual PAH, while PAHc is the concentration of the PAHs (mg/kg) (Tsai et al. 2004; Hu et al. 2014).
Cancer health risk assessment
The tendency that residence of a contaminated environment could be predisposed to cancer risk when soil particles from such environments are ingested accidentally has been reported in scholarly publications (Man et al 2013; Hu et al. 2014; Onyedikachi et al. 2019; Cao et al. 2019). The possibility of being affected by cancer, resulting from unintended contact with carcinogenic or mutagenic substances, could be explained using cancer health risk assessment (Li et al. 2014). Risk of unset of cancer due to accidental or unintended intake of soil particles could be through inhalation, dermal absorption, and ingestion (Chiang et al. 2009; Tarafdar and Sinha 2017). Health risk assessment of the frequency, intensity, and overall level of exposure as well extent of interaction with materials containing PAHs in the study area was established according to USEPA (1991). The potential risk of cancer resulting from exposure to PAHs in the investigated soil samples was determined using the incremental lifetime cancer risk (ILCR) method as described by previous publications (Hu et al. 2014; Man et al 2013; Ali et al. 2017; Tarafdar and Sinha 2017; Onyedikachi et al. 2019), according to Eqs. 3–5.
where BaPeq is the benzo (a) pyrene equivalent of the PAHs (mg/kg), RI is the rate of unintentional ingestion of soil particles (mg/day), FE is the frequency of exposure (day/year), DE means the duration of exposure (year), BW is the average body weight of the exposed individual (kg), AT indicates the averaged time of exposure (day), and OSF means the oral slope factor of the PAHs (mg/kg day)−1
where ESA implies the exposed skin area to soil particles (cm2/day), SAF is the skin adherence factor of the soil particles (mg/cm2); DAF denotes the dermal absorption factor, CF means the conversion factor, GIAF refers to the gastrointestinal absorption factor (mg/kg day)−1
where ET refers to time of exposure (h/day), IUR indicates the inhalation unit risk (mg/m3)−1, AT implies the averaging time (h); PEF is the particle emission factor (m3/kg). The values of the parameters stated in Eqs. 3–5 are presented in Table 1.
Total carcinogenic risk
The total carcinogenic risk for the inhabitants was estimated by the addition of the various pathways of exposure to PAHs according to Eq. 6 (Chiang et al. 2009; Tarafdar and Sinha 2017). Carcinogenic value > 10−6 is considered undesirable (Wang et al. 2011; Li et al. 2014).
Results
Characteristics of PAH concentrations and analysis
The result of PAH concentrations recorded in the study is shown in Table 2. A total of 14 PAHs were observed for the points sampled, with mean concentrations ranging from 1.22E−02 ± 0.00 mg/kg at sample point E to 5.60E−02 ± 0.01 mg/kg at sample point A (Table 1). In terms of depth, the PAH concentrations generally decreased with increasing depth (Fig. 2). It was observed that higher mean concentrations of the PAHs were recorded at 0–10 cm compared to other depths (Fig. 2). The mean concentration of PAHs observed at 0–10 cm is as follows; 9.56E−02 ± 0.05, 3.61E−02 ± 0.01, 9.61E−02 ± 0.03, 5.20E−02 ± 0.04, 3.38E−02 ± 0.02, 2.19E−02 ± 0.01, and 6.53E−02 ± 0.03 mg/kg, respectively, for A1, B1, C1, D1, E1, CN1.1, and CN2.1 as presented in Fig. 2. Individually, more concentrations of some PAHs were recorded in the sample points than others as presented in Fig. 3. Elevated Dbh levels were recorded in almost all the sample points as indicated in Fig. 3. Similarly, in terms of occurrence, it was observed that detection frequency varied from 1% for Ant, Apt, and Pyr to 53% for Dbh, also suggesting higher detection frequency for Dbh (Fig. 4).
Table 3 shows the PAH (mg/kg dm) concentrations associated with the study area. The PAH concentrations showed trends from little variation (CV % < 20) to high variation (CV % > 50). The coefficient of variation is in the decreasing order CN2 > E > C > D > CN1 > B > A. Also, one-way analysis of variance revealed significant variation (p < 0.05) in the PAH concentrations recorded in this study. Significant variations (p < 0.05) in the concentrations of PAHs were observed between different sample points as well as different depths. The diagnostic ratio analysis result of this study is presented in Table 4. This revealed that diagnostic ratio values ranged from 0.08 for Bbf/Bkf to 4.85 for Fla/Pyr. The PCA was used to evaluate how the PAHs are grouped, which revealed the extent of variation in the source of the PAHs. Table 5 is the result of PCA showing the component matrix, initial eigenvalues, % cumulative, and the % variance, while Fig. 5 is the pictographic representation of the PCA results.
The three principal components (PC) having initial eigenvalues > 1.0 were rotated, which revealed that PC1 accounted for 36.560% of the variation, and 26.332% of the variations were due to PC2, while 22.457% of the variations resulted from PC3. The rotated component matrix, therefore, indicated that the majority of the PAHs are from similar sources of contamination as suggested in Fig. 5.
To further establish that the PAH concentrations recorded in the study were primarily due to anthropogenic influence resulting from the activities of automobile mechanics in the investigated mechanic village, correlation analysis was conducted. Result of correlation analysis of the PAHs at a significant level of 5% is presented in Table 6. The result revealed mostly positive correlation among the PAHs as shown in Table 6.
The carcinogenic potency was estimated as the BaPeq (mg/kg) of the PAHs according to Eq. 2, and the results are presented in Table 7. The result indicated total BaPeq (mg/kg) values ranging from 0.0775 for sample E to 0.453 for sample A.
The result of carcinogenic risk analysis which was determined according to Eqs. 3–5 is shown in Table 8.
Discussion
The observed mean concentration of PAHs at 0–10 cm is in the order C1 > A1 > CN2 > D1 > B1 > E1 > CN1. This suggests that elevated mean PAH levels were recorded at C1 and A1 as well as CN1.1, indicating the presence of increased PAH levels even in some unexpected areas like the control. Increased PAH levels in such areas like the control could be associated with so many factors. PAHs are among the pollutants that are considered ubiquitous in the environment (Tobiszewski and Namiesnik 2012). They could be emitted from processes that occur naturally such as volcanic eruptions, biomass combustion, and diagenetic processes (Wang et al. 2011). Atmospheric deposition could contribute to high PAH concentrations in areas considered unpolluted by virtue of the economic and other anthropogenic activities in such areas (Marr et al. 2006; Tian et al. 2009; Opara et al. 2016; Njoku et al. 2016; Ibe et al. 2020). In addition, the low elevation of the area (control) as well as high permeability and porosity of the Benin Formation may have contributed to the increased concentration of PAHs (Onyeagocha 1980; Ibe et al. 2020; Ejiogu et al. 2019). Also, the elevated levels of PAHs in the control may be linked to flooding in the area due to the high intensity of rain events which causes water overflow and heavy flooding resulting in substantial leaching and eroding of topsoil particles containing deposited or accumulated PAHs (Chukwuocha et al. 2017; Enyoh and Isiuku 2020). This results in eventual distribution of the PAHs to the floodplains and other areas having low elevation. The above-stated factors may have influenced the PAH levels observed at the control.
The concentrations of Dbh and other PAHs were found to decrease with an increase in depth. Higher mean Dbh levels were obtained in the study compared to other PAHs. The mean PAH levels observed in the study is in the order; Dbh > Indp > Aph > Apt > Flu > Phe > Fla > Ban > Bbf > Chy > Nap > Bkf > Ant > Pyr. This suggests that a higher mean concentration of Dbh was recorded in the investigated area.
The observed increased detection frequency of Dbh may be associated with its relatively high molar mass (278.35 g/mol) and fairly low water solubility of 5.00E−4 mg/L (Hwang et al. 2003). A detection frequency of 38% was reported for Dbh in soils of Taizhou (Hu et al. 2014). The elevated mean concentration of Dbh ranging from 0.045 to 0.514 mg/kg has been reported in soils collected around a chemical plant in Shanxi, China (Jiao et al. 2017). Also, high Dbh levels were reported in soils and sediments in a related study in India (Tarafdar and Sinha 2017). However, Phe (26%), Nap (24%), and Ant (17%) were the PAHs with greater abundance in soils of the Central Himalaya as reported by a previous study (Bi et al. 2016).
The sum of PAH concentrations (∑PAHs) is shown in Table 3. The results show that ∑PAHs ranged from 1.58E−01 to 6.71E−01 mg/kg, indicating that elevated ∑PAH value (6.71E−01 mg/kg) was recorded at point A, possibly as a result of anthropogenic influence from the automobile mechanics in the study area. Sum of low molecular weight PAHs (∑LPAHs) varied from 7.16E to 03 mg/kg at CN1–1.60E−01 mg/kg at point C which suggested that elevated low molecular weight PAHs (2–3 rings) were recorded at sample point C. It was observed that the sum of concentrations of high molecular weight (∑HPAHs) ranged from 1.81E to 02 mg/kg at control point one (CN1) to 5.42E−01 mg/kg at point A. This also revealed that elevated concentrations of 4–6 rings PAHs were recorded at sample point A. Similarly, a high concentration (1.74E−01 mg/kg) of combustible PAHs (∑Comb-PAHs) which include Fla, Pyr, Ban, Chy, BkF, BbF, and Indp was recorded at sample point A. At the same point A, it was observed that the sum of concentrations of carcinogenic PAHs (∑Carc-PAHs) including Ban, Chy, BkF, BbF, Dbh, and Indp varied from 5.39E−01 to 9.74E−02 mg/kg (Table 3). The reported PAH concentrations may be associated with the activities of automobile mechanics in the study area. Reports have shown that indiscriminate spilling of petroleum products around automechanic workshops due to automobile repairs contributes to increased PAH levels in such environment (Farombi et al. 2013; Obini et al. 2013; Nwoko et al. 2017).
The mean PAH concentrations of the present study compared to related studies in Nigeria revealed that higher PAH values were reported by Obini et al. (2013), Ogoko (2014), and Ekanem et al. (2019), while comparable values were reported by Muze et al. (2020). The concentration of PAHs reported in the present study was lower than 1000 µg/kg recommended for soil cleanup by the Department of Petroleum resources, Nigeria (DPR 2002). Similarly, PAH values in the present study were within the allowed limits of 1000 µg/kg, 1500 µg/kg, and 5 mg/kg stipulated guidelines for soil cleanup by Denmark, Netherlands, and Australia, respectively (ANZECC 2000; MHSPEN 2000; DEPA 2002).
Characterization of the source of PAHs in soil samples
Diagnostic ratio of the PAH concentrations
The use of diagnostic ratios in the analysis of PAH concentrations to reveal intra-source variation as well as similarities in the polluting sources has been documented (Galarneau 2008; Jiao et al. 2017). It is widely applied to establish anthropogenic influence in the levels of PAHs in the environment (Yunker et al. 2002; Suman et al. 2016). There is a little discrepancy associated with the application of diagnostic ratio in PAH profile analysis due to some uncertainties (Muze et al. 2020). However, the distinction between emissions due to gasoline and diesel combustion has been established via PAH diagnostic ratio (Ravindra et al. 2008). Environmental contamination resulting from diverse products from crude oil processing as well as biomass combustion events such as grass, savanna, and bush fires has been distinguished with PAH diagnostic ratios (Yunker et al. 2002). It is a very useful technique used in PAH emission source identification in different environments (Manoli et al.2004; Tobiszewski and Namiesnik 2012; Onyedikachi et al. 2019).
According to Table 4, the low molecular weight PAH compounds (LPAHs) containing 2–3 ring PAHs are possibly due to natural processes or petrogenic origin, while the HPAHs (high molecular weight PAH compounds) which contain 4–6 rings PAH usually result from pyrogenic sources (Mastral and Callen 2000). The HPAHs constitute about 73.50% of the overall PAH concentrations in the soil investigated. The ratio (∑LPAHs)/(∑HPAHs) was all less than < 1, suggesting that the PAHs were of pyrogenic origin. Also, the ratio of the sum of combustible PAHs to the sum of total PAHs (∑Comb)/(∑PAHs) was all less than one, except in sample D that was 3.76, which further indicates that fossil fuel combustion was the major source of the observed PAH levels in the soils studied (Crnković et al. 2007). It was observed that the ratio of Phe/Ant was all less than ten (< 10), suggesting pyrogenic sources. This also indicates that the PAH concentrations result from the use of hydrocarbon products in the study location, which arises primarily from the activities of automechanics in the mechanic village. Similarly, the ratio of Fla/Pyr revealed values that were all greater than one (> 1) in all the samples, which suggests that the PAHs result from the combustion of hydrocarbon products (Essumang et al. 2011). However, Fla/(Fla + Pyr) ratio indicated that both petrogenic and pyrogenic sources as well as combustion of grass, wood, and coal may have contributed to the level of these PAHs (De La Torre-Roche et al. 2009). Also, the results of the ratio of Ban/(Ban + Chy) suggest that petrogenic sources and vehicular emissions contributed to the levels of PAHs in the investigated mechanic village (Pies et al. 2008). In addition, the ratio of Ant/(Ant + Phe) was all less than one (< 1), which was an indication of petrogenic source of PAH emission (Akyüz and Çabuk 2010). Contrarily, the ratio of Bbf/Bkf is suggestive of the fact that apart from petrogenic and pyrogenic sources, other sources like aluminum works may have added to the observed levels of PAHs as revealed by sample B (Table 4) (Tobiszewski and Namiesnik 2012).
Principal component analysis (PCA)
It could be concluded from the PCA results (Table 5 and Fig. 5) that apart from Phe in PC2, Aph and Flu in PC3, all other PAHs including Nap, Apt, Ant, Fla, Pyr, Ban, Chy, Bbf, Bkf, Idp, and Dbh are due to similar sources of contamination. This is in agreement with the diagnostic analysis which suggested that the PAHs were of pyrogenic origin, primarily as a result of anthropogenic influence due to the presence of the automobile mechanics in the Orji Mechanic Village.
Previous reports indicated that Chy, BbF, Indp, and Dbh are major indicators of PAH emissions associated with fossil fuel combustion in automobile engines (Guo et al. 2003; Dallarosa et al. 2008). Ban and Indp are the primary compounds that indicate combustion of kerosene and gasoline in internal combustion engines, as well as combustion of natural gas (Lim et al. 1999). Fla and Pyr are the major PAHs that result from the straw combustion (Jenkins et al. 1996).
Correlation analysis
The result (Table 6) revealed mainly strong positive correlations among the PAHs. Nap exhibited a strong positive correlation with Bkf (r = 0.960) > Ban (r = 0.936) > , Bbf (r = 0.755) > Dbh (r = 0.741) > Pyr (r = 0.553). A strong positive correlation was observed between Aph and Flu (r = 0.868), Ant (r = 0.575), while it had a strong negative correlation with Indp (r = − 0.548). Similarly, Phe exhibited a strong positive relationship with Fla (r = 0.676), Chy (r = 0.591), while it had a strong negative correlation with Indp (r = − 0.548). Pyr displayed a strong positive correlation with most of the PAHs Bbf (r = 0.735) > BKf (r = 0.638) > Chy (r = 0.599) > Ban (r = 0.545). In the same way, Fla displayed a strong positive correlation with Chy (r = 0.897) > Pyr (0.682) > Bbf (r = 0.663), Ban correlated strongly with, Bkf (r = 0.950) > Dbh (r = 0.730) > Bbf (r = 0.629). Also, Ant had a strong positive correlation with Pyr (r = 0.682) and Dbh (r = 0.593). Furthermore, a strong positive relationship was observed between Chy and Bbf (r = 0.783), Bbf and Bkf (r = 0.778) and Dbh (r = 0.533), Bkf and Dbh (r = 0.850). The observed strong positive correlations suggested similarity in the source of the PAHs which is as result of automobile repair events in the investigated mechanic village.
Health risk assessment
The result of carcinogenic potency as presented in Table 7 revealed BaPeq values in the decreasing order of A > CN1 > CN2 > D > B > C > E. This indicates elevated carcinogenic potency for sample A with sample E showing the least carcinogenic potency. The observed BaPeq values are indications that there could be a serious health risk for the inhabitants of the study area. Result of carcinogenic risk analysis (Table 8) indicated that cancer risk of the PAHs arising from ingestion of the soil particles ranged from 2.35E−05 in sample E to 3.05E−04 in sample B. Risk of cancer to human as a result of inhalation of the dust particles ranged from 7.65E−23 in sample E to 4.47E−22 in sample A. Also, the risk of cancer resulting from dermal contact with the soils containing these PAHs ranged from 2.01E−05 in sample E to 1.18E−04 in sample A. In all, it was observed that samples A and B have the highest carcinogenic risk as indicated in Table 8.
Furthermore, the total cancer risk is also presented in Table 8. It was estimated according to Eq. 6. The lifetime cancer risk could be described using numerical values as suggested by the New York State Department of Health (NYS DOH 2012). The risk is considered very low cancer risk when the risk value is ≤ 10−6 to < 10−4, moderate cancer risk (10−4 ≤ to < 10−3), high cancer risk (10−3 ≤ to < 10−1), and very high cancer risk (≥ 10−1) (NYS DOH 2012; Tarafdar and Sinha 2017). The results shown in Table 6 for the total cancer risk were all above 10–6, which is an indication of the possibility of cancer risk (Hoseini et al. 2016). The results suggested that the investigated section of Orji Mechanic Village posed a moderate cancer risk to the inhabitants of the study area. Therefore, there is a need for the regulatory agencies to intensify efforts to lessen the risk of these environmental contaminants in the study area.
Conclusion
The ecological assessment of polycyclic aromatic hydrocarbon in soils of the abandoned sections of Orji Mechanic Village, Owerri, Imo State, Nigeria, was investigated. The study revealed the presence of polycyclic aromatic compounds (PAHs) in all the sampled soils including the control samples. The results suggested that Dbh showed the highest percentage (53%) of occurrence in all the sampled soils. However, elevated concentrations of the PAHs were observed in the topsoil (0–10 cm) compared to samples collected from other depths (10–20 cm and 21–30 cm).
The diagnostic ratio, PCA, and correlation analysis indicated that pyrogenic sources were responsible for the observed level of the PAHs, likely due of automobile repair and other services rendered in the mechanic village. This mainly results from the spilling of petroleum products during automobile repair and maintenance in the area. The BaPeq values are in the decreasing order of A > CN1 > CN2 > D > B > C > E. This indicates elevated carcinogenic potency for sample A. The result of carcinogenic risk analysis revealed that samples A and B had the highest carcinogenic risk. Similarly, the total cancer risk was above 10–6, which is an indication of a greater risk of cancer due to contact with the contaminated soil samples; hence, the result of this study is a serious ecological concern. There could be a buildup of these polycyclic aromatic contaminants in the study area, which may eventually contaminate the groundwater as well as nearby surface water resources in the area. Therefore, there is a serious need to frequently monitor activities in the study area to avert possible health challenges that may arise from increased levels of the PAHs resulting from the mechanics during automobile maintenance or servicing in the area. Also, serious regulations should be enacted to assist in the control of activities of automobile mechanics in the automobile repair workshops and mechanic villages to lower contamination of the environment with PAHs.
Availability of data and materials
All data and materials are available.
Abbreviations
- PAHs:
-
Polycyclic aromatic hydrocarbons
- Nap:
-
Naphthalene
- Apt:
-
Acenaphthylene
- Aph:
-
Acenaphthene
- Flu:
-
Fluorene
- Phe:
-
Phenanthrene
- Ant:
-
Anthracene
- Fla:
-
Fluoranthene
- Pyr:
-
Pyrene
- Ban:
-
Benzo(a)anthracene
- Chy:
-
Chrysene
- Bbf:
-
Benzo(b)fluoranthene
- Bkf:
-
Benzo(k)fluoranthene
- Idp:
-
Indeno(1,2,3-cd)pyrene
- Dbh:
-
Dibenzo(a,h)anthracene
- ∑PAHs:
-
The sum of all the PAHs determined
- ∑HPAHs:
-
The sum of high molecular weight (4–6 rings) PAHs
- ∑LPAHs:
-
The sum of low molecular weight (2–3 rings) PAHs)
- ∑Comb-PAHs:
-
The sum of combustible PAHs
- STD:
-
Standard deviation
- Min:
-
Minimum
- Max:
-
Maximum
- ANOVA:
-
Analysis of variance
- PCA:
-
Principal component analysis
- IARC:
-
International Agency for Research on Cancer
- GC:
-
Gas chromatography
- USEPA:
-
United State Environmental Protection Agency
- MSD:
-
Mass selective detector
- LOD:
-
Limit of detection
- RSD:
-
Relative standard deviation
- BePeq:
-
Benzo(a) pyrene equivalent
- TEQF:
-
Toxic equivalent factor
- RI:
-
Rate of ingestion
- ESA:
-
Exposed skin area
- SAF:
-
Skin adherence factor
- SAF:
-
Skin adherence factor
- DE:
-
Duration of exposure
- ET:
-
Time of exposure
- AT:
-
Averaging time
- OSF:
-
Oral slope factor
- GIAF:
-
Gastrointestinal absorption factor
- DAF:
-
Dermal absorption factor
- IUR:
-
Inhalation unit risk
- PEF:
-
Particle emission factor
References
Abdel-Shafy HI, Mansour MSM (2016) A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet 25:107–123
Adeniyi AA, Afolabi JA (2002) Determination of total petroleum hydrocarbons and heavy metals in soils within the vicinity of facilities handling refined petroleum products in Lagos Metropolis. Environ International 28:79–82
Akyüz M, Çabuk H (2010) Gas–particle partitioning and seasonal variation of polycyclic aromatic hydrocarbons in the atmosphere of Zonguldak, Turkey. Sci Total Environ 408:5550–5558
Ali N, Imi I, Khoder M, Shamy M, Alghamdi M, Al KA (2017) Polycyclic aromatic hydrocarbons (PAHs) in the settled dust of automobile workshops, health and carcinogenic risk evaluation. Sci Total Environ 601–602:478–484
ANZECC (2000) Australian and New Zealand guidelines for fresh and marine water quality. National Water Quality Management Strategy, vol1 Chapters 1–7, p 314. https://www.waterquality.gov.au/sites/default/files/documents/anzecc-armcanz-2000-guidelines-vol1.pdf. Accessed 10th Sept 2020
ATSDR (1997) Agency for toxic substances and disease registry toxicology profile for used mineral based crankcase oil. Department of Health and Human Services, Public Health Service Press, Atlanta
Bi X, Luo W, Gao J, Xu L, Guo J, Zhang Q, Romesh KY, Giesy JP, Kang S, de Boer J (2016) Polycyclic aromatic hydrocarbons in soils from the Central-Himalaya region: Distribution, sources, and risks to humans and wildlife. Sci Total Environ 556:12–22
Cao W, Yin L, Zhang D, Wang Y, Yuan J, Zhu Y, Dou J (2019) Contamination, Sources, and Health Risks Associated with Soil PAHs in Rebuilt Land from a Coking Plant, Beijing, China. Int J Environ Res Public Health 16(670):1–16
Chiang KC, Chio CP, Chiang YH, Liao CM (2009) Assessing hazardous risks of human exposure to temple airborne polycyclic aromatic hydrocarbons. J Hazard Mater 166:676–685
Christopher M (2008) Polycyclic aromatic hydrocarbons (PAHs) in urban soil: Florida risk assessment perspective. Int J Soil Sedim Water 1(2):1–14
Chukwuocha NAC, Ngah SA, Chukwuocha AC (2017) Vulnerability studies of sensitive watershed areas of Owerri South East Nigeria using digital elevation models. J Geosci Environ Protect 5:1–10
Crnković D, Ristić M, Jovanović A, Antonović D (2007) Levels of PAHs in the soils of Belgrade and its environs. Environ Monit Assess 125:75–83
Dallarosa J, Teixeira EC, Meira L, Wiegand F (2008) Study of the chemical elements and polycyclic aromatic hydrocarbons in atmospheric particles of PM10 and PM 2.5 in the urban and rural areas of South Brazil. Atmos Res 89:76–92
De La Torre-Roche R, Lee WY, Campos-Díaz SI (2009) Soil-borne polycyclic aromatic hydrocarbons in El Paso, Texas: analysis of a potential problem in the United States/Mexico border region. J Hazard Mater 163:946–958
DEPA (2002) Danish Environmental Protection Agency, Guideline on remediation of contaminated sites, Danish Environmental Protection Agency (DEPA), Demark. https://www2.mst.dk/udgiv/publications/2002/87-7972-280-6/pdf/87-7972-281-4.pdf. Accessed 10th Sept 2020
Dong TTT, Lee BK (2009) Characteristics, toxicity, and source apportionment of polycyclic aromatic hydrocarbons (PAHs) in road dust of Ulsan, Korea. Chemosphere 74:1245–1253
DPR (2002) Environmental guidelines and standards for the petroleum industry in Nigeria. Department of Petroleum Resources, Lagos. https://dpr.gov.ng/index/egaspin/. Accessed 10th Sept 2020
Duru CE (2019) Assessment and modeling of heavy metal pollution of soil within reclaimed auto repair workshops in Orji, Imo State Nigeria. Chem J Moldova 14(1):54–60
Duru CE, Okoro IP, Enyoh CE (2017) Quality assessment of borehole water within Orji mechanic village using pollution and contamination models. Int J Chem Mater Environ Res 4(3):123–130
Duru CE, Enedoh MC, Duru IA (2019) Physicochemical assessment of borehole water in a reclaimed section of Nekede mechanic village, Imo State, Nigeria. Chem Africa 2(4):689–698
Ejiogu BC, Opara AI, Nwosu EI, Nwofor OK, Onyema JC, Chinaka JC (2019) Estimates of aquifer geo-hydraulic and vulnerability characteristics of Imo State and environs, Southeastern Nigeria, using electrical conductivity data. Environ Monit Assess 191(4):238. https://doi.org/10.1007/s10661-019-7335-1
Ekanem AN, Osabor VN, Ekpo BO (2019) Polycyclic aromatic hydrocarbons (PAHs) contamination of soils and water around automobile repair workshops in Eket metropolis, Akwa Ibom State, Nigeria. SN Appl Sci 1:447. https://doi.org/10.1007/s42452-019-0397-4
Emoyan OO, Onocha EO, Tesi GO (2020) Concentration assessment and source evaluation of 16 priority polycyclic aromatic hydrocarbons in soils from selected vehicle-parks in southern Nigeria. Sci Afr 7(e00296):1–13
Enyoh CE, Isiuku BO (2020) Characterisation of some soils from flood basin in Amakohia, Owerri. Nigeria Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1773455
Essumang DK, Kowalski K, Sogaard EG (2011) Levels, distribution and source characterization of polycyclicaromatic hydrocarbons (PAHs) in top soils and roadside soils in Esbjerg, Denmark. Bull Environ Contam Toxicol 86:438–443
Farombi AG, Adebayo OR, Oyekanmi AM (2013) Impact of petroleum product on the soil around automobile workshops in Osun State. IOSR J Appl Chem (IOSR-JAC) 4(1):13–15
Galarneau E (2008) Source specificity and atmospheric processing of airborne PAHs: implications for source apportionment. Atmos Environ 42:8139–8149
Guo H, Lee S, Ho K, Wang X, Zou S (2003) Particleassociated polycyclic aromatic hydrocarbons in urban air of Hong Kong. Atmos Environ 37:5307–5317
Hoseini M, Yunesian M, Nabizadeh R, Yaghmaeian K, Ahmadkhaniha R, Rastkari N, Parmy S, Faridi S, Rafiee A, Naddafi K (2016) Characterization and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in urban atmospheric Particulate of Tehran, Iran. Environ Sci Pollut Res 23:1820–1832
Hu G, Chen S, Shi W, Zhang B, Zhang Y, Huang J, Chen J, Giesy JP, Yu H (2014) Identification of polycyclic aromatic hydrocarbons in soils in Taizhou, East China. Geochem Health 1:13
Hwang HM, Wade TL, Sericano JL (2003) Concentrations and source characterization of polycyclic aromatic hydrocarbons in pine needles from Korea, Mexico, and United States. Atmos Environ 37:2259–2267
IARC (2002) International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention; International Agency for Research on Cancer: Beverly Hills. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Handbooks-Of-Cancer-Prevention/Breast-Cancer-Screening-2002. Accessed 13th Oct 2020
Ibe FC, Opara AI, Duru CE, Isiuku BO, Enedoh MC (2020) Statistical analysis of atmospheric pollutant concentrations in parts of Imo State, Southeastern Nigeria. Sci Afr 7(e00237):1–27
Ipeaiyeda AR, Dawodu M (2008) Heavy metals contamination of topsoil and dispersion in the vicinities of reclaimed auto-repair workshops in Iwo, Nigeria. Bull Chem Soc Ethiopia 22(3):339–348
Iwegbue CM (2007) Metal fractionation in soil profiles at automobile Mechanic Waste Dumps around Port Harcourt. Waste Manag Res 25(6):585–593
Jenkins BM, Jones AD, Turn SQ, Williams RB (1996) Emission factors for polycyclic aromatic hydrocarbons from biomass burning. Environ Sci Technol 30:2462–2469
Jia J, Bi C, Guo X, Wang X, Zhou X, Chen Z (2017) Characteristics, identification, and potential risk of polycyclic aromatic hydrocarbons in road dusts and agricultural soils from industrial sites in Shanghai. China Environ Sci Pollut Res 24:605–615
Jiao H, Wang Q, Zhao N, Jin B, Zhuang X, Bai Z (2017) Distributions and sources of polycyclic aromatic hydrocarbons (PAHs) in soils around a chemical plant in Shanxi, China. Int J Environ Res Public Health 14(1198):1–19
Kidman RL, Boehlecke R (2011) Evaluating petroleum hydrocarbon-contaminated soil. In: WM 2011 conference, February 27–March 3, 2011, Phoenix
Kinawy AA (2009) Impact of gasoline inhalation on some neurobehavioural characteristics of male rats. BMC Physiol 9:21–27
Kwon HO, Choi SD (2014) Polycyclic aromatic hydrocarbons (PAHs) in soils from a multi-industrial city, South Korea. Sci Total Environ 470:1494–1501
Lasota J, Błońska E (2018) Polycyclic aromatic hydrocarbons content in contaminated forest soils with different humus types. Water Air Soil Pollut 229:204. https://doi.org/10.1007/s11270-018-3857-3
Li H, Yu L, Sheng G, Fu J, Peng P (2007) Severe PCDD/F and PBDD/F pollution in air around an electronic waste dismantling area in China. Environ Sci Technol 41:5641–5646
Li H, Liu G, Cao Y (2014) Content and distribution of trace elements and polycyclic aromatic hydrocarbons in fly ash from a coal-fired CHP plant. Aerosol Air Qual Res 14:1179–1188
Lim LH, Harrison RM, Harrad S (1999) The contribution of traffic to atmospheric concentrations of polycyclic aromatic hydrocarbons. Environ Sci Technol 33:3538–3542
Man YB, Kang Y, Wang HS, Lau W, Li H, Sun XL et al (2013) Cancer risk assessments of Hong Kong soils contaminated by polycyclic aromatic hydrocarbons. J Hazard Mater 261:770–776
Manoli E, Kouras A, Samara C (2004) Profile analysis of ambient and source emitted particle-bound polycyclic aromatic hydrocarbons from three sites in northern Greece. Chemosphere 56:867–878
Marr LC, Dzepina K, Jimenez JL, Reisen F, Bethel HL, Arey J, Gaffney JS, Marley NA, Molina LT, Molina MJ (2006) Sources and transformations of particle-bound polycyclic aromatic hydrocarbons in Mexico City. Atmos Chem Phys 6:1733–1745
Mastral AM, Callen MS (2000) A review on polycyclic aromatic hydrocarbon (PAH) emissions from energy generation. Environ Sci Technol 34:3051–3057
MHSPEN (2000) Ministry of Housing Spatial Planning and Environment, “Circular on target values and intervention values for soil remediation,” Ministry of Housing Spatial Planning and Environment, Netherlands, p 120. http://esdat.net/Environmental%20Standards/Dutch/ENGELSE%20versie%20circulaire%20Bodemsanering%202009.pdf. Accessed 10th Sept 2020
Muze NE, Opara AI, Ibe FC, Njoku OC (2020) Assessment of the geo-environmental effects of activities of auto-mechanic workships at Alaoji Aba and Elekahia Port Harcourt, Niger Delta, Nigeria. Environ Anal Health Toxicol 35(2):1–12
Njoku P, Ibe FC, Alinnor J, Opara A (2016) Seasonal variability of carbon monoxide (CO) in the ambient environment of Imo State, Nigeria. Int Lett Nat Sci 5:40–52
Nwoko CO, Njoku-Tony RF, Nlemedim PU, Ihugba UA (2017) Assessment of the Distribution Pattern of Poly Aromatic Hydrocarbons Around Nekede Auto-mechanic Village, Imo State, Nigeria. J Chem Environ Biol Eng 1(2):27–33
NYS DOH (2012) Health consultation: hopewell precision area groundwater contamination site town of east fishkill, Dutchess County, New York, NY. Appendix C, DOH procedure for evaluating potential health risks for contaminants of concern, New York
Obidike IR, Maduabuchi IU, Olumuyiwa SSV (2007) Testicular morphology and cauda epididymal sperm reserves of male rats exposed to Nigerian Qua Iboe Brent crude oil. J Vet Sci 8(1):1–5
Obini U, Okafor CO, Afiukwa JN (2013) Determination of levels of polycyclic aromatic hydrocarbons in soil contaminated with spent motor Engine oil in Abakaliki Auto-Mechanic Village. J Appl Sci Environ Manag 17(2):169–175
Odjegba VJ, Sadiq AO (2002) Effects of spent engine oil on the growth parameters, chlorophyll and protein levels of Amaranthus hybridus L. Environmentalist 22:23–28
Ogoko EC (2014) Evaluation of polycyclic aromatic hydrocarbons, total petroleum hydrocarbons and some heavy metals in soils of NNPC Oil Depot Aba Metropolis, Abia State, Nigeria. IOSR J Environ Sci Toxicol Food Technol 8(5):21–27
Okparanma R, Mouazen A (2013) Determination of total petroleum hydrocarbon (TPH) and polycyclic aromatic hydrocarbon (PAH) in soils: a review of spectroscopic and non-spectroscopic techniques. Appl Spectrosc Rev 48(6):458–486
Ololade IA (2014) An assessment of heavy metal contamination in soils within auto-mechanic workshops using enrichment and contamination factors with geoaccumulation Indexes. J Environ Protect 5:970–982
Olugboji OA, Ogunwole OA (2008) Use of spent engine oil. AU JT 12(1):67–71
Onyeagocha AC (1980) Petrography and depositional environment of the Benin Formation, Nigeria. J Min Geol 17:147–158
Onyedikachi UB, Belonwu CD, Wegwu MO, Ejiofor E, Awah FM (2019) Sources and cancer risk exposure of polycyclic aromatic hydrocarbons in soils from industrial areas in Southeastern, Nigeria. J Chem Health Risk 9(3):203–216
Opara AI, Ibe FC, Njoku PC, Alinnor JI, Enenebeaku CK (2016) Geospatial and geostatistical analyses of particulate matter (PM10) concentrations in Imo State, Nigeria. Int Lett Nat Sci 57:89–107
Paíga P, Mendes L, Albergaria JT, Delerue-Matos CM (2012) Determination of total petroleum hydrocarbons in soil from different locations using infrared spectrophotometry and gas chromatography. Chem Pap 66(8):711–721
Peng C, Chen W, Liao X, Wang M, Ouyang Z, Jiao W, Bai Y (2011) Polycyclic aromatic hydrocarbons in urban soils of Beijing: status, sources, distribution and potential risk. Environ Pollut 159:802–808
Pies C, HoffmannB PJ, Yang Y, Ternes TA, Hofmann T (2008) Characterization and source identification of polycyclic aromatic hydrocarbons (PAHs) in river bank soils. Chemosphere 72:1594–1601
Ravindra K, Sokhi R, Van Grieken R (2008) Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmos Environ 42:2895–2921
Sadler R, Connell D (2003) Analytical methods for the determination of total petroleum hydrocarbons in soil. In: Langley et al (eds) Proceedings of the 5th national workshop on the assessment of site contamination, national environment protection council service corporation 2003, Adelaide
Sharifi M, Sadeghi Y, Akbarpour M (2007) Germination and growth of six plant species on contaminated soil with spent oil. Int J Environ Sci Technol 4(4):3–470
Suman S, Sinha A, Tarafdar A (2016) Polycyclic aromatic hydrocarbons (PAHs) concentration levels, pattern, source identification and soil toxicity assessment in urban traffic soil of Dhanbad, India. Sci Total Environ 545–546:353–360
Tarafdar A, Sinha A (2017) Cancer risk assessment of polycyclic aromatic hydrocarbons in the soils and sediments of India: a meta-analysis. Environ Manag. https://doi.org/10.1007/s00267-017-0920-6
Tian F, Chen W, Qiao X, Wang Z, Yang P, Wang D, Ge L (2009) Sources and seasonal variation of atmospheric polycyclic aromatic hydrocarbons in Dalian, China: factor analysis with non-negative constraints combined with local source fingerprints. Atmos Environ 43:2747–2753
Tobiszewski M, Namiesnik J (2012) PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut 162:110–119
Tsai P, Shih T, Chen H, Lee W, Lai C, Liou S (2004) Assessing and predicting the exposures of polycyclic aromatic hydrocarbons (PAHs) and their carcinogenic potencies from vehicle engine exhausts to highway toll station workers. Atmos Environ 38:333–343
USEPA (1991) United States Environmental Protection Agency Risk Assessment Guidance for Super Fund, Volume1, Human Health Evaluation Manual (Part B, Development of Risk-Based Preliminary Remediation Goals); OSWER, 9285.7-01B.EPA/540/R-92/003, US Environmental Protection Agency, Washington, DC
USEPA (1996a) United States Environmental Protection Agency, Method 3540C, test methods for evaluating solid waste, physically chemical methods. SW-846 Annual. US Environ. Protect. Agency. Revision 3. https://nepis.epa.gov/Exe/ZyPDF.cgi/50000U6E.PDF?Dockey=50000U6E.PDF. Accessed 6th October, 2020
USEPA (1996b) Test methods for evaluating solid waste, physical chemical methods, methods 8270-C for semi-volatile organic compounds by Gas Chromatography Mass GC-MS., SW-846, 3rd edn. US Government Printing Office, Washington, DC. https://www.epa.gov/esam/epa-method-8270e-sw-846-semivolatile-organic-compounds-gas-chromatographymass-spectrometry-gc. Accessed 6th Oct 2020
USEPA (2009) United States Environmental Protection Agency, Risk Assessment Guidance for Superfund. Vol. I: Human Health Evaluation Manual (F, Supplemental Guidance for Inhalation Risk Assessment) EPA/540/R/070/002, Office of Superfund Remediation and Technology Innovation, Washington, DC. https://www.epa.gov/sites/production/files/2015-09/documents/rags_a.pdf. Accessed 17th Oct 2020
USEPA (2011) Exposure factors handbook 2011 edition (final report). US Environmental Protection Agency, Washington, DC, EPA/600/R-09/052F. https://www.nrc.gov/docs/ML1400/ML14007A666.pdf. Accessed 17th Oct 2020
USEPA (2012) United States Environmental Protection Agency, Mid Atlantic risk assessment, Regional Screening Level (RSL) Summary Table, 2012. http://www.epa.gov/reg3hwmd/risk/human/rb-concentrationtable/usersguide.htm Accessed 16th Oct 2020
Wang J, Jia CR, Wong CK, Wong PK (2000) Characterization of polycyclic aromatic hydrocarbon created in lubricating oils. Water Air Soil Poll 120:381–396
Wang W, Huang MJ, Kang Y, Wang H-S, Leung AOW, Cheung KC, Wong MH (2011) Polycyclic aromatic hydrocarbons (PAHs) in urban surface dust of Guangzhou, China: status, sources and human health risk assessment. Sci Total Environ 409:4519–4527
Wang L, Zhang S, Wang L, Zhang W, Shi X, Li X, Li X (2018) Concentration and risk evaluation of polycyclic aromatic hydrocarbons in urban soil in the typical Semi-Arid City of Xi’an in Northwest China. Int J Environ Res Pub Heal 15(4):607–622
Wcisło E (1998) Soil contamination with polycyclic aromatic hydrocarbons (PAHs) in Poland—a review. Polish J Environ Stud 7(5):267–272
Yu XZ, Gao Y, Wu SC, Zhang HB, Cheung KC, Wong MH (2006) Distribution of polycyclic aromatic hydrocarbons in soils at Guiyu area of China, affected by recycling of electronic waste using primitive technologies. Chemosphere 65:1500–1509
Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organ Geochem 33:489–515
Acknowledgements
The authors acknowledge and appreciate TETFUND for their financial assistance. The assistance of the field technicians is acknowledged. We also appreciate Prof. Erema Darka of Rivers State University of Science and Technology, Rivers State, Nigeria, for his assistance during sample analysis.
Funding
This research was supported by the Tertiary Education Trust Fund (TETFUND) of the Federal Government of Nigeria under Grant No. TETFUND/DESS/UNI/OWERRI/2015/RP/VOL.I.
Author information
Authors and Affiliations
Contributions
FCI contributed to conceptualization, visualization, methodology, investigation, data analysis, validation, supervision, writing original—draft of the manuscript, review, and editing. ECD helped in conceptualization, validation, visualization, and editing. BOI helped in visualization, validation, writing review, and editing. JNA helped in methodology, review, and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibe, F.C., Duru, C.E., Isiuku, B.O. et al. Ecological risk assessment of the levels of polycyclic aromatic hydrocarbons in soils of the abandoned sections of Orji Mechanic Village, Owerri, Imo State, Nigeria. Bull Natl Res Cent 45, 18 (2021). https://doi.org/10.1186/s42269-021-00485-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-021-00485-2